Effect of crude brassinosteroid extract on growth and biochemical changes of Gosssypium hirsutum L. and Vigna mungo L
Автор: Syed Ali Fathima M, Johnson M, Lingakumar K
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 4 т.7, 2011 года.
Бесплатный доступ
The present study was aimed to examine the influence of BRs on seed germination and seedling growth in Gossypium hirsutumL. Var Svpr 2 Vigna mungo (L.) Hepper Var T9. application of BRs on seed germination of Gossypium hirsutum the rate of germination considerably with varied percentage from 60.4 to 99. Vigna mungoseed also showed the varied percentage of germination from 56.8 to 80.1. Both the plants exhibited high percentage of vegetative growth such as shoot length, fresh weight, dry weight and leaf area on 3% of BR supplementation. The amount of chlorophyll a, b and total chlorophyll increased under BR treatments. Among the concentration, 3% BRs caused maximum effect than the other tested concentrations. High percentage of starch 53% and 31 % was observed in Gossypiumand Vignamungorespectively. The results of the present study shows that 3% BRs promotes the growth rate of Gossypium hirsutumL. Var Svpr 2 and Vigna mungo (L.) Hepper Var. T9. The results of the present study supplemented to the previous observations and practical utilization of the new steroidal group of phytohormones for large scale production of the economically important crops Gossypium hirsutumL. Var Svpr 2 and Vigna mungo (L.) Hepper Var. T9.
Brassinosteroids, gossypium hirsutum, vigna mungo, phytohormones
Короткий адрес: https://sciup.org/14323562
IDR: 14323562
Текст научной статьи Effect of crude brassinosteroid extract on growth and biochemical changes of Gosssypium hirsutum L. and Vigna mungo L
The occurrence of brassinosteroids (BRs) has been demonstrated in almost every part of plants such as pollen, flower buds, fruits, seeds, vascular cambium, leaves, shoots and roots. BRs are required for normal development of plants. Works on higher plants suggest that they play a critical role in a range of developmental processes, e.g. stem and root growth, floral initiation, and the development of flowers and fruits (Hayat and Ahmad, 2003; Sasse,
-
2003 ). Brassinosteroids (BRs) are steroidal sixth group of phytohormones that regulate various aspects of plant growth and development, including cell elongation, photo-morphogenesis, xylem differentiation, and seed germination (Rao et al., 2002; Sasse, 2003) as well as adaptation to abiotic and biotic environmental stresses (Krishna, 2003; Divi and Krishna, 2009). The role of BRs in plant stress responses has been validated in several
studies (Dhaubhadel et al., 1999; Dhaubhadel et al., 2002; Kagale et al., 2007; Koh et al., 2007).
BR supports tolerance in plants to a wide range of stresses including heat, cold, drought and salinity. This increase is generally correlated with higher expression of stress marker genes such as heat shock protein (hsp) genes, RD29A and ERD10 (Bajguz and Tretyn, 2003; Kagale et al., 2007; Nilovskaya et al., 2001; Kamuro and Takaysuto, 1991; Kulaeva et al., 1991; Vardhini and Rao, 2003; Anuradha and Rao, 2003), indicating that increased expression of stress-responsive genes is responsible, in part, for the higher stress tolerance in BR-treated plants. To this date, about 70 BRs have been isolated from plants (Dhaubhadel et al., 1999). In view of some reports it is evident that the exogenous application of brassinosteroids as a foliar spray improves growth and yield of some plants e.g. tomato (Vardhini and Rao, 2001) and Arachis hypogaea (Vardhini and Rao, 1998). Exogenous application of brassinosteroids can also alleviate the adverse effects of various environmental stresses and produces resistance in plants against these stresses e.g., salt stress (Hathout, 1996; Vardhini and Rao, 1997), heat stress (Zhu et al., 1998), drought stress (Li and Van Staden, 1998) and chilling stress (Wilen et al., 1995). Brassinosteroids are known to act along with auxins to stimulate cell elongation (Katsumi, 1991; Sasse, 1991). A number of studies were recorded the role of BRs on seed germination, vegetative growth, elongation of shoot and root and improving the productivity of various crops such as vegetables, pulses, cereals, fruits and oil seeds (Anuradha and Rao 2003; Swamy and Rao, 2006; Anuradha and Rao, 2007; Kagale et al., 2007; Shahbaza and Ashraf, 2007; Bajguz and Hayat, 2009). But there is no report on the influence of BRs on seed germination and growth in Gossypium hirsutum L. and Vigna mungo L. To fulfill the lacuna, the present study was aimed to examine the influence of BRs on seed germination and seedling growth in Gossypium hirsutum L. Var Svpr 2 and Vigna mungo (L.) Hepper Var T9.
MATERIALS AND METHODS
Cultivation of plants
Seeds of Gossypium hirsutum L. Var Svpr 2 and Vigna mungo (L.) Hepper Var. T9 were collected from the Tamil Nadu Agricultural University, Tamil Nadu, India and washed in the running tap water for 5 min. To remove the surface contaminants, the seeds were treated with commercial detergent Triton X100 for 3 min and washed with distilled water. The washed seeds were sterilized with 0.1% HgCl 2 for 3 min and washed thrice with sterile distilled water. The sterilized seeds were soaked in water for 24 h at room temperature and germinated in dark for 40 h on moist soil. After germination, the pots were transferred to direct solar radiation. For each treatment 20 seeds were placed in the earthen pots and repeated thrice.
Isolation of BR and extract preparation
500 mg fresh cauliflower inflorescences were harvested and pulverized with 400 ml of isopropyl alcohol. The suspension was filtered through two layered muslin cloth. The filtering process was repeated thrice to obtain the maximum quantity of crude extract from the inflorescence. The filtrate was collected and stored in the refrigerator at 4°C. To study the effect of BRs on seed germination, growth and biochemical changes of Gossypium hirsutum L. Var Svpr 2 and Vigna mungo (L.) Hepper Var. T9, different percentage of BRs (0.01, 0.05, 0.1 and 3) was prepared.
Seed germination
For the seed germination studies, the healthy seeds of Gossypium hirsutum L. Var Svpr 2 and Vigna mungo (L.) Hepper Var. T9 was soaked in different percentage of BR extracts viz., 0.01, 0.05, 0.l and 3 %(v/v). The seeds soaked in distilled water were treated as control. The seeds were soaked in BRs and distilled water for 24 h at room temperature. After 24 h, seeds were planted in earthen pots for germination. The earthen pots were filled with Black soil, Red soil and sand in 1:1:1 ratio. Growth and biochemical characteristics of the plants were observed at the end of 5th, 10th, 15th, 20th and 25th days after germination.
Growth analysis
The growth of the plant was recorded on 25th and 50th day in terms of shoot and root length, fresh and dry weight of shoots and roots by random selection.
For each treatment ten plantlets were selected randomly. The leaf area was measured by the conventional graph method and leaves were selected randomly.
Biochemical analysis
Pigment was extracted by grinding the leaves in 80% acetone (v/v) and the total chlorophyll content was determined by Wellburn and Lichtenthaler (1984) method. The amount of total soluble leaf protein was calculated using Lowry et al., (1951) method. The amount of starch content was calculated by using Lugol’s iodine method.
Table 1 Influence of Crude Brassinosteroid extracts on Seed germination of Gossypium hirsutum L.
Var Svpr 2 and Vigna mungo (L.) Hepper Var T9
|
Concentration of Crude Brassinosteroid in % |
Mean % of Seed germination ± S.E. |
|
|
G. hirsutum Var Svpr 2 |
V. mungo Var. T9 |
|
|
Control |
75.5 ± 0.82 |
68.5 ± 0.82 |
|
0.1 |
78.3 ± 0.64 |
80.1 ± 0.51 |
|
0.3 |
81.4 ± 0.48 |
75.6 ± 0.43 |
|
0.5 |
85.3 ± 0.61 |
70.3 ± 0.61 |
|
1.0 |
90.8 ± 0.38 |
65.8 ± 0.44 |
|
2.0 |
92.3 ± 0.41 |
63.4 ± 0.28 |
|
3.0 |
94.3 ± 0.38 |
61.3 ± 0.38 |
|
4.0 |
73.1 ± 0.65 |
60.3 ± 0.58 |
|
5.0 |
60.4 ± 0.38 |
56.8 ± 0.41 |
Effect of BR concentration on photosynthetic pigment composition
Effect of BR concentration on metabolites
The soluble protein content and starch level was determined in Gossypium and Vigna mungo leaves subjected to BRs treatment. The influence of BRs in soluble protein and starch content are depicted in Fig. 2. G - J. High percentage of protein and starch was observed in Gossypium and Vigna mungo on 3% BR supplemented medium.
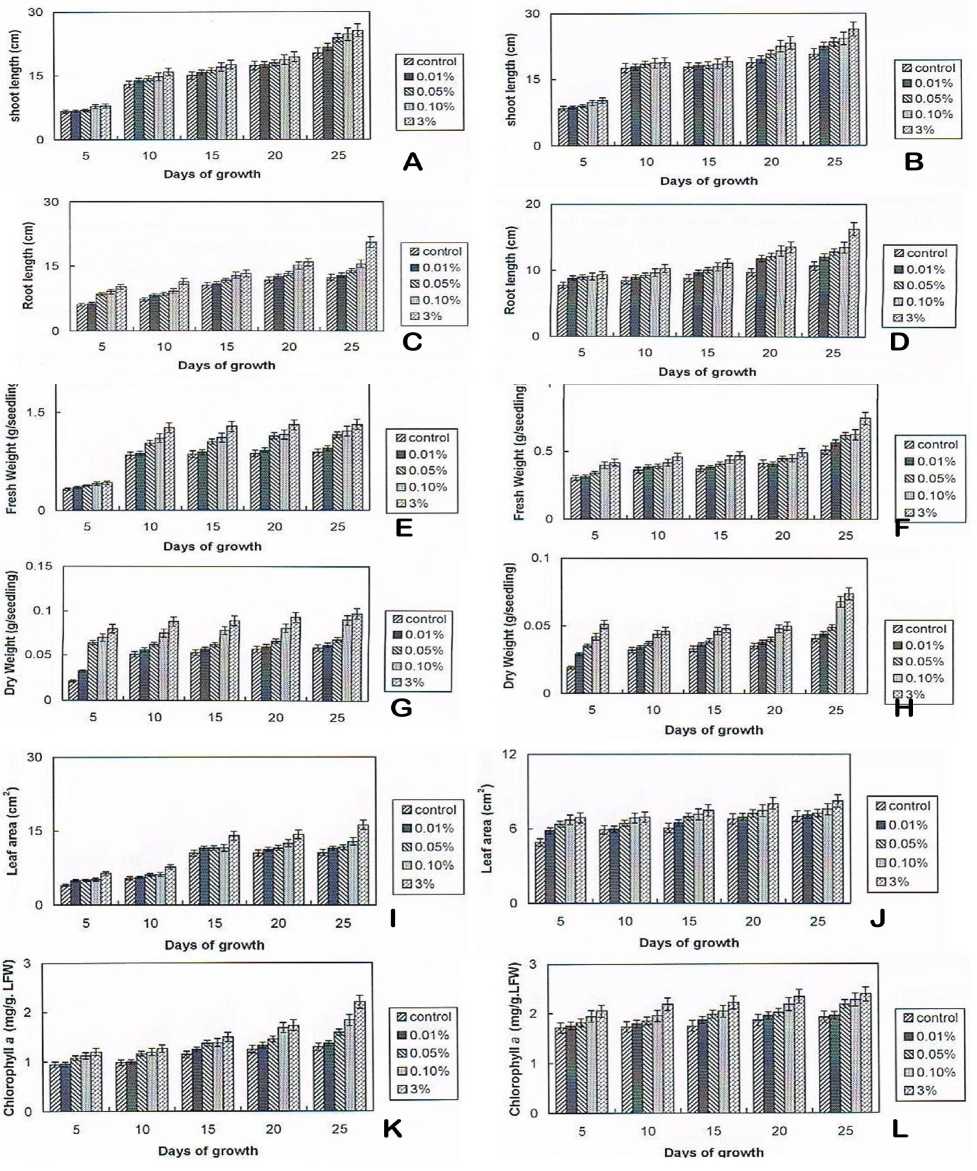
Figure 1 Influence of Crude Brassinosteroid extracts on physiological and Biochemical Changes in Gossypium hirsutum L. Var Svpr 2 and Vigna mungo (L.) Hepper Var T9 seedlings
-
A) Influence of Crude Brassinosteroid extracts on shoot length of Gossypium
-
B) Influence of Crude Brassinosteroid extracts on shoot length of Vigna mungo
-
C) Influence of Crude Brassinosteroid extracts on root length of Gossypium
-
D) Influence of Crude Brassinosteroid extracts on root length of Vigna mungo
-
E) Influence of Crude Brassinosteroid extracts on fresh weight of Gossypium
-
F) Influence of Crude Brassinosteroid extracts on fresh weight of Vigna mungo
-
G) Influence of Crude Brassinosteroid extracts on dry weight of Gossypium
-
H) Influence of Crude Brassinosteroid extracts on dry weight of Vigna mungo
-
I) Influence of Crude Brassinosteroid extracts on leaf area of Gossypium
-
J) Influence of Crude Brassinosteroid extracts on leaf area of Vigna mungo
-
K) Influence of Crude Brassinosteroid extracts on chlorophyll a content of Gossypium
-
L) Influence of Crude Brassinosteroid extracts on chlorophyll a content of Vigna mungo
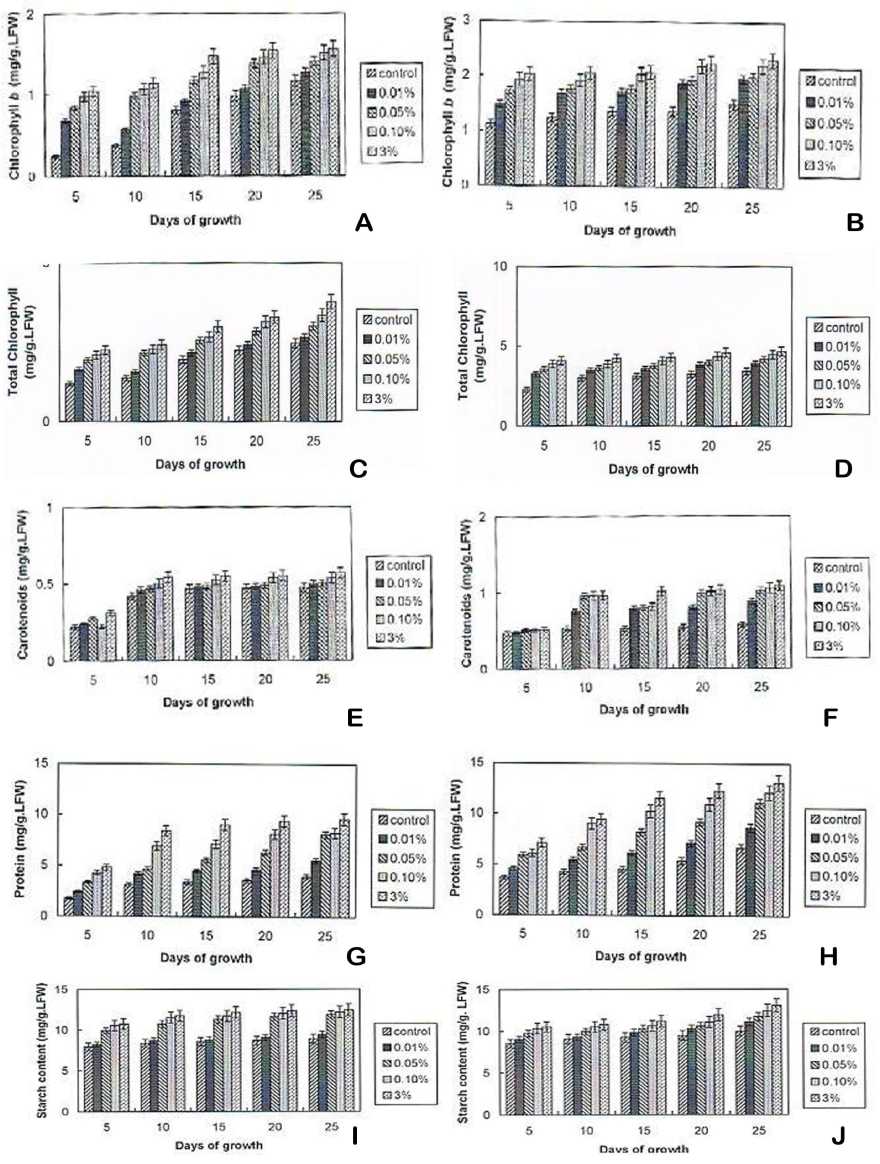
2 Effect of Crude Brassinosteroid extracts
on the Biochemical Changes
hirsutum L. Var Svpr 2 and Vigna mungo (L.) Hepper Var T9 seedlings
Figure
in Gossypium
-
A) Effect of Crude Brassinosteroid extracts on chlorophyll b content of Gossypium
-
B) Effect of Crude Brassinosteroid extracts on chlorophyll b content of Vigna mungo
-
C) Effect of Crude Brassinosteroid extracts on Total chlorophyll content of Gossypium
-
D) Effect of Crude Brassinosteroid extracts on Total chlorophyll content of Vigna mungo
-
E) Effect of Crude Brassinosteroid extracts on carotenoids content of Gossypium
-
F) Effect of Crude Brassinosteroid extracts on carotenoids content of Vigna mungo
-
G) Effect of Crude Brassinosteroid extracts on protein content of Gossypium
-
H) Effect of Crude Brassinosteroid extracts on protein content of Vigna mungo
-
I) Effect of Crude Brassinosteroid extracts on starch content of Gossypium
-
J) Effect of Crude Brassinosteroid extracts on starch content of Vigna mungo
RESULTS
Effect of various concentrations of BRs on seed germination
The application of BRs on seed germination of Gossypium hirsutum increased the rate of germination considerably with varied percentage from 60.4 to 99. Similar to that, Vigna mungo also showed the varied percentage of germination from 56.8 to 80.1. For G. hirsutum , the highest percentage (99.3 ± 0.38) of seed germination was achieved in 3% BR supplementation. For V. mungo, the highest percentage of germination (80.1) was achieved on 0.1% supplementation of BR extract (Table 1). The emergence of radical was taken as to the exogenous application of crude BR extracts. Above 3% of BRs supplementation showed the lethal effects, the high concentration of BR extract was unable to promote rate of seed germination.
Effect of BR on vegetative growth
The effect of BRs on vegetative growth was depicted on Fig. 2 and 3. The effect of various concentration of crude BRs extract on growth parameters such as shoot length, fresh weight, dry weight and leaf area of Gossypium and Vigna are demonstrated in Fig.1 A-J. Both the plants exhibited high percentage of vegetative growth on 3% of BR supplementation. Besides the role of BRs on shoot and root morphology, leaf area was also monitored at various stages of plant growth. The Gossypium and Vigna mungo showed the significant increase throughout the growth period by the augmentation of BRs. Nearly 50% increase was noticed in Gossypium at 3% BR application (Fig. 8) after 20 days of growth. The increase in leaf area was more prominent in G. hirsutum than V. mungo seedlings.
DISCUSSION
During the last few decades, the concern about the use of BRs as growth hormone has increased.
The importance of brassinosteroid as a plant growth regular was investigated by several authors either by the application to germinating seeds or by foliar application. The application of steroid hormones to plants stimulated cell elongation in roots and shoots, cell division and flowering (Braun and Wild, 1984). The results of the present study also coincide directly with previous observations. The results exhibited the increase in rate of seed germination, growth pattern and biochemical constituents in Gossypium hirsutum L. Var Svpr 2 and Vigna mungo (L.) Hepper Var. T9 by the crude BRs application. For the present study, the crude BR extract was used as the source of hormone. Moreover, the hormonal response of the extract was tested by the conventional rice lamina inclination assay. The angle between the second lamina and the leaf sheath was measured and 3% BR induced the maximum angle. The rice - lamina inclination test confirmed the hormonal activity of the crude BR extract (Takatsuto et al., 1983). Brassinosteroids have unique growth promoting activity when applied exogenously (Mandava et al., 1981). In the present study, application of crude BR extract to soaked seeds of Gossypium hirsutum L. Var Svpr 2 and Vigna mungo (L.) Hepper Var. T9 evoked positive responses. Our results were directly coincided with Mandaya et al., observations. From the experiments, it was found that the shoot length, root length, fresh weight and dry weights of both the plants increased to a greater extent throughout the experimental period (5-25 days). Stimulation of such growth parameters was reported by Braun and Wild (1984). Unlike other phytohormones, BRs induced all the growth parameters. Similar observation was made in BR treated barley by Gregory (1981). Kauschmann et al., (1996) also reported a similar kind of observation in bean plants. Vardhini and Rao (1999) correlated BR induced growth responses to changes in macromolecules such as nucleic acids and proteins. The results of the present study also confirm and supplemented the previous observations (Braun and Wild, 1984; Kauschmann et al., 1996; Vardhini and Rao, 1999). The changes in chlorophyll a, chlorophyll b, chlorophyll a + b and carotenoid content in cotton and blackgram upon BR application confirms the hormonal activity of BRs. Total chlorophyll level was elevated to a greater level in blackgram and cotton at 3% BR treatment. Although, low concentration caused increase in pigment level, a significant rise was noticed only at 3% BR. Carotenoid content also increased. Supplementation of BR to salt stressed seedlings ameliorated the stress by increasing chlorophyll content (Anuradha and Rao, 2003). Enhanced levels of chlorophyll and carotenoid in treated plants indicate a better photosynthetic level of total chlorophyll increased is in agreement with the findings of Braun and Wild (1984). They ascertained that high RuBP case (a Calvin cycle enzyme) activity and high chlorophyll content are reasons for greater photosynthetic activity. In addition to photosynthetic pigments, the level of soluble proteins and starch was also high in BR treated plants indicating an overall growth. Increase in soluble protein and starch content in the tested plants was also noticed in groundnut as reported by Prakash et al., (2003). The increase in soluble protein could be due to enhanced activity of the enzyme in nitrogen metabolism. Even though, enzyme activity like nitrate reductase was not followed, the enhancement in non-structural carbohydrate indicates the better adaptiveness of the crop plants to BR treatment. The induction of protein, DNA and RNA content in excised beans exposed to BR treatment was reported by Kalinich et al., (1985). The possible reason for enhancement of metabolite in BR treated cotton and blackgram could be high in DNA and RNA polymerase activity. Both enzyme systems were enhanced by BR treatment (Kalinich et al., 1985). Recently, leucine-rich protein (BRL 1) has been identified from Arabidopsis. This protein os considered to be the BR preceptor, located on the plasma membrane. The binding of BR molecule to the receptor causes activation of the kinase domain with subsequent phosphorylation (Clouse and Sasse, 1998).
5. Conclusion
The present study confirmed the influence of BRs on the germination and growth of Gossypium hirsutum L. Var Svpr 2 and Vigna mungo (L.) Hepper Var. T9. The results of the present study showed that the concentration of BRs has the influence on the germination and seedling growth and metabolites. The results of the present study shows that 3% BRs promotes the growth rate of Gossypium hirsutum L. Var Svpr 2 and Vigna mungo (L.) Hepper Var. T9. The results of the present study supplemented to the previous observations and practical utilization of the new steroidal group of phytohormones for large scale production of the economically important crops Gossypium hirsutum L. Var Svpr 2 and Vigna mungo (L.) Hepper Var. T9.
Список литературы Effect of crude brassinosteroid extract on growth and biochemical changes of Gosssypium hirsutum L. and Vigna mungo L
- Anuradha, S.and Rao, S.S.R. (2003). Application of brassinosteroids to rice seeds (Oryza sativa L.) reduced the impact of salt stress on growth and improved photosynthetic pigment levels and nitrate reductase activity. Plant Growth Regul. 40, 29-32.
- Anuradha, S. and Rao S.S.R. (2007). The effect of brassinosteroids on radish (Raphanus sativus L.) seedlings growing under cadmium stress. Plant Soil Environ., 53 (11), 465-472.
- Bajguz, A. and Tretyn, A. (2003). The chemical characteristic and distribution of brassinosteroids in plants. Phytochemistry, 62, 1027-1046.
- Bajguz, A. and Hayat, S. (2009). Effects of brassinosteroids on the plant responses to environmental stresses. Plant Physiology and Biochemistry, 47, 1-8.
- Braun, P. and Wild, A. (1984) Proceedings of the 6th International Congress on Photosynthesis (Sybesma, C. (Ed.)). pp. 461-463.
- Clouse, S.D. and Sasse, J.M. (1998). Brassinosteroids: essential regulators of plant growth and development. Annu Rev Plant Physiol Plant Mol Biol. 49, 427-451.
- Dhaubhadel, S., Browning, K.S., Gallie, D.R. and Krishna, P. (2002). Brassinosteroid functions to protect the translational machinery and heat-shock protein synthesis following thermal stress. Plant J. 29, 681-691.
- Dhaubhadel, S., Chaudhary, S., Dobinson, K.F. and Krishna, P. (1999). Treatment with 24 epibrassinolide, a brassinosteroid, increases the basic thermotolerance of Brassica napus and tomato seedlings. Plant Mol Biol. 40, 333-342.
- Divi, U.K. and Krishna, P. (2009). Brassinosteroids confer stress tolerance, in: Hirt, H., Wiley, V.C.H. (Eds.), Plant stress biology: genomics goes systems biology, pp. 119-135.
- Gregory, L.E. (1981). Acceleration of plant growth through seed treatment with brassins. Am. J. Bot., 68, 586-588.
- Hathout, T.A. (1996). Salinity stress and its counteraction by the growth regulator (Brassinolide) in wheat plants (Triticum aestivum L. cultivar Giza 157). Egypt. J. Physiol. Sci., 20, 127-152.
- Hayat, S. and Ahmad, A. (2003). Brassinosteroids: Bioactivity and Crop Productivity, Kluwer Academic Publishers, Dordrecht.
- Kagale, S., Divi, U.K., Krochko, J.E., Keller, W.A. and Krishna, P. (2007). Brassinosteroid confers tolerance in Arabidopsis thaliana and Brassica napus to a range of abiotic stresses. Planta., 225, 353-364.
- Kalinich, J.F., Mandava, N.B. and Jodhunter, J.A. (1985). Relationship of nucleic acid metaolosm to brassinolide induced responses in beans. J. Plant Physiol., 120, 207-214.
- Kamuro, Y. and Takaysuto, S. (1991). Capability for problems of practical uses of brassinosteroids, in: Cutler, H.G., Yokota, T., Adam, G., (Eds.), Brassinosteroids Chemistry, Bioactivity and Application, ACS Symp. Ser. 474, Am. Chem. Soc., Washington, pp. 292-297.
- Katsumi, M. (1991). Physiological modes of brassinolide action in cucumber hypocotyls growth, in: Cutler, H.G., Yokota, T., Adam, G., (Eds.), Brassinosteroids Chemistry, Bioactivity and Application, ACS Symp. Ser. 474, Am. Chem. Soc., Washington, Chap. 21.
- Kauschmann, A., Jessop, A., Konez, C., Szekeres,M., Willimitzer, L. and Altmann, T. (1996). Genetic evidence for an essential role of brassinosteriods in plant development. Plant J., 9, 701-713.
- Koh, S., Lee, S.C., Kim, M.K., Koh, J.H., Lee, S., An, G., Choe, S. and Kim, S.R. (2007). T-DNA tagged knockout mutation of rice OsGSK1, an orthologue of Arabidopsis BIN2, with enhanced tolerance to various abiotic stresses. Plant Mol Biol., 65, 453-466.
- Krishna, P. (2003). Brassinosteroid-mediated stress responses. J. Plant Growth Regul., 22, 289-297.
- Kulaeva, O.N., Burkhanova, E.A., Fedina, A.B., Khokhlova, V.A., Bokebayeva, G.A., Vorbrodt, H.M., Adam, G., 1991. Effect of brassinosteroids on protein synthesis and plant cell ultrastructure under stress conditions. In: Cutler, H.G., Yokota, T., Adam, G., (Eds.), Brassinosteroids Chemistry, Bioactivity and Application, ACS Symp. Ser. 474, Am. Chem. Soc., Washington: 141-155.
- Li, L. and Van Staden, J. (1998). Effects of plant growth regulators on the antioxidant system in seedlings of two maize cultivars subjected to water stress. Plant Growth Regul., 25, 81-87.
- Lowry, O.H., Rosenbury, N.J., Farr, A.L. and Ranalall, R. J. (1951). Protein measurement with folinphenol reagent. J. Biochem., 193, 265-275.
- Mandava, N.B., Sasse, J.M. and Yopp, J.H. (1981). Brassinolide -a growth promoting steroidal lactone. II. Activity in selected gibberellins and cytokinin bioassays. Physiol. Plant., 53,453-461.
- Nilovskaya, N.T., Ostapenko, N.V. and Seregina, I.I. (2001). Effect of epibrassinolide on the productivity and drought resistance of spring wheat. Agrokhimiya, 2, 46-50.
- Prakash, M., Saravanan, K., sunil kumar, B. and Ganesan, J. (2003). Effect of brassinosteriods on certain Biochemical parameters in groundnut (Arachis hypogaea L.). Indian J. Plant Physiol., 8, 313 -315.
- Rao, S.S.R., Vardhini, B.V.V., Sujatha, E. and Anuradha, S. (2002). Brassinosteroids -A new class of phytohormones. Curr. Sci., 82, 1239-1245.
- Sasse, J.M. (1991). The case for brassinosteroids as endogenous plant hormones, in: Cutler H.G., Yokota T., Adam G. (Eds.), Brassinosteroids: Chemistry, Bioactivity and Applications, Washington, D.C., Am. Chem. Soc., pp. 158-166.
- Sasse, J.M. (2003). Physiological actions of brassinosteroids: an update. J. Plant Growth Regul., 22, 276-288.
- Shahbaz, M. and Ashraf, M. (2007). Influence of Exogenous Application of Brassinosteroid on Growth and Mineral Nutrients of Wheat (Triticum aestivum L.) Under Saline Conditions. Pak. J. Bot., 39(2), 513-522.
- Swamy, K.N. and Rao, S.S.R. (2006). Influence of Brassinosteroids on rooting and growth of Geranium (Pelargonium sp.) stem cuttings. Asian J of Plant Sciences 5(4), 619-622.
- Takasuto, S., Yazawa, N., Ilekawa, N., Morishita, T. and Abe, H. (1983). Synthesis of 28 homobrassinolide analogues and structure activity relationship of Brassinosteroids in the Rice lamina inclination test. Phytochemistry 22, 1393-1397.
- Vardhini, B.V. and Rao, S.S.R. (1997). Effect of brassinosteroids on salinity induced growth inhibition of ground nut seedlings. Indian J. Plant Physiol., 2(2), 156-157.
- Vardhini, B.V. and Rao, S.S.R. (1998). Effect of brassinosteroids on growth, metabolic content and yield of Arachis hypogaea. Phytochemistry, 48, 927-930.
- Vardhini, B.V. and Rao, S.S.R. (1999). Effect of brassnosteriods on nodulation and nitrogenase activity in groundnut (Arachis pypogaea L.). Plant growth Regulation, 23, 165-167.
- Vardhini, B.V. and Rao, S.S.R. (2001). Effect of brassinosteroids on growth and yield of tomato (Lycopersicon esculentum Mill.) under field conditions. Indian J. Plant Physiol., 6, 326-328.
- Vardhini, B.V. and Rao, S.S.R. (2003). Amelioration of water stress by brassinosteroids on seed germination and seedling growth of three varieties of sorghum. Plant Growth Regul., 41, 25-31.
- Wellburn, A.R. and Lichtenthaler, H. (1984). Formulae and program to determine total caroteniods and chlorophyll a and b of leaf extracts in different solvents, in: Sybesma, C., Nijhoff, M., Junk, W. (Eds.), Advances in Photosynthesis Research, The Hague, 2, pp 9-12.


