Effect of acute hypoxia on the functional state of erythrocytes and hemoglobin in black scorpionfish
Автор: Soldatov A.A., Andreyeva A.Y., Kukhareva T.A., Kladchenko E.S.
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 3 т.19, 2023 года.
Бесплатный доступ
In the present work the influence of hypoxia on black scorpionfish ( Scorpaena porcus ) nucleated red blood cells has been studied at in vivo (whole blood) and in vitro (cell suspension) experiments. Experiments were conducted in the range of oxygen concentration 0,3-8,5 mg О2 l-1, water temperature 14-16 оС, and the duration of exposure period was 4 h. Deep hypoxia (less than 2 mgО2 l-1) caused hemoglobin transition to a ferry-form (MtHb), and the most substantial increase of MtHb concentration was observed at severe hypoxic conditions (less than 1 mg О2 l-1). The highest MtHb level observed was 19-32 %. The results of in vivo and in vitro experimental series were similar, indicating that mechanisms involved in MtHb formation occur within cells and are not associated with organismic responses to oxygen deficiency. Moderate hypoxia (oxygen concentration more than 2 mg О2 l-1) did not cause hemoglobin transformation. MtHb formation did not influence the level of reactive oxygen species (DCF-DA fluorescence) and the integrity of cellular membrane (double staining with SYBR Green I and Propidium iodide) in red blood cells. The level of dead cells in control and experimental groups did not differ, indicating that responses observed were in the range of physiological norm.
Hypoxia, experiments in vivo and in vitro, red blood cells, methemoglobin, flow cytometry
Короткий адрес: https://sciup.org/143180573
IDR: 143180573
Текст научной статьи Effect of acute hypoxia on the functional state of erythrocytes and hemoglobin in black scorpionfish
Red blood cells are highly specialized for transportation of gases and oxygen indeed. Hemoglobin is the most abundant protein in red blood cells. At normal physiological conditions oxyhemoglobin (HbO 2 ) deoxygenation is associated with the release of oxygen with retention of Fe in heme structure in bivalent state (ferro-form) (Schechter, 2008):
(Fe2+ )HbO 2 (Fe2+)Hb + O 2 .
However, in some cases the process may be accompanied with the formation of superoxide anion ( ∙ О 2 -) and oxidation of iron, transforming heme to ferrystate (Schechter, 2008). Such oxidation of hemoglobin leads to its transformation in ‘methemoglobin’ (MtHb), which is unable to bind oxygen. MtHb is usually produced during hemoglobin autooxidation or may form as a result of toxic load on the organism (nitrite, aniline, nitrobenzene) (Schechter, 2008). Autooxidation of hemoglobin is a slow process, mainly affecting deoxyhemoglobin and leading to the formation of ∙ О 2 -. The process can be divided into several stages (Jensen et al ., 1998):
(Fe2+ )HbO 2 (Fe2+)Hb + O 2 (Fe2+)Hb + X (Fe2+)HbX
(Fe2+)HbX + O 2 (Fe2+)HbX + ∙ О 2 -.
Where X – nucleophilic compound, which binds to Hb via donor/acceptor interactions.
Oxidation of hemoglobin is prevented by antioxidant molecular complex of erythrocyte, which involves glutathione (GSH), ascorbic acid, tocopherol (Krishna and Venkataramana, 200 ). However, the speed of MtHb interaction with these compounds is negligible. MtHb reduction in cells is achieved through a specific enzyme pathway. NADH-diaphorase plays a key role in this process, transporting electron from NADH to cytochrome b 5 , and then to MtHb (Percy and Lappin, 2008):
NADH + cyt b 5 (Fe3+) → cyt b 5 (Fe2+) + MtHb → cyt b 5 (Fe3+) + Hb.
The process leads to nearly 100 % reduction of oxidized pigment. MtHb concentration in normal human blood does not exceed 1 %.
Nucleated fish red blood cells possess antioxidant enzyme complex which is similar to that in higher vertebrates (Mather-Mihaich and Di-Giulio, 1991; Zikic et al ., 1991). They also contain NADH-diaphorase (Tucker and MacMillan, 1992; Schoore et al ., 1995). In fish red blood cells, the activity of some enzymes (for example, peroxidase and superoxide dismutase) and concentration of reductants (GSH) is higher compared to that in human (Wdzieczak et al ., 1982; Dafre and Reischl, 199 ). At the same time, susceptibility of respiration pigments to oxidation in fish is substantially higher. MtHb concentration in normal fish blood may exceed 10 % (Hardig and Hoglund, 1983; Sajiki and Takahashi, 1991).
In some cases spontaneous increase of MtHb concentration in fish blood could be observed without apparent toxic methemoglobinemia. High MtHb level was observed in fish undergoing adaptation to hyperthermia (30-40оС) (Wilson and Knowles, 198 ; Jensen et al ., 1998) or hypoxia (Affonso et al ., 2002; Chen et al ., 201 ). MtHb formation in hypoxic conditions is quite paradoxical since a low oxygen concentration suggests a lower oxidative load on red blood cells. Methemoglobinemia under hypoxia has been reported not only in fishes ( Colossoma macropomum (Affonso et al ., 2002), Megalobrama amblycephala (Chen et al. , 201 ), but also in higher vertebrates (Olander and Parr, 19 8) and human (Arnaud et al ., 19 9). It has been shown that partly deoxygenated hemoglobin can be easily oxidized and transformed to met-form (Mansouri, 1981).
It is known, that hypoxia leads to other physiological reactions, which functional role have not been determined yet. For example, catalase (CAT) and superoxide dismutase (SOD) activity increase in fishes under hypoxic conditions. The latter may induce dismutation of ∙О2-. The increase of antioxidant enzymes activity in hypoxia was observed in somatic tissues and red blood cells (Willmore and Storey, 199 ; Lushchak and Bagnyukova, 200 ; Stara et al., 2012). Since hypoxia usually occurs as periodic factor for the majority of aquatic organisms it is considered that increase of CAT and SOD activity precedes further reoxygenation (Lushchak and Bagnyukova, 200 ). On the other hand, the increase of the activity of CAT and SOD may be also related to the enhancement of oxidative processes in the circulating blood of fish. These, in turn, may cause Hb transformation to ferry-form. However, this process remains unknown.
The above information was obtained mainly under in situ or in vivo experiments, supposing indirect influence of hypoxia on target cells and the involvement of combination of other factors, which could hardly be considered. In the present work we investigated the influence of hypoxia in series of in vitro experiments (in suspensions of red blood cells) and in vivo experiments (on the whole blood). The work is focused on the quantitative analysis of MtHb level, and some other functional parameters of red blood cells, which were determined by flow cytometry.
MATERIALS AND METHODS
Capture and transportation of the material
The object of the research was benthic species Scorpaena porcus L. (black scorpionfish). Only adult specimens which did not undergo spawning (gonad maturation stage III-IV) were chosen for the experiment (body length 14.0 cm – 1 .0 cm, weight 85 g – 115 g). Fish were captured and transported in the laboratory in 60 L aerated tanks. Transportation period did not exceed 3 hours. Dissolved oxygen concentration in water was .5-8.0 mg O 2 l-1. Prior the experiment, animals were kept in tanks equipped with a flowing sea water system for an acclimation period of roughly 1 week. Specimens were daily fed with minced fish. Daily ration amounted 6- % of body mass. Fish were anesthetized with urethane as it does not cause significant changes of heart rate and respiratory rhythm (Soldatov, 2005). Blood samples obtained by caudal vein puncture were drawn into 0.5 ml plastic tubes with heparin (Richter, Hungary).
In vivo experiments
The series were performed on experimental stand, which allows to maintain given concentration of oxygen. Water temperature in the tank (13.5 l) was 15-16 оС. Experimental tank was designed to keep 1 individual. Photoperiod of the experiment was adjusted to 12 hours day : 12 hours night. After adaptation period, oxygen concentration decreased from 8.5 mgО2 l-1 (100% saturation level) by bubbling of the water with nitrogen gas for 1.5-2 hours. In the experiments the range of oxygen concentration 0.35-8.5 mgО2 l-1 has been investigated. The constant oxygen level was maintained by periodic aeration of the water in the tank and controlled on the oxygen sensor DO Meter ST300D RU (“Ohaus”, USA). The duration of experimental period was 4 h.
In vitro experiments
Blood samples obtained by caudal vein puncture were drawn into a heparinized syringe with heparin. Hemoglobin concentration in samples was 54-61 g l-1, hematocrit was 24-28 %. Hemoglobin concentration was determined by hemoglobin-cyanide method using standard reagent kit (“Agar-med”, Russia). Hematocrit was estimated by blood centrifugation in heparinized capillaries using hematocrit rotor (800 g, 15 min, MPW-310 centrifuge, Poland).
Red blood cells (RBCs) were separated from plasma and leukocytes by centrifugation (800 g , 15 min) and washed three times in an equivalent volume of the following buffer: 128 mM NaCl, 3 mM KC1. 1.5 mM CaCl 2 , 1.5 mM MgCl 2 , 15 mM Тris, 2,2 mM D-glucose (рН .8) (Tiihonen and Nikinmaa, 1991).
Cells were resuspended in the buffer solution with different oxygen concentration (the range 0-5 mgO 2 l-1) adjusting concentration 4.5x10 cells ml-1. Hypoxic conditions were achieved by bubbling the incubation suspension with nitrogen gas. Cells were incubated in hypoxic conditions for 4 hours. To keep the concentration of oxygen at the constant level vacuum tubes were used (6.0 ml).
Hemoglobin spectral analysis
After completing the incubation period cells were sedimented by centrifugation (800 g, 15 min, Elmi CM-50). Some portion of cell pellet was used for flow cytometry analysis, the rest of erythrocytes were lysed with ice-cold phosphate buffer 0.35 M, pH .3 with the appropriate oxygen concentration to prepare hemoglobin in solution. The ratio cells-to-lysing solution corresponded to hemoglobin concentration 45-52 g l1. Cell membranes and nuclei were sedimented by centrifugation (800 g, 15 min, Elmi CM-50). Hemolysates were used for further spectral analysis.
Hemoglobin concentrations were estimated spectrophotometrically with a PerkinElmer Lambda 35 spectrophotometer (USA). The concentrations of oxyhemoglobin (HbO 2 ), deoxyhemoglobin (DeoxyHb) and methemoglobin (MetHb) was measured at absorbance 560, 5 6 and 630 nm, and calculated according to the following equations at pH .3 (Benesch et al ., 19 3):
[HbO 2 ] = (1.013 A 5 6 – 0.3269 A 630 – 0. 353 A 560 ) 10-4 [DeoxyHb] = (1.3 3 A 560 – 0. 4 A 5 6 – 0. 3 A 630 ) 10-4 [MtHb] = (2.985 A 630 + 0.194 A 5 6 – 0.4023 A 560 ) 10-4
Flow cytometry analysis
After incubation in hypoxic conditions cell concentration in suspension was adjusted to 106 cells ml-1. 2’- ’-dichlorofluorescein (DCF-AM) assay was used for measuring level of reactive oxygen species in RBCs. The dye stock solution was prepared in dimethyl sulfoxide (DMSO), stored frozen at -20ºC. The final concentration of the fluorochrome in the sample was 1 mg ml-1. Cells were incubated with DCF-AM for 30 min at room temperature in the dark immediately before the experiments.
To assess RBCs viability double staining protocol with SYBR Green I (SG I) and probidium iodide (PI) was used. SG I is a highly specific DNA dye used for DNA analysis in living and dead cells as well; PI is a commonly used mortality indicator as it fluorescence is detected only in cells with damaged membrane. Erythrocytes were simultaneously dyed with both fluorochromes. The final concentration of dyes in sample was: 10 µg ml-1 for SG I and 2 µg ml-1 for PI. Cells were incubated for 40 min at room temperature in the dark.
All flow cytometric measurements were done on the flow cytometer Beckman Coulter FC500. SG I and DCF-AM fluorescence was registered in FL1 channel (green fluorescence), PI fluorescence – in FL3 channel (red fluorescence). Data analysis was performed in Flowing software 5.1.
Statistics
The normality of distribution was verified using Pearson’s criteria. The significance of differences was assessed with Student's test (р ≤ 0.05, р ≤ 0.02). Graphic representation of data was made in Grapher .0 software. Results are presented as Mean ± S.E.
RESULTS
In vivo experiments
The decrease of oxygen in medium from 8. to 2.0 mgO 2 l-1 did not significantly influence blood MtHb level, which was similar to control (1,5-4,0 %) (Fig. 1). However, under deep hypoxia (≤2.0 mg O 2 l-1) significant increase of MtHb concentration was observed. The average increase of blood MtHb was 6 times higher compared to that at normoxia (p≤0,001). The most dramatic changes were observed at oxygen level less than 1 mgO 2 l-1. In some individuals MtHb concentration reached 19 %. Analysis of specters demonstrated typical changes: the increase of optical density at 630 nm (maximum absorption for MtHb) and the decrease of oxyhemoglobin level, which was confirmed by the increase of optical density at 560 nm (Fig. 2).
Hemoglobin transition to ferry-form is associated with the formation of superoxide radical (∙О 2 -), which is neutralized via dismutation process at normal conditions. The efficacy of this process could be indirectly accessed by the level of reactive oxygen species (ROS) in cells (DCF-AM fluorescence). Figure 3 shows, that the intensity of DCF-AM fluorescence substantially decreased as hypoxia deepened. Intracellular ROS level was 3.0-3.5 times lower (p≤0,001) in hypoxic probes compared to control, indicating that antioxidant complex in red blood cells completely neutralized ∙О 2 - and hemoglobin oxidation observed may be attributed to a physiological norm.
Double staining of red blood cells with SG I and PI demonstrated that hypoxia (less than 2 mgО 2 l-1) did not influence the mortality level (Fig. 4). The total number of live cells exceeded 98 %.
Experiments in vitro
Spectrophotometric analysis allowed assessing functional state of black scorpionfish hemoglobin at different oxygen concentrations. The decrease of oxygen level in solution from -8 to 2-3 mg l-1 did not influence the spectrum shape, positions of maximum and extinction values of the protein (Fig. 5A). Under deep hypoxia (0.2-2.0 mg l-1) we observed significant increase of extinction at 560 and 630 nm, indicating formation of DeoxyHb and MetHb respectively (Fig. 5B).
Concentration of MtHb in hemoglobin solution was estimated using spectrum deconvolution (Fig. 6A). MtHb level increased as oxygen concentration in the medium declined. The highest methemoglobin concentration was observed at oxygen concentration less than 1 mg О2l-1. In some individuals MtHb level increased on 32 % comparing to control level. The ranking of the obtained values into three groups depending on the level of hypoxia (more than 2 mg l-1, 1-2 mg l-1, less than 1 mg l-1) allowed us to calculate the average values and conduct a statistical comparison between them (Fig. 6B). The differences between groups reached 4-16 times (p<0,01).
Functional state of RBCs was assessed based on estimating the number of dead cells in suspensions. Cells were simultaneously stained with two DNA-dyes: SG I and PI. On the basis of quadrant analysis of SG I and PI fluorescence no significant changes in cell’s viability was observed. For normoxic suspensions the number of living cells was 94.0±5. %, and for deoxygenated suspensions – 88.6±4.5 % (Fig. ).
The intracellular ROS level was determined on the basis of concentration of hydrogen peroxide in red blood cells. For this purpose we used flow cytometric analysis of DCF-DA fluorescence (Fig. 8). It has been shown that the decrease of oxygen concentration in the incubation medium wasn’t accompanied with ROS accumulation. In hypoxic probes the level of DCF fluorescence was significantly lower compared to that in normoxic cells.
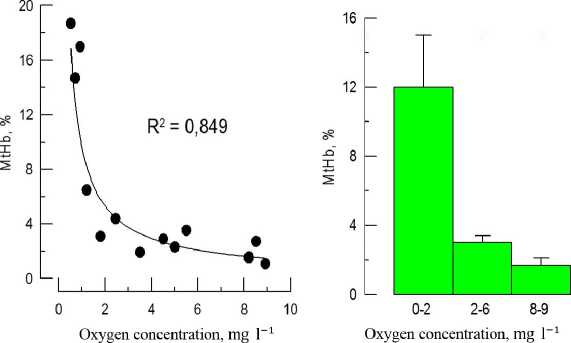
Figure 1 The influence of hypoxia ( in vivo experiments) on MtHb concentration in black scorpionfish blood (A –
distribution plot; B – ranked data)
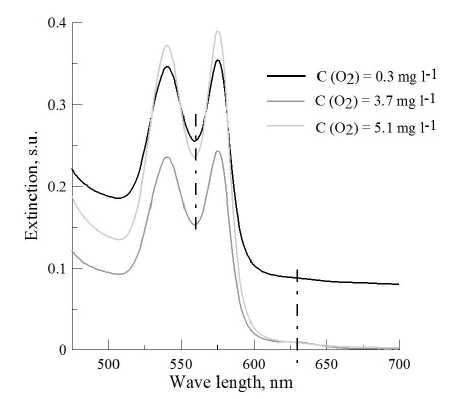
Figure 2 Hemoglobin specters of black scorpionfish ( in vivo experiments) under different oxygen regimes (dashed line shows optical density at 560 and 630 nm)
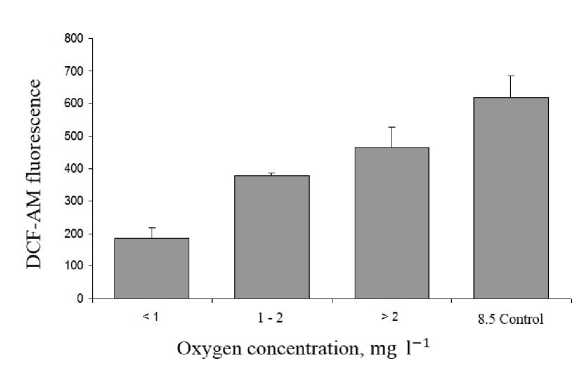
Figure 3 ROS level in lack scorpionfish red blood cells ( in vivo experiments) under hypoxia.
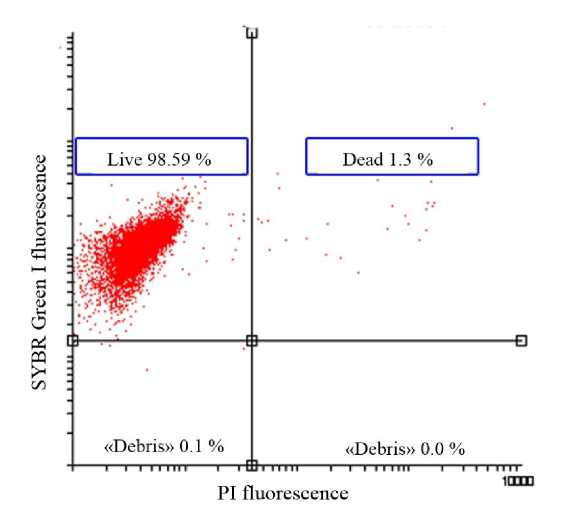
Figure 4 The ratio between live and dead cells in red blood cell suspensions ( in vivo experiments) under hypoxia (less
than 2 mg О 2 l-1)
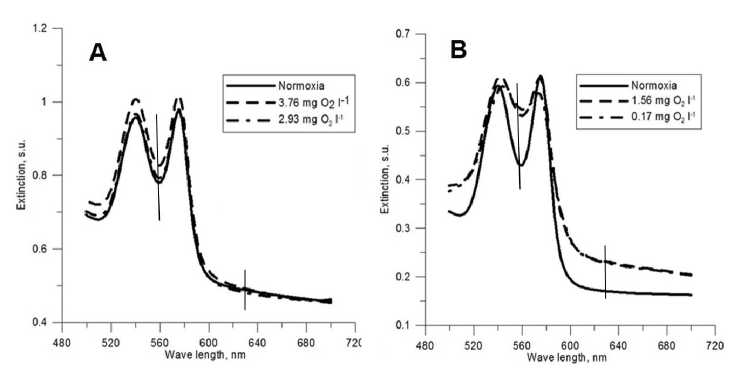
Figure 5 Hemoglobin specters of black scorpionfish under various level of hypoxia (A – comparing of normoxia and moderate hypoxia (≥2 mg О 2 l-1); B – comparing of normoxia and deep hypoxia (≤2 mg О 2 l-1)
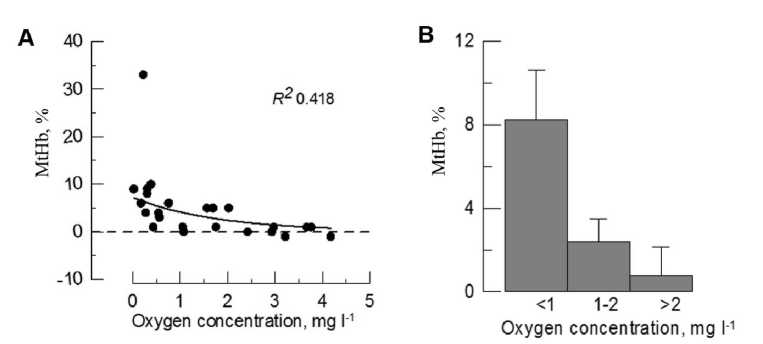
Figure 6 The influence of hypoxia on MtHb concentration in black scorpionfish hemolyzates (A – data distribution; B – data distribution after ranking).
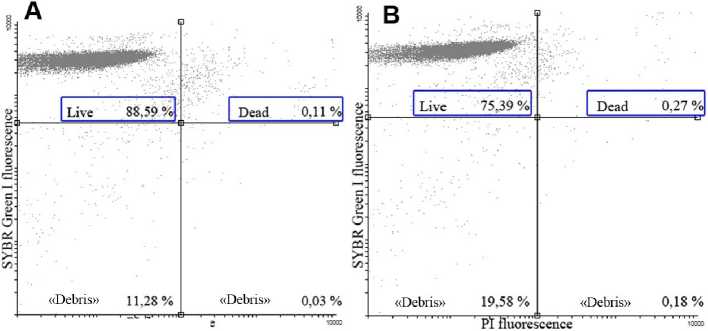
Pl fluorescence
Figure 7 The live/dead ratio in red blood cell suspensions under normoxia (( .22 mg О 2 l-1) and hypoxia (0.43 mg О 2 l-1 (A – normoxia; B – hypoxia)
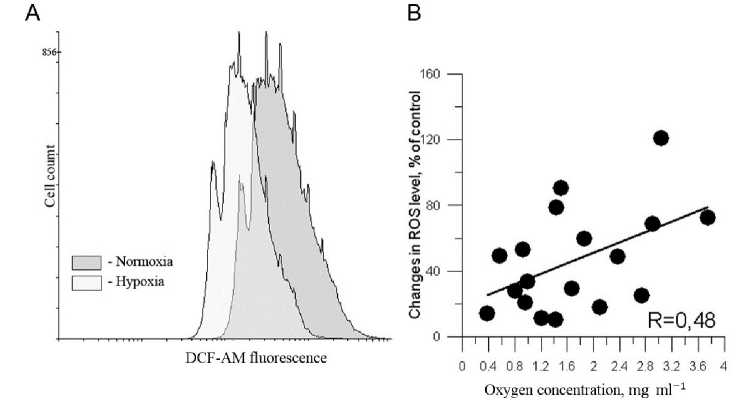
Figure 8 The level of ROS in red blood cells under normoxia and hypoxia estimated on the basis of DCF-AM fluorescence (A – flow cytometric plot; B – data distribution).
DISCUSSION
The results of the present work indicate the following patterns occurring in black scorpionfish red blood cells during hypoxia: (1) MtHb concentration increased at oxygen concentration lower than 2 mg О2 l-1. The level of methemoglobinemia was similar in cells undergoing both in vivo and in vitro hypoxia and was not accompanied with the intracellular ROS accumulation; (2) deep hypoxia do not influence red blood cell membrane integrity which is evidenced by a constant level of dead cells in control and experimental groups.
The increase of MtHb in blood and red blood cell suspensions of black scorpionfish was observed at oxygen concentration lower than 2 mg О 2 l-1 and the most substantial changes were noted at deep hypoxia (≤ 1 mg О 2 l-1). The estimated concentrations of MtHb were close for both experimental approaches, indicating that the processes associated with the oxidation of hemoglobin occurred within cells and were not determined by other organismic responses to oxygen deficiency.
Methemoglobin formation under hypoxia has been demonstrated in numerous works on higher and lower vertebrates (Olander and Parr, 19 8; Arnaud et al ., 19 9; Affonso et al ., 2002; Chen et al ., 201 ). The response is quite paradoxical as it occurs at low oxidizing load. Deoxygenated hemoglobin has been shown to be easily oxidized comparing to oxygenated protein (Mansouri, 1981; Jensen et al ., 1998). This fact can be explained by high-spin state of Fe+ ion (4 unpaired electrons). Binding of oxygen leads to converting of Fe+ to low-spin state where all electrons are paired. Therefore, any changes, which cause lowering of low-spin state of Fe+ in complex Hb-O 2 are supposed to be the reason of electron detachment and oxidizing of iron. Molecule of oxygen, in turn, may accept electron from Fe+ ion with formation of ∙ О 2 -. Hypoxia usually cause deoxy-Hb formation and enhances auto-oxidizing of hemoglobin. At the same time venous blood contain oxygen at concentration sufficient to accept electrons from Fe2+, as its diffusion in tissues is restricted by low concentration gradient in system “blood→tissues”.
In tolerant to hypoxia species of teleosts red blood cells contain several types of hemoglobin and some of them possess high affinity to oxygen, which markedly depends on pH (Bohr effect) (Soldatov et al., 2004). Functional role of these hemoglobins increases under hypoxia and those fractions, which are effective at normoxia are being deoxygenated and then oxidized. Such process should be considered normal as it is implemented in the range of species tolerance. In the present work similar responses of hemoglobin system of black scorpionfish also may occur under hypoxia. It has been shown, that hemoglobin of black scorpionfish has two components (unpublished data), although kinetic parameters of oxygen binding for each fraction remain unstudied.
However, we should also take into account that nucleated fish red blood cells possess aerobic metabolism at normoxia (Boutilier and Ferguson, 1989; Phillips et al ., 2000). Oxygen deficiency usually enhances glycolysis leading to two consequences for cells: (1) decrease of cytoplasmic pH level; (2) lack of NADH. Deficiency of NADH may decrease the activity of NADH-diaphorase, a specific electron carrier from NADH to cytochrome b 5 (and finally MtHb) (Percy and Lappin, 2008). Intracellular acidification of red blood cells has been demonstrated in several works (Adragna et al ., 2004). This should inhibit NADH-diaphorase activity and contribute hemoglobin oxidizing. Also it is known, that substantial pH decrease enhances hemoglobin autooxidizing (Wallace et al ., 1982; Perutz 1990).
We should also mention the possibility of electrons’ leakage from the respiratory chain in mitochondria under hypoxic conditions. The process may occur due to relatively low affinity of cytochrome oxidase (complex IV) to oxygen. At that moment electrons from the previous units of respiratory chain slow down and their “leakage” to oxygen enhances, which should induce ∙ О 2 - formation in cell. However, the process is not accompanied with transduction of hemoglobin to MtHb.
Hemoglobin oxidation may be also induced by another adaptive response to hypoxia observed in teleosts, red blood cells swelling. Increased volume of deoxygenated RBCs has been characterized in many species (Soivio et al ., 19 4; Nikinmaa et al ., 198 ; Holk 1996). Swelling of erythrocytes in hypoxic conditions is considered to regulate intracellular pH by the activation of Na+/H+-exchanger (Tufts, 1992). Na+/H+-exchanger is cAMP-dependent and is activated through the binding of adrenaline and noradrenaline with β-adrenoreceptors
(Ferguson and Boutilier, 1988; Salama and Nikinmaa, 1990; Val et al ., 199 ). At the same time red blood cell swelling has also been demonstrated in isolated deoxygenated suspensions (Andreyeva et al ., 201 ). It is supposed, that erythrocytes enlarge due to substantial pH decrease and Na+/H+-exchanger is activated in vitro through accumulation of Н+ in cytoplasm.
Activation of Na+/H+-exchanger leads to cytoplasm hydration and an increase of its dielectric constant, which facilitates water income into hydrophobic cavity of proteins and hemoglobin in particular (White et al ., 19 8). Water binds to imidazole functional group of histidine causing oxidation of oxy- and deoxy-forms of protein and ∙ О 2 - release. Given the importance of the process it is supposed to play a significant role in formation of methemoglobin and ∙ О 2 - under extremely low oxygen concentrations.
At the same time methemoglobin formation is not always observed during hypoxia impact (Cameron, 19 1; Soldatov and Parfenova, 2001). However, in these works oxygen concentration was higher than 2.5 mg l-1. In the present work we also did not observe significant changes in hemoglobin conformation at that level of oxygen.
The increase of methemoglobin concentration in blood and red blood cell suspensions under hypoxia may be accompanied with the formation of ∙ О 2 - and the enhancement of oxidizing processes in cells. The efficacy of superoxide degradation may be indirectly assessed on the basis of functional state of cells. The level of ROS (hydrogen peroxide) did not increase in red blood cells under hypoxia. DCF fluorescence was even lower in deoxygenated cells compared to control level. Mortality level of cells also did not change after exposure to hypoxia. Thus, we can suppose that antioxidant complex fully neutralized ∙ О 2 - and methemoglobin formation in red blood cells was in the range of physiological norm.
CONCLUSION
In conclusion, methemoglobin formation in black scorpionfish RBCs occurs at oxygen concentrations less than 2 mg О 2 l-1 with the maximal increase 19-32 % observed at deep hypoxia (less than 1 mg О 2 l-1).
Methemoglobinemia was observed in in vitro and in vivo experiments, indicating that cellular processes played a major role in hemoglobin oxidation. Moderate hypoxia (≥ 2 mg О 2 l-1) did not lead to MtHb accumulation. The increase of methemoglobin in red blood cells did not influence ROS level and the integrity of cellular membranes. The level of dead cells did not change after exposure to hypoxia indicating that processes observed were in the range of physiological norm.
ACKNOWLEDGMENT
The research was carried out at the expense of the grant of the Russian Science Foundation No. 23-2400061,
CONFLICTS OF INTEREST
The authors declare that they have no potential conflicts of interest.
Список литературы Effect of acute hypoxia on the functional state of erythrocytes and hemoglobin in black scorpionfish
- Adragna N.C., Di Fulvio M. and Lauf P.K. (2004). Regulation of K-Cl cotransport: from function to genes. J. Memb. Biol., 201, 109-137.
- Affonso E.G., Polez V.L., Correa C.F., Mazon A.F., Araujo M.R., Moraes G. and Rantin F.T. (2002). Blood parameters and metabolites in the teleost fish Colossoma macropomum exposed to sulfide or hypoxia. Comp. Biochem. Physiol. C., 133, 375382.
- Andreyeva A.Y., Soldatov A.A. and Mukhanov V.S. (2017). The influence of acute hypoxia on the functional and morphological state of the black scorpionfish red blood cell. In Vitro Cell Develop. Biol. - Animal., 53, 312-319.
- Arnaud J., Quilici J.C., Gutierrez N., Beard J. and Vergnesa H. (1979). Methaemoglobin erythrocyte reducing systems in high-altitude natives. Annual Human Biology., 6, 585-592.
- Benesch R.E., Benesch R. and Yung S. (1973). Equations for the spectrophotometric analysis of hemoglobin mixtures. Anal. Biochem., 55, 245-248.
- Boutilier R.G. and Ferguson R.A. (1989). Nucleated red cell function: metabolism and pH regulation. Canadian J. Zool., 67, 2986-2993.
- Cameron J.N. (1971). Methemoglobin in erythrocytes of rainbow trout. Comp. Biochem. Physiol. A., 40, 743-749.
- Chen N., Wu M., Tang G-P., Wang H-J., Huang C-X., Wu X-J., He Y., Zhang B., Huang C-H., Liu H., Wang W-M. and Wang Y-L. (2017). Effects of Acute Hypoxia and Reoxygenation on Physiological and Immune Responses and Redox Balance of Wuchang Bream (Megalobrama amblycephala Yih, 1955). Frontiers in Physiol., 8, 1-9.
- Dafre A.L. and Reischl E. (1997). Asymmetric hemoglobins, their thiol content, and blood glutathione of the scalloped hammerhead shark, Sphyrna lewini. Comp. Biochem. Physiol. B.,116, 323-331.
- Ferguson R.A. and Boutilier R.G. (1988). Metabolic energy production during adrenergic pH regulation in red cells of the atlantic salmon, Salmo salar. Resp. Physiol., 74, 65-76.
- Hardig J. and Hoglund L.B. (1983). Seasonal and ontogenetic effects on methaemoglobin and reduced glutathione contents in the blood of reared baltic salmon. Comp. Biochem. Physiol. A., 76, 2734.
- Holk K. (1996). Effects of isotonic swelling on the intracellular Bohr factor and the oxygen affinity of trout and carp blood. Fish Physiol. Biochem., 15, 371-375.
- Jensen F.B., Fago A. and Weber R.E. (1998). Hemoglobin structure and function Fish Physiology. 17 (ed. S.F. Perry and B.L. Tufts). San Diego: Acad. Press.
- Krishna M.S. and Venkataramana G. (2007). Status of lipid peroxidation, glutathione, ascorbic acid, vitamin E and antioxidant enzymes in patients with pregnancy-induced hypertension. Indian J. Physiol. Pharmacol., 51, 284-288.
- Lushchak V.I. and Bagnyukova T.V. (2007). Effects of different environmental oxygen levels on free radical processes in fish. Comp. Biochem. Physiol. B., 144, 283-289.
- Mansouri A. 1981. Methemoglobin formation and reduction in relation to hemoglobin oxygen affinity. Experientia., 37, 95-96.
- Mather-Mihaich E. and Di-Giulio R.T. (1991). Oxidant, mixed-function oxidase and peroxisomal responses in channel catfish exposed to a bleached kraft mill effluent. Arch. Environ. Contam. Toxicol., 20, 391397.
- Nikinmaa M., Cech J.J., Ryhaenen L. and Salama A. (1987). Red cell function of carp (Cyprinus carpio) in acute hypoxia. J. Exp. Biol., 47, 53-58.
- Olander C.P. and Parr C.E. (1978). Methemoglobin in hypoxic rats. Experientia., 33, 1656-1657.
- Percy M.J. and Lappin T.R. (2008). Recessive congenital methaemoglobinaemia: cytochrome b(5) reductase deficiency. Brazilian J. Haematol., 141, 298-308.
- Perutz M.F. (1990). Mechanisms regulating the reactions of human haemoglobin with oxygen and carbon monoxide. Ann. Rev. Physiol., 52, 1-25.
- Phillips M.C.L., Moyes C.D. and Tufts B.L. (2000). The effects of cell ageing on metabolism in rainbow trout (Oncorhynchus mykiss) red blood cells. J. Exp. Biol., 203, 1039-1045.
- Sajiki J. and Takahashi K. (1991). In vitro formation of methemoglobin by lipophilic fractions in fishes and the causative substance. Eisei-Kagaku., 37, 467472.
- Salama A. and Nikinmaa M. (1990). Effect of oxygen tension on catecholamine-induced formation of cAMP and on swelling of carp red blood cells. Amer. J. Physiol. - Cell Physiol., 259, C723-C726.
- Schechter A.N. (2008). Hemoglobin research and the origins of molecular medicine. Blood.,112, 39273938.
- Schoore E.J., Simco B.A. and Davis K.B. (1995). Responses of blue catfish and channel catfish to environmental nitrite. J. Aquat. Anim. Health., 7, 304-311.
- Soivio A., Nyholm K. and Westman K. (1974). Changes in haematocrit values in blood samples treated with and withoutoxygen: a comparative study with four salmonid species. J. Fish. Biol., 6, 763-769.
- Soldatov A.A. (2005). Physiological Aspects of Effects of Urethane Anesthesia on the Organism of Marine Fishes. Hydrobiol. J, 41, 113-126.
- Soldatov A.A., and Parfenova I.A. (2001). The methemoglobin blood level and stability of circulating erythrocytes of the rockfish Scorpaena porcus to osmotic shock under conditions of experimental hypoxia. J. Evolut. Biochem. Physiol., 37, 622-625.
- Soldatov A.A., Parfyonova I.A. and Konoshenko S.V. (2004). Haemoglobin system of black sea round goby under experimental hypoxia conditions. Ukrain'skyi BiokhimichnyiZhurnal., 76, 85-90.
- Stara A., Machova J. and Velisek J. (2012). Effect of chronic exposure to prometryn on oxidative stress and antioxidant response in eatly life stages of common carp (Cyprinus carpio L.J. Neuro. Endocrinol. Lett., 33, 130-135.
- Tiihonen K. and Nikinmaa M. (1991). Short communication substrate utilization by carp (Cyprinus carpio) erythrocytes. J. Exp. Biol., 161, 509-514.
- Tucker C.S. and MacMillan J.R. (1992). Effect of short-term starvation on methemoglobin levels in nitrite-exposed channel catfish. J. Appl. Aquacult., 1, 2128.
- Tufts B. (1992). In vitro edivence for sodium-dependent pH regulation in sea lamprey (Petromyzon marinus) red blood cells. Can. J. Zool., 70, 411-416.
- Val A.L., De Menezes G.C. and Wood C.M. (1997). Red blood cell adrenergic responses in Amazonian teleosts. J. Fish. Biol., 52, 83-93.
- Wallace W.J., Houtchens R.A., Maxwell J.C. and Caughey W.S. (1982). Mechanism of autooxidation for haemoglobins and myoglobins: Promotion of superoxide production by protons and anion. J. Biol. Chem, 257, 4966-4977.
- Wdzieczak J., Zalesna G., Bartkowiak A., Witas H. and Leyko W. (1982). Comparative studies on superoxide dismutase, catalase and peroxidase level in erythrocytes and livers of different fresh water and marine fish species. Comp. Biochem. Physiol. B, 73, 361-365.
- White A., Handler Ph., Smith E.L., Hill R.L. and Lehman R. (1978). Principles Biochemistry. McGRAW-Hill. NJ.
- Willmore W.G. and Storey K.B. (1997). Antioxidant systems and anoxia tolerance in a freshwater turtle, Trachemys scripta elegans. Mol. Cell. Biochem., 170, 177-185.
- Wilson R.R.Jr. and Knowles F.C. (1987). Temperature adaptation of fish haemoglobins reflected in rates of autoxidation. Arch. Biochem. Biophys., 255, 210213.
- Zikic R.V., Stajn A. and Petrovic V.M. (1991). Effect of dexamethasone on the activity of superoxide dismutase and catalase in the tissue and erythrocytes of goldfish. Acta Biol. Jugoslavica., C 27, 45-51.


