Ecotoxicological implications of Cascabela thevetia (L.) Lippold seed aqueous extract-mediated genetoxicity in Lathyrus sativus Nucleolus, Chana punctatus, and Gallus gallus RBC: a comparative biomarker-based toxicological bioassay
Автор: Adhikari Dipan, Ghosh Rahul, Pal Sarmila
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 3 т.20, 2024 года.
Бесплатный доступ
Background : Cascabela thevetia (L.) Lippold, a popular member of family Apocynaceae, (Yellow Oleander), contains pharmacologically active constituents include terpenoids, a-vonoid, steroids and glycosides in seeds. This plant is being the preferred tool for suicides in villages of India.
Suicide plant, alkaloids, hemolysis, cytotoxicity, membrane leakage, root oxidizability, osmolytic shock, bi- to multi-nucleolation, micronucleoli, apoptosis, hemolysis and cellular death
Короткий адрес: https://sciup.org/143182815
IDR: 143182815
Текст научной статьи Ecotoxicological implications of Cascabela thevetia (L.) Lippold seed aqueous extract-mediated genetoxicity in Lathyrus sativus Nucleolus, Chana punctatus, and Gallus gallus RBC: a comparative biomarker-based toxicological bioassay
Plants have interacted with humans in direct and indirect ways for aeons, passing along their beneficial effects on the growth of contemporary society. Plants are tasked with being the repository of various and similar phytochemicals that are now regarded as "panaceas" for a number of human illnesses, as well as investigational tools/agents that can be used to elucidate complex biochemical reactions/pathways responsible for the manifestation of pathological symptoms. Out of the enormous diversity of unexplored floral populations around the world, only a small percentage of medicinal plants have been subjected to careful exploration, and even then, the proper identification, vis-à-vis bioactivity-guided purification, and elucidation of their mechanism of action on target organisms have not yet attained a commendable state as part of their routine biological and pharmacological screening journey. Additionally, random screening using various model organisms, such as plants, animals, microbes, etc., in vitro should be used to carry out their various mechanisms of action for a class of related compounds, as crude/bulk drugs, which might open up new vistas of hopeful templates for discovering powerful drugs with substantive activity. Today, the use of less common/toxic plants for this purpose has increased globally in an effort to advance our knowledge base and replace some well-known phytochemicals with newer candidates that are more potent target drugs. This will speed up the never-ending search for appropriate drugs with therapeutic potential. Particularly in this era of synthetic polyherbal formulations, preclinical evaluation for the goal of safety for human consumption is ostensibly required for the general principles of pharmaceutical toxicology. An evaluation of cytotoxicity/mutagenicity, particularly determining the genotoxic end points, becomes absolutely necessary to pinpoint their narrow dose for safe consumption from the point of view of commercial use of polyherbal extracts (containing complex mixture of biologically active compounds) as potential source of future drugs (Celik 2012).
In addition to fixed oils and fats, tannins, and flavonoids, the well-known yellow oleander (Cascabela thevetia (L.) Lippold) is a rich source of phytoconstituents (Rajbhar and Kumar, 2014). These include alkaloids, glycosides, saponins, flavonoids, and phenolic compounds. (Kumar et al., 2011). A variety of pharmacological effects, including anti-HIV, antiinflammatory, antidiarrheal, antimicrobial, and cytotoxic effects, have been reported from time to time. These effects are in addition to anti-spermatogenic, antitermite, antifungal, antioxidant, and antimicrobial effects. These effects are used to treat various human conditions, including diabetes, liver toxicity, fungal infection, microbial infection, inflammation, pyrexia, and anal (Sharma et al., 2022). As a source of cytotoxic medications to treat cancer, the latex of T. peruviana is abundant in metabolites (Al-Rajhi et al., 2022; El-Sawi et al., 2020). The most lethal component of this plant, however, is its seed, which has long been used by both rural and urban Indian populations as the most convenient and affordable method of suicide. The plant's various parts are rich in active ingredients, with the leaf having 0.07% of them, the fruit having 0.045%, the seeds having 4.8%, and the milky sap having 0.036% of cardiac glycosides (thevetin), which are the main causes of intoxication for nearly all vertebrate animals (Kohls et al., 2012).
Because Cascabela thevetia has so many distinct traditional uses, it is important to first identify the possible toxicity and popularity of certain herbs in traditional systems of medicine. To acquire a deeper understanding of toxic casualties utilising higher model systems, it becomes automatically important to evaluate its toxicological parameters (particularly from an agricultural point of view). Therefore, it is evident that different portions of the same plant contain phytoconstituents that are strikingly distinct from one another and may have pharmacological effects that are strikingly divergent in both in vitro and in vivo settings (Phuse and Khan, 2018).
Understanding the mechanism of the interaction of plant extracts and their components with the biological membrane at the cellular level will allow us to understand their agonistic/antagonistic effects on the human tissue systems. In this connection, a study has been undertaken aimed at understanding the effects of extracts from the Cascabella thevetia dried fruit on the structure of the biological and lipid membrane. RBC lacks the typical protein synthesizing machinery but carrying all vital functions in common with comparatively less structural complexities in relation to other specialized cells, erythrocyte membranes are thus popularly chosen simple cell model as a substitute to study the stress-mediated complicated biochemical phenomena in intact living body (Asaro and Zhu, 2020). In our studies, washed erythrocytes were treated as a model of the cell, and their membrane as a model of the biological membrane. To detect this objective washed RBC of poultry chicken (Gallus gallus) was taken. In vitro hemolytic activities are being nowadays being considered as a potential bioassay in the area of research dealing with drug lead discovery. Researchers always employ this assay as an important tool to explore ethno botanically important plants to find out potential natural products with cytotoxic/cytostatic actions.
Based on literature review it was found that investigations on developmental toxicity and lethality bioassays are really few (Siby et al., 2020) on dried fruits of Cascabela thevetia affecting higher plant development (from seed germination to chromosomal/genome toxicity). Apart from plant systems scientists have shown great interest in exploring genotoxicity caused by environmental pollutants that can be evaluated with cheap and reliable biological tests incorporating animal models containing RBC for detecting and identifying the mode of action of genotoxicants present in the ecosphere (Grisolia and Cordeiro, 2000). Advantages of fishes are manifold as fishes can metabolize xenobiotics and accumulate pollutants which make them a very sensitive and suitable model for monitoring aquatic genotoxicity (Grisolia and Corderio, 2000). Freshwater food fish Channa punctatus has a wide distribution in tropical and subtropical countries and easy availability throughout the year with almost zero maintenance in wet lab, the presence of 32 well-differentiated diploid chromosomes and easy ways to collect RBC has made this model immensely popular toxicity studies throughout the world (Kumar et al., 2010). Moreover, micronucleus tests are reliable, sensitive and easy to perform with Channa punctatus making it a widely employed bioassay to assess impacts of aquatic pollutants on the fish population (De Flora et al., 1993, Ayllon and Garcia-Vazquez, 2000; Vigano et al., 2002).
Therefore, the present study was undertaken to assess the extent of genotoxic potential on Chana punctatus (in vivo) and in vitro haemolysis induction in washed RBC of Gallus gallus (domestic Chicken) for a better understating of the mode and mechanism of action toxic phytotchemcials present in Cascabella thevetia dried seed in addition to Lathyrus sativus L., root tip cell nucleolus index assay ( in vivo ).
MATERIALS AND METHODS
Experimental materials:Collection of the samples and experimental design for test material treatments:
Certified seeds of Lathyrus sativus L. (variety Mahatora), was procured from the seed testing officer, State Seed testing Laboratory, Govt of West Bengal, India.
Fresh and young seeds of Cascabela thevetia (L.) were collected from adjoining areas of Hooghly Mohsin College, Chinsurah, Hooghly, washed thoroughly under tap water, patted on filter paper to semidryness and then kept under bright sunlight during the summer months of April and May 2022.The dried seeds were grinded to find powder and kept in air-tight clean glass jars for future use. Required amount of powdered sample was brought to boiling for 3 min with adequate amount of distilled water. Infusion of the powdered plant material (dried fruit powder) in different concentrations (5mg, 10 mg, 20 mg, 30 mg and 40 mg/ ml respectively) as these concentrations produced visible alterations in both physiological and cytological changes in different plant models employed in toxicity studies (Patel et al., 2019) and filtered in Whatman 1 filter paper for experimental use as Cascabela thevetia (L.) Lippold seed aqueous extract (CTSAE ) . Distilled water was used as negative control.
Hemolysis Assay: Fresh domestic chicken Gallus gallus) blood was collected from local slaughter houses in citrated tubes and brought immediately to laboratory for further processing. The ability of CTSAE to alter the physiologic state of RBC (erythrocytes lysis) was evaluated by monitoring the release of haemoglobin after membrane damaged caused by haemolytic process (by CTSAE, if any) following the method of Adhikari et al. (2007) with minor modifications. Briefly, 2% erythrocyte suspension was pre-incubated at 37°C for 30 min with the above mentioned concentration of CTSAE followed by incubation for 3h in an incubator shaker (37°C; 100 rotations per min). After incubation an aliquot was taken out from the reaction mixture and was diluted 10 times with the Phosphate buffer saline (PBS). The optical density of the supernatant (A) was measured at 540 nm in Hitachi U-2000 spectrophotometer. Complete haemolysis (B) was obtained by adding distilled water (instead of PBS) to the erythrocyte suspension (Sarkar et al., 2017). The percentage of hemolysis was determined as follows: (A/B)*100.
Micronuclei Assay in Fish Blood : Alive, healthy and disease free fish ( Channa punctatus weight 22-30 gm) were procured from local market Chinsurah, Hooghly, for the present study. Fish were disinfection with dipping in 2% KMnO4 solution. Then the fish were acclimatized in aquaria for 10 days before starting the treatment for experiment. Fish fed was made in lab and fed (cooked mixture of fish meal, soybean meal, mustard oil cake in 1:1:1 ratio) on alternate days. A minimum of 4 fish per group were exposed to each concentration of CTSAE ((5mg, 10 mg, 20 mg, 30 mg and 40 mg/ ml respectively) for 4 days (96 h) and triplicate experiment was conducted in each group. One group of 4 fish served as control. Water of each tank containing toxicant was changed daily to remove faecal matter.
Micronucleus test : Micronucleus test was performed as per method of Göney and Gazeloğlu (2020). By heart puncturing with sterilized needle fresh blood from fishes of each group was collected in a heparinised syringe. Thin smear in cleaned grease free slide was made immediately. Slides were fixed by dipping it in absolute methanol for 5-10 min, air dried for at least 1 hr and stained with Giemsa for 10 min. Under running distilled water from squeeze bottle slides were washed, air dried overnight, mounted with DPX and observed under Olympus CH20i microscope) fitted with CMOS Camera (IS 500, 5.0 MP) and its attachment with a computer with the aid of VIEW 7 image analysis software.
Scoring criteria for micronuclei Following criteria, as suggested by Carrasco et al. (1990), were used for identification of micronuclei: (a) the MN must be smaller than one-third of the main nuclei, (b) MN must be clearly separated from the main nuclei, and (c) MN must be on the same plane of focus and have the same color as of main nuclei. Cells with more than four MN were discarded to exclude apoptotic phenomena. From each slide, 1500 cells were scored under light microscope (microscope (Olympus CH20i microscope) fitted with CMOS Camera (IS 500, 5.0 MP) and its attachment with a computer with the aid of VIEW 7 image analysis software, oil immersion lens, 100/1.25). The MN frequency was calculated as follows:
No of cells showing micronuclei formation MN% = -----------------------------------------------x 100
Total number of cells
Determination of Membrane Permeability/ Electrolyte Leakage after NaCl treatment on etiolated roots of Lathyrus sativus L. : Ions that were leaking into deionized water from tissue were used to measure membrane permeability or electrolyte leakage (EL). Test tubes containing 10 mL of deionized water and segments of fresh root samples (processed and controlled sets of 100 mg root tissues in each tube) were used. The tubes were immersed in water that was 32 °C-heated for 6 hours. Following incubation, the bathing solution's electrical conductivity (EC1) was measured using an electrical conductivity metre (Systronics M-308, Kolkata, India). After that, the samples were autoclaved for 30 minutes at 121°C to totally destroy the tissues and liberate all electrolytes. The final electrical conductivity (EC2) of the samples was then calculated after they had been cooled to 25 ° C. The formula EL%=EC1/EC2X100 was used to convert the EL into a percentage (Adhikari, 2021).
Evaluation of root metabolic/mitochondrial activity in germinating roots of Lathyrus sativus L.: The best method for determining a cell's viability is TTC (2,3,5-Triphenyl tetrazolium chloride) staining. Lathyrus sativus L. seeds were subjected to 24 hours of treatment with various NaCl solution concentrations. The same procedure was followed while using pure water as the positive control and 0.1% hydrogen peroxide as the negative control. In 0.5% (w/v) TTC stain for five hours in the dark, all the roots were submerged. After that, distilled water was used to cleanse the roots. Using a spectrophotometer and 95 % ethanol as a blank, absorbance was measured at 490 nm. The test O.D.s had been translated into percentages representing the following rise or fall in metabolic activity, and the positive control (hydrogen peroxide O.D.) was taken to represent 100% metabolic/respiratory activity (dehydrogenase) activity, out of root mitochondrial activity (Adhikari, 2021).
Detection of morphometric changes in nucleolus after CTSAE treatment in germinating root tips of Lathyrus sativus L. variety Bidhan Khesari using Hematoxylin staining:
Seeds of Lathyrus sativus L., were allowed to germinate after 24 hrs after priming with different concentrations of test sample (500, 400, 300, 200 and 100 mM respectively) and after 72 hrs of germination the root tips were cut and fixed in FAA (4% formalin: Glacial Acetic Acid: Ethanol=1:2:7) and kept overnight at 4˚C. The very next day the root tips were hydrolyzed in 45% Acectic acid for 45 mints at water bath not allowing the temperature to rise above 85°C. After acid hydrolysis the root tips were cooled, washed in distilled water and incubated in saturated solutions of iron alum (ferric ammounium sulphate) for 10 minutes followed by staining in 0.5% aqueous hematoxylin solution for 45 minutes. The root tips were then washed and one drop of 0.2% orcein was applied and squashed in 45% acetic acid and observed under a compound microscope (Olympus CH20i microscope, Japan) outfitted with a CMOS Camera (IS 500, 5.0 MP) and its attachment to a computer with the aid of VIEW 7 image processing software.
Studies on nucleolar morphometric changes: treatment groups having cells with different numbers of nucleoli were manually scored and in different groups apart from control groups showing different numbers of nucleoli with or without nuclear membranes tabulated. Nuclolar volume was measured using the formula 4/3πr3 using stage micrometer, Erma, Japan to measure the nuclear and nucleolar diameters. Among different shaped nuclear morphological alignments four distinct morphometric parameters were chosen as “cytological markers” of endpoint cytotoxicity i.e., (i) big vacuolated nucleus with translucent centres, (ii) Elongated nuclei, (iii) dumbbell shaped nuclei, (iv) nuclei in chain, and (v) micronucleoli (one fourth of diameter than control nuclei) in scattered conditions throughout the cytoplasm. The following formula
NF% (Nucloloar Frequency)=
1*(no of Mononucleolus)+2* (no of Bi-nucleolus)
+3* (no of Tri-nucleolus)+4* (no of Tetranucleolus) + 5*(no of Penta--nucleolus) + 6*(no of hexa--nucleolus)
------------------------------------------------------------------X 100
Total no of nucleoli/nucleus counted
RESULTS
Haemolytic activity of CTSAE in washed chicken blood
The differential haemolytic activity of aqueous extract of dried seed of Cascabella revealed differential activity. At lower concentrations (5 mg/ml and 10 mg/ml treatments) the chick RBC hemolysis was less than 10% (i.e., 6.25 and 3.5% RBC lysis respectively) in comparison to both negative (NS treated) and positive control (Distilled water treated) groups. Only at higher dose treatments i.e., at 40, 30 and 20 mg/ml treatment groups there were 60.21, 46.32 and 25.51% erythrolysis in comparison to PC groups. The phytoextract contained lot of metabolites (Cardiac glycosides, tannins, saopnins, terpenoids and flavonoids; detected by preliminary phytochemical screening of CSTAE) in addition to the alkaloids isolated (Table: 2) which all together accounted together for the observed haemolytic activity of CSTAE. In this experimental set up an almost same pattern could be observed where the aqueous extract having the lower concentrations exhibited very mild erythrolysis and with increasing concentrations there accounted a rise in the erythrolysis which could be the outcome of differential concentrations of phytoconstituents forming a consortium with the extracted alkaloids (Figure 1) which in together could impart a physical imbalance in the RBC membrane integrity thereby altering the membrane phospholipid structures causing osmotic fragility and subsequent
The effect different concentrations of CSTAE on root erythrolysis
metabolic activity (dehydrogenase) activity on
Micronuceli Assay of CTSAE in Channa punctatus:
A total of 2,500 cells were scored for each group to study the micronuclei. Mean frequency of micronuclei observed was 0.08, 0.10, 0.14, 0.24 ,0.29 , 0.33 and 0.38 in control, 5, 10, 20, 30 and 40mg/ ml CSTAE, respectively (Table 4). The result indicated that the percentage of micronuclei increased (P<0.05) with increase in concentration of cadmium chloride.
Determination of Membrane Permeability/ Electrolyte Leakage after CTSAE treatment on etiolated roots of Lathyrus sativus L.,:
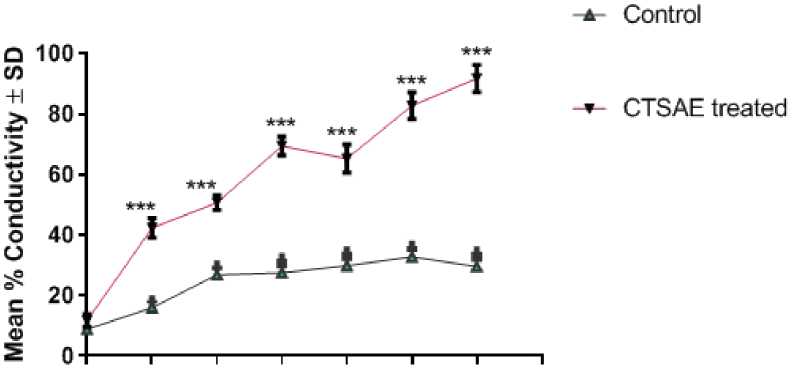
In untreated seeds of Lathyrus sativus L., the root electrolyte leakage was minimal which was below 20% (Figure 3). After incubation at different CTSAE pretreatments (2.5 to 40 mg/ml; 24 hrs) when the seeds were germinated after 72 hrs the etiolated roots could show a differential response in terms of electrolyte leakage/membrane permeability. There was a concentration dependent disruption of membrane leakage in a significant manner in etiolated roots of Lathyrus sativus L., in comparison to control (Graph:2) showing membrane hydrolyzing/disruptive properties of CTSAE.
Evaluation of root metabolic/mitochondrial activity after CTSAE in germinating roots of Lathyrus sativus L.:
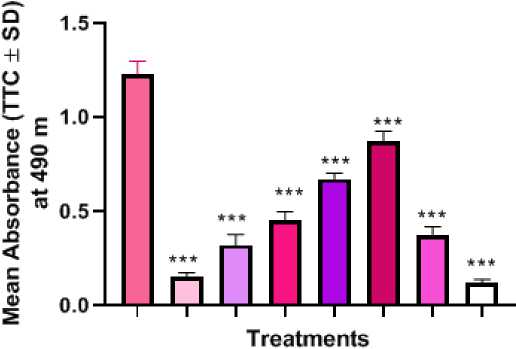
germinating roots of Lathyrus sativus L. TTC staining was employed in the present study as an indicator to evaluate the effect of plant extract on mitochondrial metabolism. In TTC staining 2, 3, 5-triphenyl tetrazolium chloride is reduced to red formazan by mitochondrial enzymes. The result showed a dose dependent decrease in mitochondrial activity as visualised by decrease in staining and absorbance in comparison to positive control (p< 0.001) (Figure 4). Positive control remained unstained with minimum absorbance indicating least mitochondrial activity and negative control showed maximum activity. In the case of treatments, it was found that at 2.5, 5 and 10 mg there was an increase in root metabolic activity (dehydrogenase activation). But at 20 mg/ml treatment there was significant higherst root metabolic activity in comparison to positive control. This trend is in a dosedependent fashion. But at the highest concentration i.e. at 30 and 40 mg/ml pretreatments treatment there was a total loss of root metabolic activity probably due to cellular poisoning and disruption of dehydrogenase activity in comparison to both negative and positive control sets (72 hrs).
Detection of morphometric changes in nucleolus after CTSAE treatment in germinating root tips of Lathyrus sativus L. variety Bidhan Khesari using Hematoxylin staining:
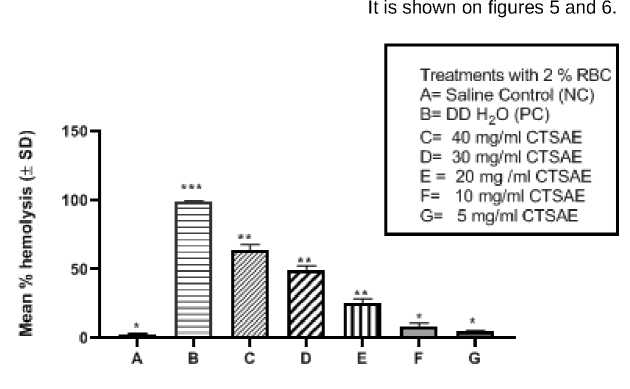
Figure 1. Effect of different concentrations of CSTAE in washed chicken RBCs. all the results are expressed as Mean % hemolysis± SD, with ANOVA coupled with one-sample t test (where all the mean values are compared with a theoritical mean against two tailed P value summary), P<0.05 was considered as statistically significant,
followed by Post-hoc Kruskal-Walis test taking the variance of each group medians into significance, showing Kruskal-Wallis statistic= 19.64 and P<0.01 in each test groups.
Table 1 : Micronuclei frequency induced by different concentration of CTSAE in Channa punctatus exposed for 96 hrs
|
Treatment |
Total cells |
Total MN count |
Micronuclei Index |
|
Control |
2500 |
145 |
0.058±0.014 |
|
5 mg/ ml |
2500 |
200 |
0.08±0.025 |
|
10 g/ml |
2500 |
325 |
0.13±0.05* |
|
20 mg/ ml |
2500 |
455 |
0.18±0.07** |
|
30 mg/ml |
2500 |
595 |
0.238±0.82*** |
|
40 mg/ ml |
2500 |
696 |
0.278±0.95*** |
Values are mean of replicates ±SE. followed by one-way ANOVA followed by post –hoc Dunnet’s multiple comparison test. Micronuclei at *p<0.05; *** p< 0.001, **p< 0.01, differ significantly from the control in Dunnet multiple comparisions test.
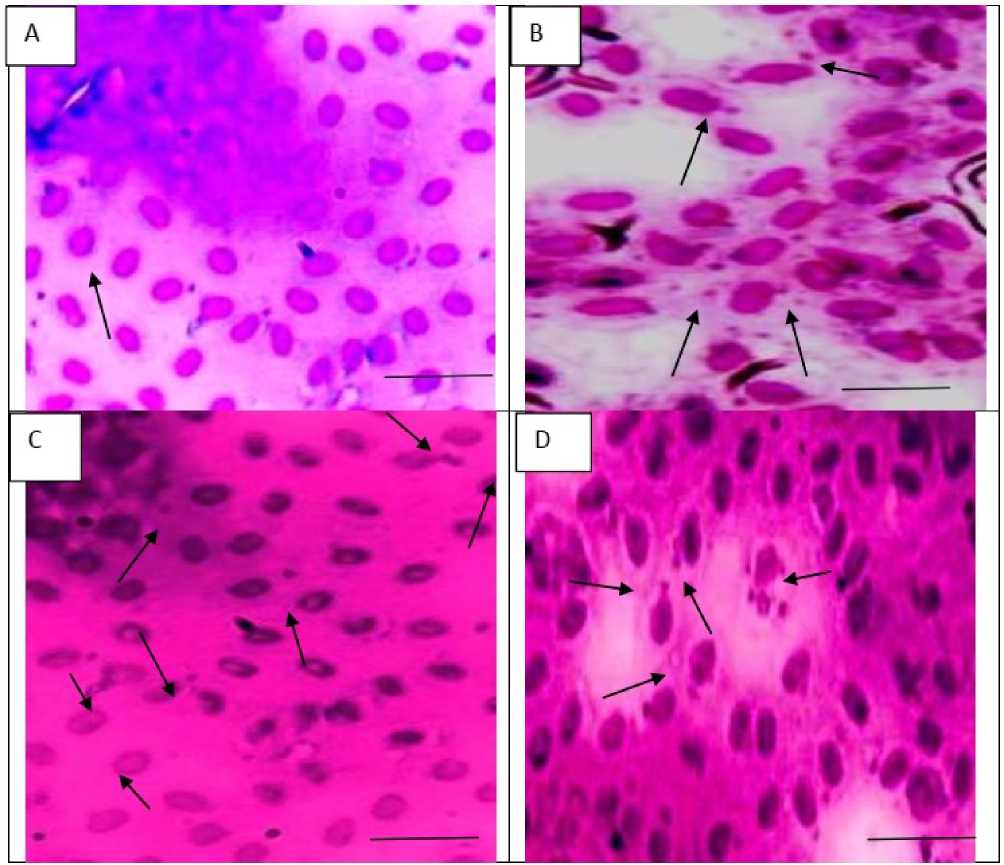
Figure 2. (Plate A-D ): Nuclear abnormalities in erythrocytes of C. punctatus after exposure to CTSAE (Control and 20, 30 and 40 mg/ml concentrations) for 96 h. Plate A : Showing normal morphometry RBC of control groups. In Plate B (20 mg/ml treatment): different levels of nuclear budding and micronuclei and nuclear bridges with binucleate formation are visualized. Plate C (30 mg/ml treatment): Nuclear vacuolation, nuclear protusion and nuclear lesions, forming Lobed nuclei and Notched nuclei. Plate D (40 mg/ml treatment): showing nuclear blebbing and multi-fragmented nucleus in erythrocytes of C. Punctatus .
CSTAE Treatment (mg/ml)
Figure 3. Graphical presentation of the relationship between concentration of PD, time and mortality
a Positive Control
□ Negative Control a CTSAE treated (2.5 mg/ml) a CTSAE treated (5 mg/ml) □ CTSAE treated (10 mg/ml) □ CTSAE treated (20 mg/ml) a CTSAE treated (30 mg/ml) D CTSAE treated (40 mg/ml)
Figure 4. Determination of root metabolic activity (dehydrogenase activity by TTC staining) after chromium treatment in germinating roots of Lathyrus sativus L. TTC staining - Graph showing the effect of different concentrations of CSTAE on mitochondrial activity. (NC) Negative control - Distilled water; (PC) Positive control - 0.1% hydrogen peroxide showing the mean O.D. of formazan produced in root tissues of L. sativus L. after 48 hours of growth due to root dehydrogenase activity. P verses negative control (**=<0.001, ***=<0.0001) following ANOVA and Dunnet’s multiple comparison test with each treatment with negative control.
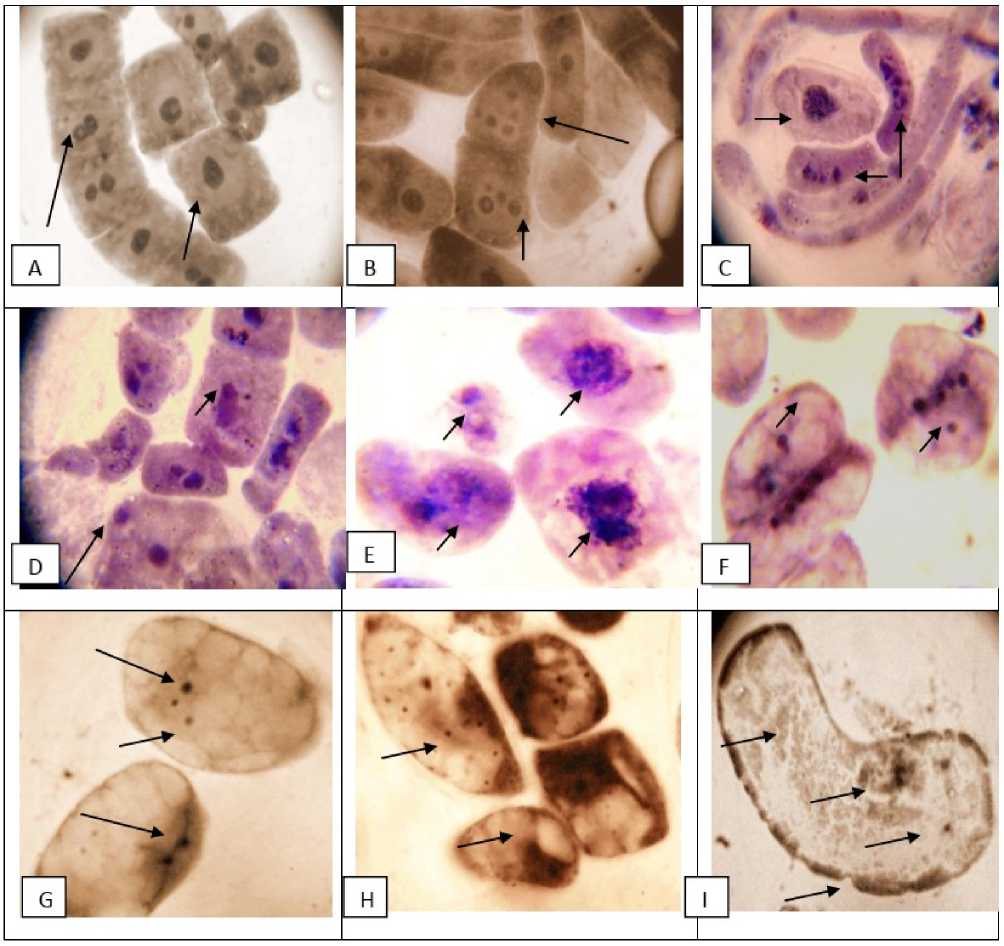
Figure 5. Meristematic root tip cells of Lathyrus sativus L., representing nucleolar alterations (frequency and volume) after 72 hrs of germination after increasing concentrations of CTSAE. Photomicrophotograhs showing different shapes and states of nuclear morphometrics after different concentrations of CTSAE priming in germinating root tips of Lathyrus sativus L., A = Control root tip cells showing intact nuclear membranes with double nuclei. B = root cells after 2.5 g/100ml CTSAE pretreatment showing all 2-4 with disappearing nuclear membrane. C = root cells after 5 g/100 ml CTSAE pretreatment showing no nuclear membranes with small round to oblate and pear-shaped nucleoli. D = root cells after 5 g/100 ml CTSAE pretreatment showing disintegrating nuclear chromatin with multifragmentations, E = root cells after 10 g/100 ml CTSAE pretreatment showing dismantling of nucleolus with small irregular bodies adhered together. F = root cells after 10 g/100ml CTSAE pretreatment showing multi-fragmented micronucleoli floating in translucent cytoplasm upto 7 in number, G = root cells after 20 g/100 ml CTSAE pretreatment giant ghost cells with diminishing volume of dispersed multi-nucleoli floating in the cytoplasm. H = root cells after 30 g/100 ml CTSAE pretreatment showing numerous heteromorphic micronucleoli with diminishing volume dispersed throughout cytoplasm, I = root cells 40 g/100 ml CTSAE pretreatment almost no-cytoplasm and multifragmented micronucleoli scattered around the corners with ruptured cell walls showing aopototic appearances.
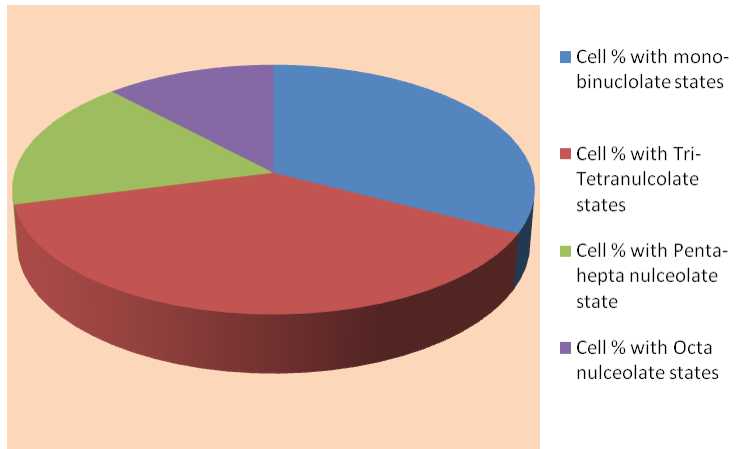
Figure 6. Changing percentages of root tip cells having different number of nucleoli (1-8) after pre-treatment with increasing CTSAE in Lathyrus sativus L.
DISCUSSION
In vitro measurement of hemolytic activity of different phytoconstituents can serve “Bioassay-based indicator” for cytotoxicity. Performance of different crude extracts during hemolytic assay could be considered as important determinant to deipher whether a drug possessing any antioxidant/pro-oxidant poteintialities and if these bioactivities can be employed in pharmacological therapeutics. It has been suggested that, the potential phytoconstituents present in different extracts in vitro and in vivo reactions might bring out damaging to plant cell chromosomes (Adhikari et al., 2023) could also potentially impart genotoxicity for mammalian cells too (Feretti et al., 2007). Reports are already available related to the haemolytic action of the Ethyl acetate fraction of the dried flowers extract of Cascabella on human RBC, which could be due to the presence of phenolics and flavonoids which happen to be responsible for probable osmolyte imbalance in the RBC suspension disrupting the membrane integrity of RBC (Phuse and Khan, 2018). Almost in all crude phytoextracts substantive amounts of bioactives, i.e., Saponins, alkaloid and tannins at high doses could become pro-oxidant, thereby increasing lipid peroxidation in the testes (Emmanuel et al., 2018). Various classes of lytic compounds may bring about hemolysis after getting penetrated deeply into the membrane bilayers, weakening the interaction between its components, thus disturbing structure of the membrane and facilitated diffusion of water swells the cell, and the membrane is permanently damaged (Bonarska-Kujawa et al., 2014) leading to membrane fragility leading to erythrolysis. Saponin (secondary metabolite; terpenic in nature) is a major component of CSTAE (as per preliminary phytochemical screening) as like Lantana camara leaf extracts (Talhi et al., 2021) which reported to be modifying the surface tension of the extracellular medium (Salada et al., 2015; Musyimi et al., 2017,). Since saponins easily impart hemolytic effect inducing membrane pores in lipid bilayers, resulting copious release of hemoglobin (Adhikari and Karmakar, 2018) into plasma (Makkar and Becker, 1997). The aglycone fraction saponins (Francis et al., 2002) has affinity to membrane sterols (especially cholesterol) thereby forming an insoluble complex (saponin-cholesterol micelles), which leads pore formation in the membrane as a direct outcome of destabilization of the lipid bilayer leading to erythrolysis (Yang et al., 2005). As per investigational conformity haemolytic activity is evaluated using the mortality rate observed: 0% to 9%= non-toxic; 10% to 49% = slightly toxic; 50% to 89% = toxic; and 90% to 100% = highly toxic (Pagano and Faggio 2015) and in this experimental set up CSTAE at its highest dose (40 mg/ml) produced nearly 64% hemolysis (Graph: 1) establishing it as a cytotoxic consumable not only from human subjects but other vertebrate species as well. Interestingly Mono, di, tri and tetra sulphides derived from Allium cepa and Allium sativum have been recorded with hemolytic activities (Munday et al., 2003) whereas saponins in the butanolic faction of leaf powder of Allium stracheyi possessed maximum haemolytic activity in human washed erythrocytes (Mukherjee and Rajasekaran, 2010) whereas the active principles vicine and covicine from aqueous extracts of broad bean (Vicia faba) were correlated with strong hemolytic activity (Vural and Sardas, 1984).
Preliminary phytochemical tests also revealed the strong presence of tannins in CSTAE. Functionally tannins react with proteins as multidentate-ligands (active phenolic rings reacting with proteins, forming -stacking with aromatic side-chains of proteins forming hydrophobic interactions by van der Waals interactions), ultimately leading to protein precipitation (Maatsola et al., 2020). Tannins including tannic acid (water soluble complex polyphenols) have been reported to be stimulators of apoptois via mitochondrial depolarization (Chen et al., 2009), upregulating apoptosis-inducing factors (Chen et al., 2009, Sun et al., 2012), DNA fragmentation by activation of caspases (Labieniec and Gabryelak, 2006). Unlike nucleated cells, Erythrocytes are devoid of mitochondria and nuclei, erythrocytes may enter suicidal death or eryptosis, which is characterized by cell membrane scrambling and cell shrinkage (Lang et al., 2008) responsible for the machinery underlying apoptosis uncovering its significance of suicide mechanisms independently. Apart from direct hemolysis by membrane pore formation via membrane protein dissolution (Adhikari et al., 2007) or ROS –induced indirect hemolysis attacking the lipid bilayer through hydrolyis of 2-acyl ester bonds of 3-sn-phospholipids producing arachidonic acid and lysophospholipids (Adhikari and Karmakar, 2018). Tannin-induced eryptosis could be a viable mechanism of action of CSTAE and could prove out to be the one the possible reason for RBC poisoning activating Ca2+-sensitive K+ channels (Brugnara, Franceschi and Alper 1993) with subsequent cell shrinkage due to K+ exit, hyperpolarization, Cl- exit and thus cellular loss of KCl and osmotically driven water (Lang et al., 2003) formidably during cardiac arrests after committed suicidal attempts.
The MN test is a regular bioassay for detecting genotoxic and clastogenic substances on living cells especially when present in aqueous media. This test can be regularly empolyed for assessment of toxicogenomic potential of wide range of bioorganic compounds, which is suggestive of confirmatory application to detect the biological markers of contamination toxicology out of clastogenic pollutants whatsoever in the aquatic environments involving fishes (Göney and Gazeloğlu, 2020). CSTAE produced at varying doses produced MN formation (Table: 4, Figure 2 Plate: 2, 3, 4) which would have been the direct reflection of structural chromosomal aberrations arising out of the toxic metabolites, equipotentially active in fish systems (in vivo). This is the first report of Cascabella toxicity in fish ( Chana punctatus RBC in vivo assay) proving it to be a sensitive organic xeno-toxicant in animal models after short-term exposure of 24 to 96 h. These current findings could suggest that CSTAE has equipotential ability to affect the chromosomal integrity fish cells and could act as an early warning for ecological damage in the aquatic food chain, overall biotic stress for the organic health of the fish population which might hinder the commercial yield in a greater extent.
According to earlier research, membrane leakage may be a direct result of membrane lipid peroxidation, leading to an osmolytic imbalance in plant cells (Adhikari, 2019). In intact plant cells, electrolyte leakage is a defining feature of the stress response. This phenomenon is frequently employed as "a biophysical marker" of plant stress tolerance and as a test for damage to plant tissues caused by stress (Ghosh et al., 2020; Adhikari 2021). Every major stressor, such as an outburst of reactive oxygen species (ROS) that results in oxidative stress, can cause electrolyte leakage, which occurs often in a variety of species, tissues, and cell types (Demidchik et al., 2003, 2010). After a stress factor is applied, the electrolyte leak is recognized very immediately and can remain for several hours or a few minutes. Even though electrolyte leakage has a direct association with stress tolerance and is highly physiologically significant, the mechanisms underlying it are still poorly understood. This process is susceptible to the production of reactive oxygen species (ROS), as shown by pharmacological investigations. In this case, we suggest that ROS-activated plant cells cause PCD, or programmed cell death (Demidchik, 2012). Numerous things, including oxidative lipid bilayer breakdown, can cause electrolyte leakage. When potassium efflux from roots exposed to heavy metals was first seen, it was first believed that lipid peroxidation-induced membrane holes were the source of this reaction (De Vos et al., 1993). One of the components of the plant's stress response is electrolyte leakage. ROS production is always present when stress-induced electrolyte leakage occurs, and PCD is frequently the result.. According to recent research, ROS (hydroxyl radicals and H2O2) can activate annexins, which are responsible for catalyzing the K+ efflux from plant cells. Furthermore, under oxidative stress, K+ efflux has been demonstrated to produce PCD. Potassium ions appear to inhibit intracellular endonucleases and proteases; hence, PCD is caused by the stimulation of these hydrolytic enzymes by their efflux. (Demidchik et al., 2014). In addition, Na+/K+-dependent ATPase and plasma membrane H+-ATPase activity were probably hampered by the several alkaloids found in CSTAE (Adhikari et al,, 2023). The increased electrolyte leakage, which indicates membrane disintegration, further demonstrated the oxidative damage brought on by CSTAE exposure. Influence of CSTAE stress on the selectively permeable root cell membrane of Lathyrus sativus L, has the ability to regulate and control intracellular chemical exchange and transport. It is the initial site of stress injury at the cellular level. Both the permeability values and the assessment index of how plants respond to environmental harm brought on by phototoxicants show how much soluble material leaks through the cell membrane. Exposure to CSTAE increased stress, which directly injured the plant cell (Figure 3) and disrupted the structure and function of the cell membrane. From this point on, when membrane permeability rises, membrane stability falls, resulting in the passive leakage of macromolecules and ion cells. Thus, among the cytotoxic alkaloids found in CSTAE, the enhanced membrane penetrability—a biophysical indicator of cytotoxicity—is the clearest indication of cell membrane damage (Adhikari et al., 2023). After entering the cellular microcosm, the CSTAE alkaloids would have physiologically caused lipid perioxidation and disruptor of plasmamembrane causing physioligical death by acting as osmolyte-mediated shocks.
A higher diffusion of oxygen from the roots is indicated by increased root oxidizability (RO), mainly to counteract the toxic chemicals surrounding the site of action. Actually absorbing electrons from the mitochondrial transport is the TTC salt used in RO measurements. Stated differently, increased RO is a sign of increased ROS production as well. According to the current investigation, roots at greater concentrations had less root oxidizability as determined by TTC-reduction (Figure 4). A conspicuous decrease in root oxidizability indicated a reduction in the rate of root Root oxidizability and respiration indicate that mitochondrial toxicity is likely the cause of cellular death (apoptosis) (Figure 4). A reduction in root oxidizability further suggested that the increased CSTAE concentrations modified cellular stress in seedlings via generating reactive oxygen species. Following ROS-induced damage or disruption of the root cell membrane (following CSTAE treatment), organic compounds and intracellular ions leak significantly (Fig. 6). This leads to a malfunction of physiological metabolism (Adhikari et al. , 2020). It is evident that the physiological and biochemical properties of Lathyrus roots are impacted by CSTAE stress. Lathyrus root activity rose considerably when the seeds were exposed to CSTAE at a concentration of 20 g/100 ml; however, there was also a noticeable decline in this response at 30 and 40 g/100 ml. Furthermore, we can assume that deeper damage to germinated roots resulted from increasing concentrations of CSTAE exposure, which would have accelerated the physiological metabolism disorder leading to root cell death and caused nutrient deficiency due to electrolyte leakage (Figure 5: G-I).
Boulon et al. (2010) stated that nuclei are thought to be the place where cellular toxicity in response to abiotic stress manifests. Abiotic stress has a direct impact on the nucleolus's structure and functionality. These modified alterations could involve adjustments to the RNA creation and protein synthesis processes in proliferating cells. Therefore, it is noteworthy to mention that modifications to the nuclear structure and its proteins may function as a potent cytological marker. These markers have been widely used in environmental studies to examine the effects of various stressors on plants and animals (Liu et al., 1994). According to Arkhipehuk and Garanko (2002), a variety of nucleolar metrics, including nuclear volume and the number of nucleoli per cell, have been identified as nuclear biomarkers in plants due to their high rate of reproducibility, affordability, and ease of use. The cytological examination of rRNA genetic alterations is more favorable for cells with a small number of NOR and fixed nucleoli characteristics than for other species with a large number of nucleoli, according to Arkhipehuk (1995). Thus, Lathyrus sativus L., which fits this description, can therefore be used as a model plant system to bioassay the effects of environmental contaminants for the investigation of nuclear parameters in the capacity as a "cytotoxic-endpoint biomarker." When compared to chromosomal abnormalities, the mitotic index, and the development of micronuclei, the fluctuation in the number of nucleoli in L. sativus meristamatic cells has been identified as "the most sensitive biomarker" measure with respect to cytotoxicity. According to Lima et al. (2019), this specific parameter, or "changes in nucleolar activity," emerges as the most important assay for determining and understanding the full range of information regarding the toxic, genotoxic, and cytotoxic effects of various pollutants and cytotoxic substances, including our test substance, CSTAE. Our results clearly showed the significant variations in nuclear activity number as a function of nucleolar features primarily found in this specific bioassay for CSTAE cytogenotoxicity assessment. As the concentration of CSTAE pretreatment increased, changes were noted in the number of nucleoli per cell and the proportion of cells with decreasing nuclear volume relative to control cells. As a result, the amount of active NORs and the level of their transcriptional activity in the nucleus at rest changed, causing ROS production and cytotoxic stress. The decrease in nuclear volume relative to the change in nuclear frequency could have been a direct result of the ROS burst, and the resulting reduction in the cell division cycle may have led to cellular death in the root tip cells' actively developing region. The nucleus's nucleoli serve various essential purposes and are the source of ribosomes for the cells. As the well-known location of ribosomal gene transcription (Adhikari, 2021), the nucleolus is known to include NORs, which are defined as nucleolar components that contain the r-DNA gene pool (Trere, 2000). Nucleolus plays critical roles in the regulation of numerous essential cellular processes, such as cell cycle regulation, apoptosis, telomerase production, RNA processing, monitoring, and response to cellular stress, and these roles changed after CSTAE induced ROS formation. As a result of ROS outburst leading to genotoxicity, nucleolar dysfunction, and cellular toxicity leading to cell death, the altered expression of nucleolus in Lathyrus sativus L., may reflect an enhancement and dysfunctional nucleolar activity, which is a crucial component of the cellular/nucleolar response to CSTAE stress. These findings could also throw newer insights into the occurrence and process of cellular apoptosis in plants.
CONCLUSION
Ultimately, it can be concluded that CSTAE exhibits cytotoxic and genotoxic activity in both in vitro and in vivo tests (plant and animal) at high concentrations (40, 30 and 20 mg/ml). Different parts of Cascabella thevetia have been exploited as crude extracts to uncover the pharmacological properties per say, and in practice, crude Cascabella fruits are consumed during suicidal attempts as a functional source of poison. This is why CSTAE crude extract has been assayed in these experimental setups. Dealing with crude extracts, however, also entails discovering the potency of intricate combinations or consortiums of physiologically active substances that cooperatively impart multi-cascade hazardous responses not only in human but in other animals also. Certain chemicals possess pro-oxidant potency, which can render them cytotoxic or genotoxic.
Conversely, other compounds may confer cytoprotective or antigenotoxic activities due to their antioxidant qualities. Alkaloids, tannins, saponins, phenolics, and other phyto-components that are rich in pharmacological diversity and rich in CSTAE must be investigated as possible bioactive-therapeutic agents for future drug development. Receptor kinetics studies in conjunction with bioactivity-guided purification and bioassays using structure-activity relationship studies (SAR) with various biomarkers can yield conclusive evidence-based pharmaco-mapping, even though improper use of CSTAE can result in serious issues and harm to cells.
Список литературы Ecotoxicological implications of Cascabela thevetia (L.) Lippold seed aqueous extract-mediated genetoxicity in Lathyrus sativus Nucleolus, Chana punctatus, and Gallus gallus RBC: a comparative biomarker-based toxicological bioassay
- Adhikari D, (2019) Augmentation of Mitodepressive and cytogenetic effects of Lead upon Acute exposure on Grass Pea (Lathryrus sativus L.,) root tip cells. American Journal of Biological Sciences, 1(1), 1422.
- Adhikari D, (2021) Chromium induces genotoxicity in root tip cells of Grass Pea (Lathyrus sativus L., Variety Nirmal): A ROS mediated acute toxicity study. Journal of Stress Physiology and Biochemistry, 17(2): 98-120.
- Adhikari D, Ghosh T., and Ghosh R, (2023) A Comprehensive Comparative Study on Cascabelathevetia (L.) Lippold, Seed Aqueous Extract-mediated Escalation of Abiotic Stress and Cellular Genotoxicity: Insights from Multivariate Allelochemical Analysis vis-a-vis Employment of Plant Bioassays (Lathyrus sativus L., and Allium sativum L., germinating root tip cells). Journal of Stress Physiology & Biochemistry, 19(4). 178-202 ISSN 1997-0838.
- Adhikari D, Karmakar S, (2018) In vitro Pro-inflammatory Activity and Associated Pharmacological Activities of Paracondylactis indicus Dave. Nematocyst Venom. Pharmacologia, 9, 114-121.
- Adhikari D., Samanta S, Roy A, Dutta A, Vedasiromoni J, Sen T, (2007) In vitro hemolysis and lipid peroxidation inducing activity of the tentacle extract of the sea anemone (Paracondylactis indicus Dave.) on rat erythrocytes. Indian Jr of Pharmacology, 39 (3): 155-159. DOI: 10.4103/0253-7613.33436.
- Al-Rajhi AM, Yahya R, Abdelghany TM, Fareid MA, Mohamed AM, Amin BH, Masrahi AS (2022) Anticancer, anticoagulant, antioxidant and antimicrobial activities of Thevetia peruviana latex with molecular docking of antimicrobial and anticancer activities. Molecules, 27(10), 3165. DOI: 10.3390/molecules27103165.
- Arkhipchuk, V.V. (1995) The use of nucleolar characteristics in biotesting. Tsitol Genet., 29, 612.
- Arkhipchuk, V.V. and Garanko, N.N. (2002) A novel nucleolar biomarker in plant and animal cells for assessment of substance cytotoxicity. Environ Toxicol, 17, 187-194.
- Asaro RJ, Zhu Q, (2020) Vital erythrocyte phenomena: what can theory, modeling, and simulation offer? Biomech Model Mechanobiol 10, 19, 1361e88. https://doi.org/10.1007/s10237-020-01302-x.
- Ayllon F, Garcia-Vazquez, E (2000) Induction of micronuclei and other nuclear abnormalities in European minnow Phoxinus phoxinus and mollie Poeclia latipinna: an assessment of the micronucleus test. Mutat. Res., 467, 177-186. DOI: 10.1016/s1383-5718(00)00033-4
- Bonarska-Kujawa D, Cyboran S, Gybka R, Oszmianski J, Kleszczynska H, (2014) Biological Activity of Blackcurrant Extracts (Ribes nigrum L.) in Relation to Erythrocyte Membranes, BioMed Research International, 1-13,
- Boulon, S., Westman, B.J., Hutten, S., Boisvert, F.M. and Lamond, A.I. (2010) The nucleolus under stress. Mol Cell, 40, 216-227.
- Brugnara C, de Franceschi L, Alper SL, (1993) Inhibition of Ca2+-dependent K+ transport and cell dehydration in sickle erythrocytes by clotrimazole and other imidazole derivatives. J Clin Invest. 92, 520-526. doi: 10.1172/JCI116597.
- Celik TA (2012) Potential Genotoxic and Cytotoxic Effects of Plant Extracts. A Compendium of Essays on Alternative Therapy, Dr. Arup Bhattacharya ed ISBN:978- 953307-863-2.
- Chen KS, Hsiao YC, Kuo DY, Chou MC, Chu SC, Hsieh YS, Lin TH, (2009) Tannic acid-induced apoptosis and -enhanced sensitivity to arsenic trioxide in human leukemia HL-60 cells. Leuk Res. 33, 297307.
- De Flora, SL, Vigano F, Agostini AD', Camoirano M, Bagnasco C,.Bennecelli F, Melodia F, Arillo, A (1993) Multiple genotoxicity biomarkers in fish exposed in-situ to polluted river water. Mutat. Res., 319, 167-177.
- De Vos, C., Bookum, W T., Vooijs, R., Schat, H. and DeKok, L. (1993) Effect of copper on fatty acid composition and peroxidation of lipids in the roots of copper tolerant and sensitive Silene cucubalus. Plant Physiology and Biochemistry., 31, 151-158.
- Demidchik V, (2010) Reactive oxygen species, oxidative stress and plant ion channels. In: Demdichik V, Maathuis FJM, eds. Ion channels and plant stress responses. Heidelberg: Springer- Verlag., pp 207232.
- Demidchik V, (2012) Reactive oxygen species and oxidative stress in plants. In: Shabala S, ed. Plant stress physiology. Wallingford, UK: CAB International., pp 24-58.
- Demidchik V., Shabala, S.N., Coutts, K.B., Tester, M.A. and Davies, J.M. (2003) Free oxygen radicals regulate plasma membrane Ca2+- and K+- permeable channels in plant root cells. Journal of Cell Science., 116, 81-88.
- Demidchik, V., Straltsova, D., Medvedev, S.S., Pozhvanov, G.A., Sokolik, A. and Yurin, V. (2014) Stress-induced electrolyte leakage: the role of K+-permeable channels and involvement in programmed cell death and metabolic adjustment, Journal of Experimental Botan., 1-12.
- El-Sawi SA, Maamoun AA, Salama AH, Farghaly AA (2020) Chemical profiling of Thevetia peruviana leaves cytotoxic active extracts enhanced by microemulsion formulation. Bulletin of the National Research Centre, 44(1), 1-14.
- Emmanuel OY, Obasib KK, Lawal I, (2018) Spermatogenic and spermatotoxic effects of Telfairia occidentalis (Ugu) aqueous leaves extract in adult male Wistar rats (Rattus novergicus). Toxicol Rep 5, 954-958.
- Feretti D, Zerbini I, Zani C, Ceretti, Moretti M, Monarca S. (2007) Allium cepa chromosome aberration and micronucleus tests applied to study genotoxicity of extracts from pesticide-treated vegetables andgrapes. Food Addit Contam 24(6), 561-572, DOI: 10.1080/02652030601113602
- Francis G, Kerem Z, Makkar HP, Becker K, (2002) The biological action of saponins in animal systems: a review, British Journal of Nutrition, 88, 580-605. doi: 10.1079/BJN2002725.
- Ghosh T, Mukherjee S, and Adhikari D, (2020) Evaluation of acute toxicity studies on Copper-induced oxidative stress in Lathryrus sativus L., (variety Ratan) germinating seeds: A Biomarker based risk assessment. Journal of Advanced Scientific Research, 11(04), 243-254.
- Goney G, Gazeloglu C, (2020) Evaluation of fish micronucleus results in Turkish ecogenotoxicological studies. COMU J. Mar. Sci. Fish, 3(1): 1-10. DOI: 10.46384/jmsf.654156.
- Grisolia CK, Cordeiro CMT, (2000) Variability in micronucleus induction with different mutagens applied to several species of fish. Gen. Mol. Biol., 23, 235-239.
- Kohls S, Scholz-Bottcher BM, Teske J, Zark P, Rullkotter J (2012) Cardiac glycosides from Yellow Oleander (Thevetia peruviana) seeds. Phytochemistry; 75: 114-127. doi: 10.1016/j.phytochem.2011.11.019. Epub 2011 Dec 22.
- Kumar A, Singh S, Mahour K, Vihan VS, Gururaj K (2011) Phytochemical analysis of some indigenous plants potent against ectoparasite. Asian Journal of Experimental Biological Sciences; 2(3): 506-9. e-ISSN 0976-7614.
- Kumar R, Nagpure NS, Kushwaha B, Srivastava SK, Lakra WS (2010) Investigation of the genotoxicity of malathion to freshwater teleost fish Channa punctatus (Bloch) using the micronucleus test and comet assay. Arch. Environ. Contam. Toxicol., 58, 123-30.
- Labieniec M, Gabryelak T, (2006) Oxidatively modified proteins and DNA in digestive gland cells of the fresh-water mussel Unio tumidus in the presence of tannic acid and its derivatives. Mutat Res, 603, 48-55. doi: 10.1016/j.mrgentox.2005.10.013.
- Lang F, Gulbins E, Lerche H, Huber SM, Kempe DS, Foller M, (2008) Eryptosis, a window to systemic disease. Cell Physiol Biochem. 22:373-380. doi: 10.1159/000185448.
- Lang PA, Kaiser S, Myssina S, Wieder T, Lang F, Huber SM, (2003) Role of Ca2+-activated K+ channels in human erythrocyte apoptosis. Am J Physiol Cell Physiol. 285, C1553-C1560. doi: 10.1152/ajpcell.00186.2003.
- Lima, M.G.F., Rocha, L.C., Silveira, G.L., Alvarenga,I.F.S. and Andrade-Vieria, L.F. (2019) Nucleolar alterations are reliable parameters to determine the cytogenotoxicity of environmental pollutants. Ecotox. Environ. Safe., 174, 630-636.
- Liu, D., Jiang, W., Guo, L., Hao, Y., Lu, C. and Zhao, F. (1994) Effects of nickel sulfate on root growth and nucleoli in root tip cells of Allium cepa. Isr J Plant Sci, 42, 143-148.
- Maatsola S, Kurkinen S, Engstrom MT, Thomas KMN, Pentikainen O, Juha-Pekka S, Haataja S, (2020) Inhibition of Pneumolysin Cytotoxicity by Hydrolyzable Tannins. Preprints (www.preprints.org). https://doi.org/10.20944/preprints202011.0408.v1
- Mukherjee A, Rajasekaran C, (2010) In-vitro hemolytic activity of Allium stracheyi Baker. Journal of Pharmacy Research 2010, 3(5), 1160-1162.
- Munday R, Munday JS, Munday CM, (2003) Comparative effects of mono-, di-, tri-, and tetrasulfides derived from plants of the Allium family: redox cycling in vitro and hemolytic activity and Phase 2 enzyme induction in vivo, Free Radical Biology and Medicine, 34, 1200-1211.
- Musyimi DM, Opande GT, Jane C, Sikuku PA, Buyela, DK, (2017) Antimicrobial potential and screening of phytochemical compounds of Lantana camara Linn, Journal of Biological Science, 1(2), 24557676. ISSN: 2455-7676.
- Pagano, M., & Faggio, C. (2015). The use of erythrocyte fragility to assess xenobiotic cytotoxicity. Cell biochemistry and function, 33(6), 351-355. DOI: 10.1002/cbf.3135.
- Phuse SS, Khan ZH (2018) Antioxidant and haemolytic quantification of Thevetia flowers in different extracts. IJPBS 8(2): 110-116.
- Rajbhar N, Kumar A (2014) Pharmacological importance of Thevetia peruviana. International Journal of Pharmaceutical and Chemical Sciences, 3(1), 2603. 5005ISSN: 2277.
- Salada, J. A. T., Balala, L. M., & Vasquez, E. A. (2015). Phytochemical and antibacterial studies of Lantana camara L. leaf fraction and essential oil. International Journal of Scientific and Research Publications, 5(3), 1-5.
- Sarkar R, Kumar A, Divya L , Samanta SK, Adhikari D, Karmakar S, Sen T, (2017) Antioxidant Properties of Kalanche blossfeldiana-a focus on Erythrocyte Membrane Stability and Cytoprotection. Current Traditional Medicine, 3: 51-58.
- Sharma T, Kaur J, Singh G. (2022) Phytochemistry and Pharmacological activities of Thevetia peruviana: A review. International Journal of Pharmaceutical Sciences and Research. 13(6): 2274-2282.
- Siby N, Jacob S, Thoppil JE (2020) Cytotoxic giant-cell induction & phytoconstituents of Yellow Oleander, Cascabela thevetia (L.) Lippold [Apocynaceae],.
- LAP LAMBERT Academic Publishing. ISBN: 978620-2-51678-5
- Sun Y, Zhang T, Wang B, Li H, Li P, (2012) Tannic acid, an inhibitor of poly(ADP-ribose) glycohydrolase, sensitizes ovarian carcinoma cells to cisplatin. Anticancer Drugs. 23: 979-990. doi: 10.1097/CAD.0b013e328356359f.
- Talhi, F., Gherraf, N., Zellagui, A., Boumaza, A., & Meghlaoui, A. Phytochemical screening and hemolytique activity of some leaves extracts of L. Acta Scientifica Naturalis, 8(3), 1-9.
- Trere, D. (2000) AgNOR staining and quantification. Micron, 2, 127-131.
- Vigano, L, Camoirano A, Izzotti AA, Agostini FD', Polesello S, Francisci C, De Flora S (2002) Mutagenicity of sediments along the Poriver and genotoxicity biomarkers in fish from polluted areas. Mutat. Res., 515, 125-134.
- Vural N, Sardas S, (1984) Biological activities of broad bean (Vicia faba L.) extracts cultivated in South Anatolia in Favism sensitive subjects. Toxicology 31, 175-179.
- Yang ZG, Sun HX, Fang WH (2005), Haemolytic activities and adjuvant effect of Astragalus membranaceus saponins (AMS) on the immune responses to ovalbumin in mice, Vaccine, 23(44), 5196-5203.


