Динамика содержания общего белка, гемоцианина и активности антиоксидантных ферментов в условиях острой гипертермии у легочного моллюска Lymnaea stagnalis
Автор: Хомич А.С., Голубев А.П., Аксенов-грибанов Д.В., Бодиловская О.А., Широкова И.А., Лубяга И.А., Шатилина З.М.
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 1 т.14, 2018 года.
Бесплатный доступ
Была оценена динамика содержания общего белка и гемоцианина в гемолимфе, а также активность антиоксидантных ферментов (пероксидаза, каталаза и глутатион-S-трансфераза) в легочном моллюске Lymnaea stagnalis (Linnaeus, 1758) под воздействием острого температурного стресса. , Показано, что воздействие острого термического стресса (30 ° С) приводит к активации физиологических механизмов стрессоустойчивости и не влияет на активность антиоксидантных ферментов в исследуемой популяции моллюсков.
Короткий адрес: https://sciup.org/143165193
IDR: 143165193
Текст научной статьи Динамика содержания общего белка, гемоцианина и активности антиоксидантных ферментов в условиях острой гипертермии у легочного моллюска Lymnaea stagnalis
Возрастающее загрязнение водоемов представляется серьезной экологической и социальной проблемой. Несмотря на ряд проведенных мероприятий по очистке и сохранению уникальных природных экосистем, спектр загрязнителей различной природы постоянно расширяется. Эффекты антропогенного воздействия в первую очередь проявляются на пресных континентальных водоемах, так как именно благодаря своей значимости в жизни человека, они чаще попадают в сферу промышленного освоения (Camargo & Alonso, 2006; Zuykov et al. , 2013).
Повышение температуры водоемов, как в результате глобального изменения климата, так и сброса в них подогретых вод ГРЭС и АЭС («тепловое загрязнение»), отрицательно сказывается на состоянии их биоты и приводит к снижению видового разнообразия (Courrat et al. , 2009; Kennish, 2002; Vasconcelos et al. , 2007). Значительные температурные колебания вызывают нарушения в сложных системах биотических связей в водных экосистемах и, как следствие, приводят к снижению их стабильности (Niinemets et al. , 2017). Обитающие в данных условиях организмы вынуждены приспосабливаться к воздействию таких колебаний, что, по-видимому, ведет к повышению их стресс-резистентности на биохимическом и на популяционном уровне.
Одним из распространенных объектов биомониторинга для изучения последствий антропогенного и температурного загрязнения водоемов является легочной моллюск Lymnaea stagnalis (Linnaeus, 1758) (Gnatishina et al., 2011; Gust et al., 2013; Khomich et al., 2017; Reategui-Zirena et al., 2017). Данный вид, широко распространенный по всей умеренной зоне Евразии, способен существовать в водоемах с высоким уровнем загрязнения различной природы (Golubev et al., 2005). L. stagnalis населяет мелкие стоячие и малопроточные водоемы с сильно различающимися температурными, гидрологическими и лимнологическими характеристиками. Он является одним из немногих беспозвоночных, способных существовать в водоемах-охладителях ГРЭС и АЭС при температурах до 30–33 оС.
В связи с этим, целью настоящего исследования являлась оценка влияния острой гипертермии на некоторые физиологические и биохимические стресс-реакции, а именно – изменение содержания общего белка и гемоцианина в гемолимфе, а также оценка активности ферментов антиоксидантной системы (пероксидазы, каталазы и глутатион S-трансферазы) в мышечной ткани у L. stagnalis .
MATERIALS AND METHODS
Для исследования использованы половозрелые особи L. stagnalis (высота раковины в пределах 35 мм), отловленные в октябре 2017 года в заводи реки Ангара на острове Юность в черте г. Иркутска (52,26775292º с. ш. и 104,28403115º в. д.).
Отловленных животных перед экспериментальной экспозицией акклимировали в лабораторных условиях в аэрируемых аквариумах объемом 4 л по 10-15 особей на протяжении 10 суток. Температура воды составляла 6,5 ºС и соответствовала температуре отлова. Корм задавали с избытком, смену воды и корма производили раз в 3 суток. Отсутствие гибели в акклимационный период позволило предположить, что содержание в лабораторных условиях не являлось моллюсков стрессовым.
Перед проведением экспериментов моллюсков случайным образом разделяли на две группы. Первую группу (экспериментальная экспозиция) помещали в термостатируемые аквариумы при температуре воды 30 ºC, где их экспонировали в течение 28 ч. Данная температура близка к верхнему пределу зоны температурной толерантности для пресноводных моллюсков (Khmeleva et al. , 1984; Golubev, 1995) и при ее воздействии L. stagnalis подвергаются сильному тепловому стрессу, хоть и способны существовать довольно продолжительное время в природных водоемах при 30 оС (Sidorov, 2003). Через час после начала экспозиции, а затем каждые 4 ч, то есть через 4, 8, 12,16 и так далее до 28 ч, у моллюсков отбирали гемолимфу и мышечную ткань, которую тут же фиксировали в жидком азоте.
Контрольных особей фиксировали до начала проводимого эксперимента.
Оценку содержания общего белка и гемоцианина в гемолимфе осуществляли по методике Никерсона и ван Холде (Nickerson & Van Holde, 1971). Активность ферментов антиоксидантной системы (пероксидазы, каталазы и глутатион S-трансферазы) исследовали с применением спектрофотометрических методов (Habig et al. , 1974; Aebi, 1984; Drotar et al. , 1985) с модификациями М.А. Тимофеева (2006, 2010). Измерения проводили на спектрофотометре Cary 50 (Varian, США) при λ=280 нм для оценки содержания общего белка и при λ=335 нм для измерения концентрации дыхательного пигмента гемоцианина. Активность ферментов антиоксидантной системы (пероксидазы, каталазы и глутатион S-трансферазы) определяли при λ=340 нм, λ=240 нм и λ=436 нм соответственно. Для оценки удельной активности ферментов в образцах определяли содержание суммарного белка по методу М. Бредфорд (Bradford, 1976). Все измерения проведены в 3-х аналитических повторностях.
Проверку нормальности распределения проводили, используя критерий Шапиро-Уилка (P > 0,05), анализ достоверных различий проводили по методу Манна-Уитни (P < 0,05) с применением метода Холма для множественных сравнений, в пакете программ STATISTICA 8.0.
RESULTS
Содержание общего белка в контрольных образцах гемолимфы составило 1,24±0,21 мг/мл. Через 4 ч экспериментального воздействия количество белка составляло 2,70±0,8 мг/мл (P = 0,007) и двукратно превышало значение в контрольной группе. В ходе последующей экспозиции содержание белка снижалось до контрольных величин (рис. 1).
При оценке содержания гемоцианина установлено, что экспозиция в условиях острой гипертермии вела к полуторакратному повышению его концентрации от уровня в контрольных образцах (P=0,018) после 4 ч экспозиции (рис. 2). Затем содержание гемоцинанина возвращалось к контрольному уровню. При достижении 28 ч эксперимента отмечали пятикратное снижение содержания дыхательного пигмента (P = 0,035).
Среднее значение активности пероксидазы, глутатион S-трансферазы и каталазы у L. stagnalis составило 0,68±0,14 нКат/мг белка, 6,50±0,63 нКат/мг белка и 226,3±35,81 нКат/мг белка соответственно (рис. 3). Статистически значимых изменений активности исследуемых ферментов при стрессовом воздействии не наблюдали.
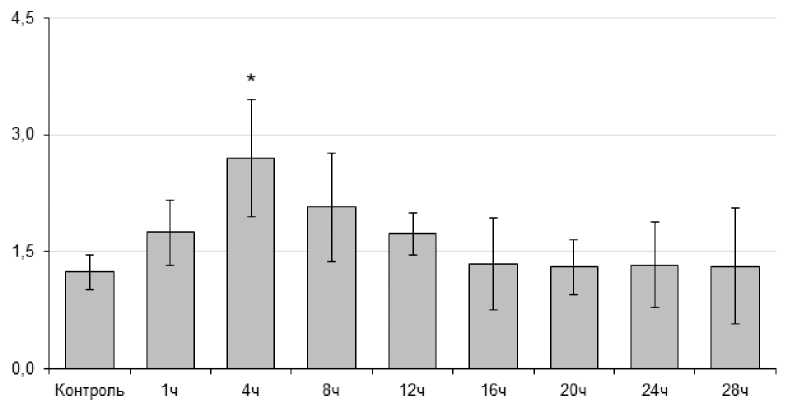
Figure 1. Динамика содержания общего белка (в мг/мл) у L. stagnalis при острой гипертермии (30 ºС).
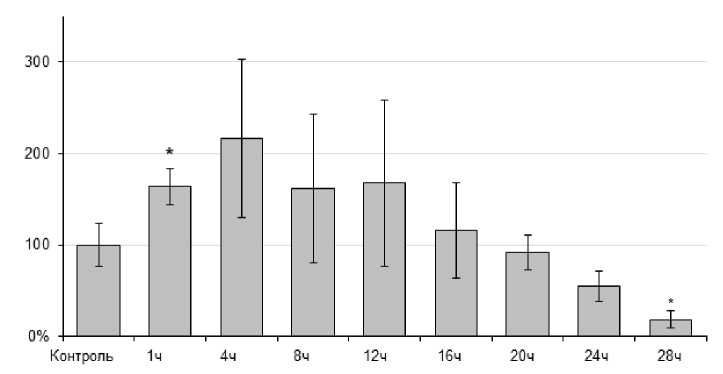
Figure 2. Динамика содержания гемоцианина (в % )у L. stagnalis при острой гипертермии (30 ºС)
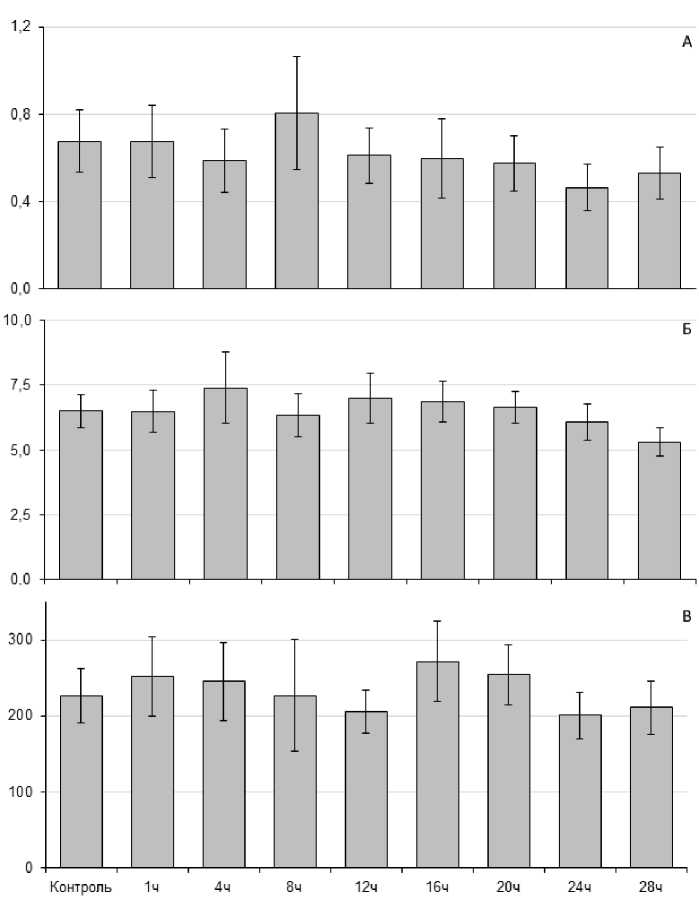
Figure 3. Оценка активности ферментов АОС (в нКат/мг белка) у L. stagnalis , при острой гипертермии (30 ºС) (А - пероксидаза; Б - глутатион S-трансфераза; В - каталаза).
DISCUSSION
Как следует из полученных результатов, в условиях острой гипертермии у L. stagnalis происходит быстрое повышение содержания общего белка и гемоцианина в гемолимфе уже через 4 ч экспозиции при стрессовой температуре. Это свидетельствует об активации физиологических реакций стресс-адаптации (Giomi & Portner, 2013). Установлено, что общее содержание белка сильно коррелировало с содержанием гемоцианина (r=0,84 P<0,05). Учитывая, что у моллюсков на долю гемоцианина приходится 90-98% от всей массы белков в гемолимфе (Coates et al. , 2012), повышение содержания общего белка косвенно указывает на интенсификацию физиологических механизмов направленных на преодоление последствий недостатка кислорода.
Выявленное увеличение концентрации гемоцианина в начале экспозиции свидетельствует о развитии тканевой гипоксии и повышенной потребности в снабжении тканей кислородом с помощью дыхательного пигмента гемоцианина. Гемоцианин является важнейшим белком гемолимфы лимнеид и представляет собой хромопротеин, состоящий из глобина и медьсодержащей простетической группы (Jürgen, 2013). Данный белок осуществляет транспорт кислорода, однако помимо этого, участвует в поддержании гомеостаза, транспорте гормонов, осморегуляции и выполняет защитную функцию для других белков (Coates & Nairn, 2014; Becker et al. , 2014; Paul & Pirow, 1998).
Тканевая гипоксия часто развивается на фоне воздействия повышенной температуры (Conley et al., 2007). Естественной физиологической реакцией для моллюсков является усиление легочной аспирации с целью восполнения недостатка кислорода, одного из ключевых компонентов системы энергообеспечения (Sidorov, 2005). Снижение концентрации гемоцианина после 28 ч экспозиции, указывает на замедление метаболизма исследуемого вида и косвенно свидетельствует об активации менее энергозатратных механизмов сохранения гомеостаза (Hochachka, Somero, 2014).
Острая гипертермия оказывает прямое влияние на состояние водных организмов и приводит к развитию оксидативного стресса (Lushchak, 2011; Madeira et al. , 2013; Vinagrea et al. , 2012). Образующиеся активные формы кислорода и их производные имеют высокую реакционную способность и повреждают биологические молекулы (Ray et al. , 2012). Одним из защитных механизмов, способных противостоять таким стрессовым нагрузкам, являются ферменты антиоксидантной системы (Axenov-Gribanov et al. , 2015; Verlecar et al. , 2007). Однако в нашем исследовании предъявляемое температурное воздействие не приводило к изменению активности антиоксидантных ферментов: пероксидазы, глутатион-S трансферазы и каталазы, что свидетельствует о достаточно высокой адаптивной способности исследуемой популяции моллюсков.
Раннее было показано, что острая гипертермия приводит к изменению активности указанных ферментов у популяций L. stagnalis , обитающих в водоемах с более стабильным температурным режимом (Axenov-Gribanov et al. , 2016). Вероятно, отсутствие биохимических изменений у моллюсков в нашем исследовании связано с довольно сильным нагревом воды в литоральной зоне водоема, где обитают представители вида L. stagnalis . В таких водоемах температура воды в летний период может прогреваться до 25-30 oC, что значительно превышает верхний предел зоны температурного комфорта у L. stagnalis (Foster et al. , 2015). По-видимому, обитание в этих условиях на протяжении длительного периода времени способствовало усилению существующих механизмов температурной адаптации.
Таким образом, в ходе настоящего исследования показано, что острая гипертермия приводит к активации физиологических механизмов стресс-резистентности и не оказывает влияние на активность ферментов антиоксидантной системы у исследуемой популяции моллюсков.
ACKNOWLEDGMENT
Исследование выполнено при основной финансовой поддержке гранта РФФИ в рамках научных проектов № 17-34-50012 (выплата заработной платы Хомичу А.С.), № 16-34-60060 (выплата заработной платы Аксенову-Грибанову Д.В.), при частичной финансовой поддержки гранта РНФ 17-14-01063 (выплата заработной платы Широковой Ю.А.), а также базовой части Госзадания 6.9654.2017/8.9 (выплата заработной платы Шатилиной Ж.М., Лубяга Ю.А.) и задания № 5.3.14 Государственной программы научных исследований Республики Беларусь «Химические технологии и материалы, природно-ресурсный потенциал».
Список литературы Динамика содержания общего белка, гемоцианина и активности антиоксидантных ферментов в условиях острой гипертермии у легочного моллюска Lymnaea stagnalis
- Aebi H. (1984) Catalase in vitro. Methods Enzymol., 105, 121-126
- Axenov-Gribanov D., Vereshchagina K., Lubyaga Y., Gurkov A., Bedulina D., Shatilina Z., Homich A., Golubev A. and Timofeyev M. (2015) Stress response at the cellular and biochemical levels indicates the limitation of the environmental temperature range for Eastern Siberia populations of the common gastropod Limnaea stagnalis. Malacologia, 59(1), 33-44
- Axenov-Gribanov D.V., Khomich A.S., Bodilovskaya O.A., Kondratieva E.S., Lubyaga Y.A., Shatilina Z.M., Emshanova V.A. and Golubev A.P. (2016) The estimation of the antioxidant enzymes activity in representatives of different populations of Lymnaea stagnalis differ in the degree of infestation under temperature stress. J. Stress Physiol. Biochem., 12(3), 84-91
- Becker M.I., Arancibia S., Salazar F., Del Campo M. and De Ioannes A. (2014). Mollusk hemocyanins as natural immunostimulants in biomedical applications, immune response activation, Dr. Ht Duc (Ed.), InTech. Available from: https://www.intechopen.com/books/immune-response-activation/mollusk-hemocyanins-as-natural-immunostimulants-in-biomedical-applications
- Bradford M.M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem., 72(1-2), 248-254
- Camargo J.A. and Alonso Á. (2006) Ecological and toxicological effects of inorganic nitrogen pollution in aquatic ecosystems: A global assessment. Environ. Int., 32(6), 831 -849
- Coates C. J. and Nairn J. (2014). Diverse immune functions of hemocyanins. Dev. Comp. Immunol., 45(1), 43-55
- Coates C.J., Bradford E.L., Krome C.A. and Nairn J. (2012) Effect of temperature on biochemical and cellular properties of captive Limulus polyphemus. Aquaculture, 334-337, 30-38
- Conley D. J., Carstensen J., Aertebjerg G., Christensen P.B., Dalsgaard T., Hansen J.L.S. and Josefson A.B. (2007) Long-term changes and impacts of hypoxia in Danish coastal waters. Ecol Appl., 17, 165-184
- Courrat A., Lobry J., Nicolas D., Laffargue P., Amara R., Lepage M., Girardin M. and Le Pape O. (2009) Anthropogenic disturbance on nursery function of estuarine areas for marine species. Estuar Coast Shelf Sci., 81, 179-190
- Drotar A., Phelps P. and Fall R. (1985) Evidence for glutathione peroxidase activities in cultured plant cells. Plant Sci., 42, 35 -40
- Foster N.L., Lukowiak K. and Henry T.B. (2015) Time-related expression profiles for heat shock protein gene transcripts (HSP40, HSP70) in the central nervous system of Lymnaea stagnalis exposed to thermal stress. Commun. Integr. Biol., 8, DOI: 10.1080/19420889.2015.1040954
- Giomi F. and Poertner H. (2013) A role for haemolymph oxygen capacity in heat tolerance of eurythermal crabs. Front. Physiol., 4, 110-121
- Gnatishina L.L., Falfushinskaya G.I., Golubev O.P., Dallinger R. and Stoliar O.B. (2011) Role of metallothioneins in adaptation of Lymnaea stagnalis (Mollusca: Pulmonata) to environment pollution. Hydrobiol. J., 47(5). 56-66
- Golubev A. P., Bodilovskaya O. A., Khomich A. S., Korotchikova N. V., Vereshchagina K. P., Lubyaga Y. A., Shchapova E. P., Shatilina Z. M. and Axenov-Gribanov D. V. (2015) The influence of trematode invasion on the thermoresistance of Lymnaea stagnalis (Gastropoda, Pulmonata) population from the floodplain reservoir of Angara river. J. Stress Physiol. Biochem., 11(2), 28-39
- Golubev A., Afonin V., Maksimova S. and Androsov V. (2005) The current state of pond snail Lymnaea stagnalis (Gastropoda, Pulmonata) populations from water reservoirs of the Chernobyl nuclear accident zone. Radioprotection, 40(1), 511-517
- Golubev A.P. (1995) Thermotholerance and radioresistance in population of Lymnaea stagnalis (Gastropoda, Pulmonata) from reservoir with different forms of anthropogenic load. Rep. Acad. Sci., 342(2), 280 -283
- Gust M., Fortier M., Garric J., Fournie M. and Gange F. (2013) Immunotoxicity of surface waters contaminated by municipal effluents to the snail Lymnaea stagnalis. Aquat. Toxicol., 126, 393-403
- Habig W.H., Pabst M.J. and Jakoby W.B. (1974) Glutathione S-Transferases: The first enzymatic step in mercapturic acid formation. J. Biol. Chem., 249, 7130 -7139
- Hochachka P. W. and Somero G. N. (2014) Biochemical Adaptation. Princeton Legacy Library, 560 p
- Jürgen M. (2013) Evolution of molluscan hemocyanin structures. BBA Proteins and Proteomics, 1834(9), 1840-1852
- Kennish M.J. (2002) Environmental threats and environmental future of estuaries. Environ. Conserv., 29, 78-107
- Khmeleva N.N., Golubev A.P. and Laenko T.M. (1985) Ecology of pulmonate mollusks from heat sources of Kamchatka. J. General Biol., 46(2), 230-240
- Khomich A.S., Axenov-Gribanovb D. V., Bodilovskaya O. A., Shirokova Y. A., Shchapova E. P., Lubyaga Y. A., Shatilina Z. M., Emshanova V. A. and Golubev. A. P. (2017) Assessment of the joint effect of thermal stress, pollution, and parasitic infestation on the activity of antioxidative enzymes in pulmonate mollusk Lymnaea stagnalis. Contemp. Probl. Ecol., 10(2),184-192
- Lushchak V.I. (2011) Environmentally induced oxidative stress in aquatic animals. Aquat. Toxicol., 101(1), 13-30
- Madeira D., Narciso L., Cabral H.N., Vinagre C. and Diniz M.S. (2013) Influence of temperature in thermal and oxidative stress responses in estuarine fish. Comp. Biochem. Physiol. A Mol. Integr. Physiol., 166(2), 237-243
- Nickerson K. W. and Van Holde K. E. (1971) A comparison of molluscan and arthropod hemocyanin. I. Circular dichroism and absorption spectra. Comp. Biochem. Physiol., 39B, 855-872
- Niinemets Ü., Kahru A., Nõges P., Tuvikene A., Vasemägi A., Mander Ü. and Nõges T. (2017) Environmental feedbacks in temperate aquatic ecosystems under global change: why do we need to consider chemical stressors? Reg. Environ. Change., 17(7), 2079-2096
- Paul R.J. and Pirow R. (1998) The physiological significance of respiratory proteins in invertebrates. Zoology, 100, 298-306
- Ray P.D., Huang B-W. and Tsuji Y. (2012) Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal., 24(5), 981-990
- Reategui-Zirena E. G., French A. D., Klein D. M., and Salice C. J. (2017) Cadmium compartmentalization in the pulmonate snail Lymnaea stagnalis: improving our understanding of exposure. Environ. Contam. Toxicol., 72(4), 575-585
- Sidorov A. V. (2005) Effect of acute temperature change on lung respiration of the mollusc Lymnaea stagnalis. J. Therm. Biol., 30. 163-171
- Sidorov A.V. (2003) The effect of temperature on respiration, defensive reactions and the locomotor behavior of the freshwater pulmonate mollusk Lymnaea stagnalis. Pavlov J. High. Nerv. Act., 4, 513-517
- Vasconcelos R.P., Reis-Santos P., Fonseca V., Maia A., Ruano M., Franza S., Vinagre C., Costa M.J. and Cabral H. (2007) Assessing anthropogenic pressures on estuarine fish nurseries along the Portuguese coast: A multi-metric index and conceptual approach. Sci. Total Environ., 374, 199-215
- Verlecar X.N., Jena K.B. and Chainy G.B.N. (2007) Biochemical markers of oxidative stress in Perna viridis exposed to mercury and temperature. Chem. Biol. Interact., 167(3), 219-226
- Vinagrea C., Madeira D., Narciso L., Henrique N. and Diniz C. M. (2012) Effect of temperature on oxidative stress in fish: Lipid peroxidation and catalase activity in the muscle of juvenile seabass, Dicentrarchus labrax. Ecol Indic., 23, 274-279
- Zuykov M., Pelletier E. and Harper D.A.T. (2013) Bivalve mollusks in metal pollution studies: From bioaccumulation to biomonitoring. Chemosphere, 93(2), 201-208