Effect of artificial photoperiod on the blood cell indices of the catfish, Clarias batrachus
Автор: Srivastava S, Choudhary Sanjeev K
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 1 т.6, 2010 года.
Бесплатный доступ
The present study is aimed to assess the influence of artificial photoperiod on the blood cell indices of an Indian catfish, Clarias batrachus(Linn.). Blood samples taken from adult fishes exposed to artificial photoperiod of 24L:0D and 0L:24D for a short period of 24 hrs, were analyzed for total RBC, total WBC, differential leukocyte count (DLC) and some physiological variables - glucose and chloride. The total RBC and WBC counts were unaffected by both the artificial photoperiod regimes. However, lymphopenia (p
Leukocyte profile, lymphopenia, neutrophilia, photoperiod, stress
Короткий адрес: https://sciup.org/14323480
IDR: 14323480
Текст научной статьи Effect of artificial photoperiod on the blood cell indices of the catfish, Clarias batrachus
Haematological parameters and physiological profiles can be useful indicators of the physiological disturbances in animals and so can be crucial in providing vital information on the general well-being of fish (Barton and Iwama 1991; Wendelaar Bonga
1997; Tavares-Dias and Morares 2004, 2007). Stoskopf (1993) considered evaluation of blood cells, blood biochemistry as useful for the diagnosis of diseases and assessing the physiological status of fish. Blood cell indices (RBC, WBC and DLC counts) are good indicators of systemic response to external stimulus and any changes are therefore reflected in their morphology and distribution in the blood. Lymphocytes numbers are known to show variability according to the physiological condition of the fish (Klontz 1972). Decreased lymphocyte numbers were observed under stressed conditions – hypoxia, cortisol induced or during handling and transport (Ellsaesser and Clem 1986, 1987). Lymphocytes numbers were also found to be diminished in trouts exposed to constant illumination (Valenzuela et al. 2008). Neutrophil numbers, on the other hand tend to increase under stressful condition.
It is now agreed that in all the five vertebrate taxa including fish, natural stressors or exogenous administration of stressors in the environment elicit a stress response in their leukocyte profile (Davis et al. 2008). From an ecological standpoint, change in ratio between lymphocytes and neutrophils reliably suggest stressful conditions of the environment, and therefore serve to assess stress.
Most of the fishes exhibit a daily light/dark rhythm tuned to the natural photoperiod with melatonin playing the key light perception hormone (Ekstrom and Meissl 1997; Porter et al. 1999; Bromage et al. 2001). The diel rhythms have been known in activities like feeding (Boujard et al. 1991; Hossain et al. 1999), locomotion and gonadal cycles (Fenwick 1970; Hontela and Peter 1980). The fact that day-length has an important role is well documented. In a number of studies on juvenile fishes, exposure to increased day-length have produced such responses as stimulated growth, better feed conversion efficiency and early maturation (Mason et al. 1992; Boeuf and Le Bail, 1999; Purchase et al. 2000; Boeuf and Falcon 2001; Davie at al. 2007) which hold lot of potential in fishery practices. Intensive fish culture practices widely utilize photoperiodic manipulation for obtaining desired growth rates, spawning and early maturation of larval fish. In adult fishes, influence of photoperiod has been observed as changes in light dependant circadian rhythimicity, melatonin production and its secretory patterns (Meissl et al. 1978; Pevet 1979; Iigo et al. 1991), anatomical and biochemical changes in the pineal organ - the chief transducer of environmental light in fish through melatonin (Hafeez et al. 1978; McNulty 1982; Srivastava 2003 a ). Artificial photoperiod regimes are alterations in the natural light:dark cycles and any alteration or manipulation of environmental parameters such as temperature or light results in abrupt changes in the environment which may cause stress thus compromising the welfare and general well-being of the fish (Barton and Iwama 1991; Wendelaar Bonga 1997). Recently some studies have focused on stress related changes in fishes exposed to artificial photoperiod. Physiological changes in blood cell indices, levels of lactate, glucose, plasma proteins, cortisol, FFA have been observed in fish exposed to altered photoperiod conditions (Biswas et al. 2004; Almazan Rueda et al. 2005; Valenzuela et al. 2008 ). However, the results are varied and species – specific in most cases and observed after the photoperiodic exposures that usually ranged from 30 days to 3 months.
Investigations of photoperiodic influences with respect to their stressor influence on adult catfishes is scarce. The only catfish which has been thoroughly investigated is Clarias gariepinus –the African catfish, in which the effect of photoperiod was observed on growth, behaviour and stress variables (Appelbaum and Kamler 2000; Almazan-Rueda et al. 2005). Some studies have been done in Ictalurus punctatus (Stickney and Andrews 1971) where growth and feed conversion under artificial photoperiod was examined. However, these studies are confined to larvae and juveniles and the species are not nocturnal. Clarias batrachus is a crepuscular and nocturnal Indian catfish species inhabiting dim- light environment. Therefore, the present study was aimed to assess the effect of artificial photoperiod of short duration (24hr) on the adults of this fish to assess their stressor influence, if any. Primarily blood cell indices and some physiological variables – plasma glucose (stress indicator) and chloride were examined in the Indian catfish species Clarias batrachus (Linn.) exposed to two artificial photoperiod conditions - 24L:0D i.e. continuous light and 0L:24D i.e. continuous darkness, each for a period of 24 hours.
MATERIALS AND METHODS
Experimental animals
Live adult specimens of Clarias batrachus of average body weight 50-70g were purchased from the local market and maintained in the laboratory under natural photoperiod, 14L:10D. The fishes were maintained in aerated aquaria (dissolved O 2 -7.5-11.5 mg/l; pH 7.03-8.5) and water temperature (25-30ºC) in disease free condition. The fishes were fed with chopped goat liver on alternate days and were acclimatized for 2-3 months before they were put on experiment.
Blood sampling
Fishes were gently netted out of the aquarium, weighed and measured. They were anaesthetized with ethyl p aminobenzoate (Acros, Germany) at a concentration of 0.35g/l. Blood was drawn from the caudal vein of the anaesthetized fishes, collected in heparinized tubes and aliquots were used for blood cell count and smear preparations. About 2ml of the blood was centrifuged at 6000g for 5min. and the plasma obtained was used for glucose and chloride analysis. After the blood samples were taken, depuration of the fishes was carried out in freshwater for 3-4 hours after which the fish were returned to the aquaria.
Determination of blood cell count
Blood cell counts - total erythrocyte (RBC) and total leukocyte (WBC) were done optically using improved Neubaur’s haemocytometer. Blood smears were prepared immediately from whole blood. Air dried smears were then stained with Wright’s and Leishman’s stain. Differential leukocyte counts (DLC) were done by counting 200 cells and the leukocytes were expressed as a percentage. The lymphocytes, thrombocytes and neutrophils were identified according to Ellis (1977). All the counts (RBC, WBC and DLC) were performed in triplicates which were then averaged for agreement within a 15% difference.
Plasma analysis
Plasma glucose was determined according to glucose–oxsidase method using diagnostic kits (Siemens Medical Solutions Diagnostics Ltd.,India). For determination of plasma chloride ( Siemens Medical Solutions Diagnostics Ltd., India) kits were utilized based on the thiocyanate method.
Experimental Protocol
Three batches (for each n=5) of Clarias batrachus (b wt 50-70g and length 25 ± 5cm) were separated. While one batch was employed as control, the other two batches were used for the experimental exposures-one for artificial photoperiod 24L:0D (continuous illumination) and the second for 0L:24D (continuous darkness), each for a period of 24 hrs. Three replicates were performed for each experimental exposure.
For continuous illumination experiments, aquaria were fitted with hoods (cover) containing CFL light (25W; 2900 lux intensity). For continuous darkness, the aquaria were kept in dark chambers. After the experimental exposures, the fishes were anaesthetized. While anaesthetizing the fishes exposed to artificial photoperiod, care was taken that anaesthesia was given under the same photic conditions as the experimental condition. The fishes were not fed during the experiment.
Statistical Analysis
All the values are given as mean ± s.d. Analysis of variance using one way ANOVA was carried out for the two treatments. A difference of p<.05 was accepted as significant.
RESULTS AND DISCUSSION
Blood cell counts
Total erythrocyte counts in fishes exposed to 24L:0D photoperiod (continuous illumination) and 0L:24D (continuous darkness) for 24 hrs did not show any significant difference. The total leukocyte counts (WBC) were also not affected by photoperiodic changes. (Table 1) .
Differential leukocyte counts (DLC) counts showed that percentage of lymphocytes were affected by photoperiod. A significant (p<.05) reduction in the percentage of lymphocytes (lymphopenia) in 24L:0D from control was observed and the values varied significantly with that in 0L:24D photoperiod. Percentage of thrombocytes were unaffected by both the artificial photoperiods (24L:0D and 0L:24D). Significant increases in the percentage of neutrophils (neutrophilia) was observed in 24L:0D photoperiod (p< 0.05), whereas in 0L:24D the percentage of neutrophils decreased.(Table 1).
Plasma Analysis
Plasma analysis showed that mean plasma glucose values varied with the photoperiods but the differences were not significant. (Fig.1a ). Mean plasma chloride levels increased significantly in 24L:0D (p<0.05) whereas decreased in 0L:24D as compared to control. (Fig.1b).
Table I . Haematological parameters of the catfish, C.batrachus under control and exposure to artificial photoperiod conditions.
Values are expressed as mean ± s.d. Different superscript lower case letters in lines indicate significant differences (p<0.05).All results include data for three replicates.
|
Parameters |
14L: 10D Control |
24L: 0D continuous illumination |
0L: 24D continuous darkness |
|
Total RBC (x106µl-1) |
6.31 ± 1.41a |
5.71 ± 1.35a |
6.12 ± 1.33a |
|
Total WBC (x105ul-1) |
1.18 ± 5.9a |
1.47 ± 6.2a |
1.64 ± 2.02a |
|
DLC Lymphocytes (%) Thrombocytes (%) Neutrophils (%) |
65.31 ± 9.2ac 24.74 ± 8.9a 9.73 ± 5.08ac |
57.74 ± 11.4b 24.40 ± 8.27a 17.02 ± 7.8b |
75.37 ± 5.0ac 17.65 ± 3.82a 5.80 ± 0.64ac |
(a)
(b)
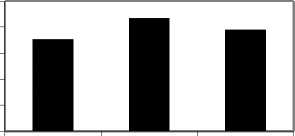
24L :0D 14L :10D 0L :24D
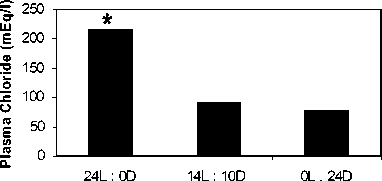
Fig.1. Mean plasma glucose (a) and chloride (b) concentrations in C. batrachus exposed to normal (14L:10D) and artificial (24L:0D and 0L:24D) photoperiod. Asterisk indicate significant difference (p<.05) from the control. All results include data for three replicates.
Discussion
The present study indicates that photoperiod has an influence upon the leukocyte profile of the catfish, Clarias barachus . While the total erythrocyte and total leukocyte counts remain unaffected by the photoperiod changes, the distribution of leukocytes exhibits marked alterations. Differential WBC counts of the adult fishes exposed to continuous illumination for 24 hrs show significant increase in the percentage of neutrophils (p<.05) and decrease in the percentage of lymphocytes (p<.05) as compared to the control, causing change in the neutrophil / lymphocyte ratio. Thrombocyte numbers showed minor variations but no significant change. In contrast, continuous darkness did not elicit any significant response in lymphocyte, thrombocyte and neutrophil numbers in the exposed fishes.
Studies relating to the influence of photoperiod on haematological parameters (including blood cell indices) are rather few in fishes and responses observed are quite variable. In a study combining the effects of different artificial photoperiods and temperatures on the haematological parameters in rainbow trout (Valenzuela et al. 2008) it was found that the fishes when exposed to a photoperiod of 24L:0D (independant of temperature) for a period of 14 days demonstrated an increase of haematocrit, number of erythrocytes but lower number of lymphocytes. Nile tilapias when exposed to different photoperiod for 3 months showed higher lymphocytes in shorter light periods (6L:6D ) than in 12L:12D while other variables such as neutrophils, plasma glucose, chloride, and haematocrit remained unaffected ( Biswas et al. 2004).
This is the first report on the effect of prolonged light (24L:0D) of short duration -24hrs on the blood cell indices of an adult crepuscular and nocturnal catfish. In other catfish species examined before -Ictalurus punctatus (Stickney and Andrews 1971) and Clarias gariepinus (Almazan-Rueda et al. 2005), the effect of photoperiod was observed on growth and feed conversion on the larval and juvenile forms, and the species concerned were not nocturnal. Under prolonged illumination, however C. gariepinus larvae and juveniles became stressed and aggressive and their feed demand increased apart from the effect on growth and survival. In the present investigation, the leukocyte profile of the adults of nocturnal catfish, C.batrachus demonstrated increased number of neutrophils and decreased number of lymphocytes under prolonged light regime (24L:0D) resulting in higher neutrophil : lymphocyte ratio. An association between leukocyte profiles and glucocorticoids levels have been demonstrated to exist in many vertebrates and higher neutrophil : lymphocyte ratio is considered to reliably indicate higher glucocorticoid levels (Davis et al. 2008) i.e. stressful condition. In this study plasma cortisol was not determined, however altered neutrophil:lymphocyte ratio which is indicative of high glucocorticoid levels in the blood indicates that continuous light (24L:0D) causes stress to the fish. The findings for C.batrachus also appear to match with that of its photic requirements : continuous light elicits a stress response whereas continuous darkness does not. The present findings for adult nocturnal C. batrachus also compare well with the findings on the larvae and juveniles of “ no pattern” C. gariepinus (Hocutt 1989) which showed increased plasma levels of cortisol during extended periods of light (Almazan-Rueda et al. 2005). In both the cases, prolonged light regimes elicit increase in plasma levels of stress hormone. Whereas the stress effect on crepuscular and nocturnal catfish is understandable, it is not clear how it becomes stressful in catfish that has no apparent pattern – day or night. It is significant that while the stress effect was observed after 6 weeks in the juveniles and semiadults of C. gariepinus, it was only after 24 hrs. in adult C. batrachus. Reduction in lymphocyte counts has been reported in case of catfishes- Ictalurus (Ellsaesser and Clem 1987) and Rhamdia quelen (Barcellos et al. 2004) but while it was a case of leucocytosis in the former, in the latter a decrease in the lymphocytes was concomitant with an increase in neutrophils. However in both the cases, the stressor was not a photoperiod.
It is interesting that exposure to artificial photoperiod (24L:0D and 0L:24D) for 24 hr did not show any significant change in plasma glucose concentrations in C. batrachus . In case of C. gariepinus, the increase in plasma glucose levels was attributed to increased swimming and not due to the stressor effect of prolonged light while that of lactate level to anaerobic glycolysis in white muscle due to frequent burst activity. In C. batrachus prolonged light is known to decrease locomotor activity (Singh and Srivastava 1971; Srivastava 2003 b) therefore not necessitating increase in plasma glucose levels. Blood glucose levels have long been used as indicators of stress in fish (Hattingh,1976; Donaldson,1981; Wedemeyer and McLeay,1981). Yet, in many studies (Adams et al.1985; Brown et al., 1986; Goss and Wood,1988; Pottinger et al., 2002), under stress blood glucose either remained unchanged or took a longer duration of stress to show the change. It remains to be determined whether these apparent discrepancies are related to the nature of the stressor, duration of the stressor or inter-species differences in glucose utilization and turnover during stress. Possibly further experiment with longer duration of prolonged light may provide information in this regard. However, the short exposure to the artificial photoperiod was sufficient to elevate glucocorticoid levels as indicated by the high neutrophil:lymphocyte ratio due to the observed neutrophilia and lymphopenia conditions. Plasma chloride levels were significantly higher in 24L:0D treatments as compared to that in controls and this can be attributed to increased corticosteroids which are known to stimulate ion-transporting mechanisms.
In the current study, exposure to 24L:0D photoperiod condition, characteristically produced lymphopenia and neutrophilia in the catfish. The two events are known to be invariably associated in mammals and have been observed in fish, too. It was reported by Weinreb (1958) in trouts under stress; in killifish, exposed to cold shock/injection of saline or ACTH (Slicher 1961), in channel catfish following handling stress as well as in response to cortisol administration (Ellsaesser and Clem 1986,1987) and in jundia after usual aquacultural management stress (Barcellos et al. 2004). In fact, Weinreb (1958) concluded in his studies that the lymphopoietic tissue was under the control of corticosteroids whereas granulopoeitic tissue was not. It has been established that lymphopenia and neutrophilia constitute one of the consistent and characteristic changes observed in the haematological pattern of peripheral blood of fishes under stress, regardless of the nature of stress (Mazeaud et al.,1977; Pickering et al., 1982; Tomasso et al., 1983). Newts exposed to constant illumination have also demonstrated lymphopenia and neutrophilia (Bennett and Reap 1978). Espelid et al. (1996) reported the two events to be consequences of stress which can be interpreted either as a direct cytolytic effect of cortisol on the lymphocytes or as a distribution of immunological cells in lymphoid tissues.
Recently much emphasis has been put to the enumeration of white blood cells from blood smears to assess stress in animals (Davis et al. 2008). The findings indicate the rapidity of the stress-induced changes in the leukocyte profile to a relatively mild stress treatment and strongly support the role of leukocyte profiles as stress indicators in vertebrates.
ACKNOWLEDGEMENTS
The authors thankfully acknowledge the University Grants Commission, New Delhi, India for providing financial assistance to this work and Principal, MM(PG) College, Modinagar for providing laboratory facilities.
Список литературы Effect of artificial photoperiod on the blood cell indices of the catfish, Clarias batrachus
- Adams, M. A., Burtis, C. A. and Beauchamp, J. L. (1985) Integrated and individual biochemical responses of rainbow trout (Salmo gairdneri) to varying durations of acidification stress; Comp. Biochem. Physiol. 82C, 301-310.
- Almazan-Rueda, P., Helmond, A. T. M. and Verreth, J. A. J., Schrama, J. W. (2005) Photoperiod affects growth, behaviour and stress variables in Clarias gariepinus; J Fish Biol 67, 1029-1039.
- Appelbaum, S. and Kamler, E. (2000) Survival, growth, metabolism and behaviour of Clarias gariepinus (Burchell 1822) early stages under different light conditions; Aquaculture Engineering 22, 269-287.
- Barcellos, L. J. G., Kreutz, L. C., Souza, C., Rodrigues, L. B., Fioreze, I., Quevedo, R. M., Cericato, L., Soso, A. B., Fagundes, M., Conrad, J., Lacerda, L. A., and Terra, S. (2004) Hematological changes in jundia (Rhamdia quelen Quoy and Gaimard Pimlodidae) after acute and chronic stress caused by usual aquacultural management, with emphasis on immunosuppressive effects; Aquaculture 237, 229-236.
- Barton, B. and Iwama, G. K. (1991) Physiological changes in fish from stress in aquaculture with emphasis on the response and effects of corticosteroids; An Rev Fish Dis. 1, 3-26.
- Bennet, M. F. and Reap, L. E. (1978) Photoperiod, stress and the distribution of leukocytes in the peripheral blood of Notophthalamus viridescens; J Comp Physiol (A), 205-207.
- Biswas, A. K., Maita, M., Yoshizaki, G. and Takeuchi, T. (2004) Physiological responses in Nile tilapia exposed to different photoperiod regimes; J Fish Biol 65(3), 811-821.
- Boeuf, G. and Falcon, J. (2001) Photoperiod and growth in fish; Vie Milieu 51, 247-266.
- Boeuf, G. and Le Bail, P. Y. (1999) Does light have an influence on fish growth? Aquaculture 177, 129-152.
- Boujard, T., Moreau, Y. and Luquet, P. (1991) Entrainment of the circadian rhythm of food demand by infradian cycles of light-dark alternation in Hoplosternum littorale (Teleostei); Aquatic Living Resources 4, 221-225.
- Bromage, N., Porter, M. and Randall, C. (2001) The environmental regulation of maturation in farmed finfish with special reference to the role of photoperiod and melatonin; Aquaculture 197, 63-98.
- Brown, S. B., Evans, R. E. and Hara, T. J. (1986) Interrenal, thyroidal, carbohydrate and electrolyte responses in rainbow trout (Salmo gairdneri) during recovery from the effects of acidification; Can. J. Fish Aquat. Sci. 43, 714-718.
- Davie, A., Mazora de Quero, C., Bromage, N., Treasurer, J. and Migaud, H. (2007) Inhibition of sexual maturation in tank reared haddock (Melanogramnus aeglefinus) through the use of constant light photoperiods; Aquaculture 270, 379-389.
- Davis, A. K., Maney, D. L. and Maerz, J. C. (2008) The use of leukocyte profiles to measure stress in vertebrates: a review for ecologists; Functional Ecology 22(5), 760-772.
- Donaldson, E. M. (1981) The pituitary-interrenal axis as an indicator of stress in fish. In Pickering, A.D. (ed.), Stress and fish Academic Press, London and New York, pp.11-47.
- Ekstrom, P. and Meissl, H. (1997) The Pineal organ of Teleost Fishes, Vol.7. Chapman and Hall, London, pp.199-284.
- Ellis, A.E. (1977) The leukocytes of fish: a review; J Fish Biol 11, 453-491.
- Ellsaesser, C. F. and Clem, L. W. (1986) Haematological and immunological changes in channel catfish stressed by handling and transport; J Fish Biol 28, 511-521.
- Ellsaesser, C. F. and Clem, L.W. (1987) Cortisol-induced haematological and immunological changes in channel catfish (Ictalurus punctatus); Comp Biochem Physiol 87A(2), 405-408.
- Espelid, S., Lokken, G. B., Steiro, K. and Bogwald, J. (1996) Effects of cortisol and stress on the immune system in Atlantic salmon (Salmo salar L); Fish Shellfish Immunol 6, 95-110.
- Fenwick, J.C. (1970) The pineal organ: photoperiod and reproductive cycles in the gold fish, Carassius auratus L; J Endocrinol 46, 101-111.
- Goss, G. G. and Wood, C. M. (1988) The effects of acid and acid/aluminium exposure on circulating plasma cortisol levels and other blood parameters in the rainbow trout, Salmo gairdner;i J. Fish Biol. 32, 63-76.
- Hafeez, M. A., Wagner, H. H. and Quay, W. B. (1978) Mediation of light induced changes in pineal receptor and supporting cell nuclei in steel head trout (Salmo gairdneri); Photochem Photobiol 28, 213-218.
- Hattingh, J. (1976) Blood sugar as an indicator of stress in the freshwater fish, Labeo capensis (Smith); J. Fish Biol. 10, 191-195.
- Hocutt, C. H. (1989) Seasonal and diel behaviour of radio-tagged Clarias gariepinus in Lake Ngezi, Zimbabwe (Pisces: Clariidae); J Zool 219, 181-199.
- Hontella, A. and Peter, R. E. (1980) Effects of pinealectomy, blinding and sexual condition on serum gonadotropin levels in the goldfish; Gen Comp Endocrinol 40, 168-179.
- Hossain, M., Batty, R. S., Haylor, G. and Beveridge, M. (1999) Diel rhythms of feeding activity in African catfish, Clarias gariepinus (Burchell 1822); Aquaculture Research 30, 901-905.
- Iigo, M., Kezuka, H., Adia, K. and Hanyu, I. (1991) Circadian rhythms of melatonin secretion from superfused goldfish (Carassius auratus) pineal glands in vitro; Gen Comp Endocrinol 83, 152-158.
- Klontz, G. W. (1972) Haematological techniques and the immune response in rainbow trout.In Mawdesley-Thomas, L.E. (ed.), Diseases of Fish Symp Zool Soc Lond No.30. Academic Press, London, pp. 89-99.
- Mason, E. G., Gallant, R. K. and Wood, L. (1992) Productivity enhancement of rainbow trout using photoperiod manipulation; Bull Aquaculturists Assoc Can 91, 44-46.
- Mazeaud, M. M., Mazeaud, F. and Donaldson, E. M. (1977) Primary and secondary effects of stress in fish: some new data with a general review; Trans. Am. Fish. Soc. 106, 201-212.
- McNulty, J. A. (1982) The effect of constant light and darkness on daily changes in the morphology of the pineal organ in the gold fish, Carassius auratus; J Neural Trans 53, 277-292.
- Meissl, H., Donley, C. S. and Wissler, J. H. (1978) Free amino acids and amines in the pineal organ of the rainbow trout, Salmo gairdneri. Influence of light and dark; Comp Biochem Physiol 61C, 401-405.
- Pevet, P. (1979) Secretory processes in the mammalian pinealocytes under natural and experimental conditions; Progr Brain Res 53, 277-292.
- Pickering, A. D., Pottinger, T. G. and Christie, P. (1982) Recovery of the brown trout, Salmo trutta L., from acute handling stress: a time-course study; J. Fish Biol. 20, 229-244.
- Porter, M. J. R., Duncan, N. J. and Mitchell, D. (1999) The use of cage lighting to reduce plasma melatonin in Atlantic salmon (Salmo salar) and its effects on the inhibition of grilsing; Aquaculture 176, 237-244.
- Pottinger, T. G., Carrick, T. R. and Yoemans, W. E. (2002) The three-spined stickleback as an environmental sentinel: effects of stressors on whole-body physiological indices; J. Fish Biol. 61, 207-229.
- Purchase, C. F., Boyce, D. L., Brown, J. A. (2000) Growth and survival of juvenile yellowtail flounder Pleuronectes ferrugineus (Storer) under different photoperiods; Aquaculture Research 31, 547-552.
- Singh, K. P. and Srivastava, C. B. L. (1991) A simple device to record the locomotory activity of Indian catfish, Clarias batrachus under laboratory conditions; Proc Nat Acad Sci India 61, 43-48.
- Slicher, A. M. (1961) Endocrinological and haematological studies in Fundulus heteroclitus (Linn); Bull Bingham Oceangr Coll 17, 3-55.
- Srivastava, S. (2003a) Influence of continous light and darkness on the secretory pinealocytes of Heteropneustes fossilis; J Biosci 28(5), 613-622.
- Srivastava, S. (2003b). Two morphological types of pineal window in catfish in relation to photophase and scotophase activity: a morphological and experimental study; J. Exp. Zool. 295A, 17-28.
- Stickney, R. R. and Andrews, J. W. (1971) The influence of photoperiod on growth and feed conversion of channel catfish; Progr Fish Cult 33, 204-205.
- Stoskopf, M. K. (1993) Clinical pathology. In Stoskopf, M.K. (ed.). Fish Medicine Saunders, Philadelphia, pp. 113-131.
- Tavares-Dias, M. and Moraes, F. R. (2004) Haematology in Teleost fish. Sao Paulo: Ribeirao Preto (in Portuguese)
- Tavares-Dias, M. and Moraes, F. R. (2007) Leukocyte and thrombocyte reference values for channel catfish (Ictalurus punctatus Raf.), with an assessment of morphological, cytochemical and ultrastructural features; Veterinary Clinical Pathology 36, 49-54.
- Tomasso, J. R., Simco, B.A. and Davis, K. B. (1983) Circulating corticosteroid and leukocyte dynamics in channel catfish (Ictalurus punctatus) during net confinement; Tex. J. Sci. 35, 83-88.
- Valenzuela, A. E., Silva, V. M. and Klempau, A.E. (2008) Effects of different artificial photoperiods and temperatures on haematological parameters of rainbow trout (Oncorhynchus mykiss); Fish Physiol. Biochem. 34(2), 159-167.
- Wedemeyer, G. A. and McLeay, D. J. (1981) Methods for determining the tolerance of fishes to environmental stressors. In Pickering, A.D.(ed.). Stress and Fish. Academic Press, New York, pp 247-275.
- Weinreb, E. L. (1958) Studies on the histology and histopathology of the rainbow trout, Salmo gairdneri iridius. I. Haematology under normal and experimental conditions of inflammation; Zoologica NY 43, 145-154.
- Wendelaar Bonga, S. E. (1997) The stress response in fish; Physiol Rev 77, 591-625.


