Effect of Cu and Mn toxicity on chlorophyll fluorescence and gas exchange in rice and sunflower under different light intensities
Автор: Hajiboland R., Hasani B.d
Журнал: Журнал стресс-физиологии и биохимии @jspb
Рубрика: Original article
Статья в выпуске: 1 т.3, 2007 года.
Бесплатный доступ
Copper (Cu) and manganese (Mn) are essential micronutrients for plants, but toxic at high concentrations. Responses of rice (Oryza sativa L.) and sunflower (Helianthus annuus L.) to toxic concentrations of Mn and Cu (up to 100 µM) were studied under three light intensities including low (LL, PPFD=100), intermediate (IL, PPFD=500) and high (HL, PPFD=800) light intensities in hydroponic medium. Rice plants showed higher susceptibility than sunflower to both heavy metals concerning dry matter of shoot and root. Growing under higher light intensity strengthened the effect of Cu toxicity while ameliorated that of Mn, the latter was attributed to the lower Mn accumulation of HL plants in both shoot and root. Chlorophyll content of leaves was influenced negatively only by Cu treatment and that at the highest concentration in the medium (100 µM). Similar with growth results, reduction of net assimilation rate (A) was higher in HL than LL plants treated by excess Cu, but in contrast to growth response, reduction was more prominent in sunflower than rice. Excess Mn-induced reduction of A was similar between LL and HL plants and was greater in sunflower than rice. Reduction of A was partly attributable to stomatal limitation, but non-stomatal mechanisms were also involved in this reduction. Copper and Mn treatment did not change the optimal quantum efficiency of PSII in dark-adapted chloroplasts (Fv/Fm ratio), but Fv/F0 was influenced particularly by Cu treatment, the reduction was higher in rice than sunflower and in HL compared to LL plants. Regarding excess Cu and Mn-mediated alterations in chlorophyll concentration, Fv/F0 and Tm values, it was suggested that, Cu and Mn toxicity depress the leaf photosynthetic capacity primarily by causing a significant alteration of the composition and functional competence of the photosynthetic units rather a reduction in the number of photosynthetic units (PSUs) per unit leaf area.
Cu toxicity, chlorophyll fluorescence, gas exchange, light intensity, mn toxicity
Короткий адрес: https://sciup.org/14323458
IDR: 14323458
Текст обзорной статьи Effect of Cu and Mn toxicity on chlorophyll fluorescence and gas exchange in rice and sunflower under different light intensities
Heavy metals are present in trace concentrations in terrestrial ecosystems, however, agricultural and industrial activities are important factors producing phytotoxic amounts of metals in soils. Plants grown on heavy metal contaminated soils, may take up an excess of these elements, which affect different physiological processes.
Usual causes of high levels of copper (Cu) in the soil are mining activities or the prolonged application of Cu-based fungicides. Plants in general are very sensitive to copper toxicity, displaying metabolic disturbances and growth inhibition at Cu contents in the tissues only slightly higher than normal levels. Toxic concentrations of Cu inhibit metabolic activity, which leads to suppressed growth and slow development (Fernandes and Henriques, 1991).
Although manganese (Mn) is not a common pollutant in soils, various soil conditions often present in acid and volcanic soils or submergence can lead to Mn reduction and create Mn toxicity in many natural and agricultural systems (Foy et al., 1978). The deleterious effect of Mn toxicity is often observed in the shoot as stunted growth, chlorosis, crinkled leaves and brown lesions (Marschner, 1995).
Toxic trace pollutants can induce many alterations in plant cells (Woolhouse, 1983), but it is difficult to draw a general mechanism about the physiology of stress, since metal toxicity results from complex interaction of metal ions with several metabolic pathways. However, one of the underlying causes of tissues injury following exposure of plants to Cu (Weckx and Clijsters, 1997) and Mn (González et al., 1998) is the increased accumulation of reactive oxygen species mediated-oxidative stress (De Vos and Schat, 1991).
One of the most important effects of reactive oxygen species is the loss of membrane integrity (Vichnevetskaia and Roy, 1999). Thylakoid lipids are susceptible to oxidation due to predominance of polyunsaturated fatty acids (Gounaries et al., 1986) and the availability of oxygen producing free radicals around functional PSII (Kyle, 1987). Therefore, photosynthetic membranes in chloroplasts are the most susceptible structures in plants grown under conditions of oxidative stresses. Reactive oxygen species not only are produced in response to heavy metal stress in shoot and root, but also higher light intensity alone or in combination with heavy metal toxicity could led to a severe oxidative damage of leaves in stressed plants.
It was reported that, response of plants to excess Mn is affected by light intensity (González et al., 1998). However, reports on the effect of high light intensity on Mn toxicity are contradictory, including increasing (Nable et al., 1988; Horiguchi 1988) or lessening toxicity symptoms (Wisssemeier and Horst, 1992). A factor that complicates the interpretation of previous studies of the effect of light intensity on Mn-toxicity symptoms is the fact that plants grown in low light usually accumulate less foliar Mn than those grown at a higher light intensity (Mc Cain and Markley, 1989). In contrast to Mn, there is no report on the effect of growth under various light intensities on Cu tolerance of plants.
Copper is a potent inhibitor of chlorophyll synthesis (Fernandes and Henriques 1991, Pätsikkä et al., 1998; Quartacci et al., 2000) in some plants such as wheat, barley and spinach. However, no significant effect of Cu toxicity on chlorophyll synthesis in maize was reported (Stiborová et al., 1986). Similarly, reports on the effect of Mn toxicity on chlorophyll content of plants, is contradictory, from no change (Wisssemeier and Horst, 1992) to severe chlorosis (Nable et al., 1988; Horiguchi 1988) depending on plant species.
Copper has been shown to increase susceptibility to photo-inhibition particularly in intact leaves (Pätsikkä et al., 1998) but the underlying mechanism has remained unclear. It has been known that high concentrations of copper when added to the incubation medium of isolated thylakoids, inhibit PSII electron transfer activity on the acceptor side (Yruela et al., 1996) and finally cause the release of the external polypeptides of the oxygen-evolving complex on the donor side of PSII (Pätsikkä et al., 2001). Some authors suggested that excess copper in the growth medium did not cause loss of photoprotection, but reduced chlorophyll content causes the high photosensitivity of PSII in copper treated plants (Pätsikkä et al., 2002). Very limited data are available concerning photochemistry of leaves under Mn toxicity.
A considerable part of the radiation absorbed by leaf pigments is reemitted back as fluorescence. It has been known that changes in chlorophyll fluorescence emissions are indications of changes in photosynthetic activity (Kautsky et al 1960). Chlorophyll fluorescence gives information about the state of Photosystem II and about the extent to which PSII is using the energy absorbed by chlorophyll and the extent to which it is being damaged by excess light (Maxwell and Johnson, 2000). The flow of electrons through PSII is indicative of the overall rate of photosynthesis and is an estimation of photosynthetic performance. To our knowledge, this is the first study of the potential impact of Cu and Mn toxicity in combination with high light intensity on photosynthesis.
There are only limited data available concerning response of photosynthesis capacity of plants to heavy metal toxicity. It is still an open question as to whether stomatal closure is the main factor inhibiting photosynthesis and biomass production under heavy metal stress.
In this work we studied the response of two important crop species as influenced by dual effect of metal toxicity and high light intensity. The main objective of this work was the evaluation of the importance of various physiological traits of photosynthesis in growth and biomass production under heavy metal toxicity..
MATERIALS AND METHODS
Two crop species were used in this study including rice ( Oryza sativa L. cv. Amol 3) and sunflower ( Helianthus annuus L. cv. Azarghol). Seeds were provided by the Rice Research Institute (Guilan, Iran) and Seed and Plant Improvement Institute (SPII) (Karaj, Iran) respectively.
Plants culture and treatments
The experiments were conducted in a growth chamber with a temperature regime of 25°/18°C day/night, 14/10 h light/dark period and relative humidity of 70/80%. Surface-sterilized seeds were germinated in the dark on sand, moistened with distilled water and CaSO 4 at 0.05 mM. The 7-days-old seedlings with uniform size were transferred to hydroponic culture in plastic container with 2L of nutrient solution (50%) and pre-cultured for 3 days. Copper and Mn treatments were started for 10-days-old plants, consisted of three levels of CuSO 4 or MnSO 4 at 0 (control), 50 and 100 µM. Composition of the nutrient solutions (pH=6.5) were used according to Yoshida et al (1972) for rice and Dannel et al (1995) for sunflower plants. Nutrient solutions were changed every 3 days and the pH was adjusted every day.
For study of the effect of different light intensities, plants were grown simultaneously under three light intensities: low light (LL=100 µmol m-2s-1 PPFD), intermediate light (IL=500 µmol m-2s-1
PPFD) and high light (HL=800 µmol m-2s-1 PPFD) intensities. The incident photosynthetic photon flux density (PPFD) was measured by a quantum sensor attached to the leaf chamber of the gas exchange unit.
After 14 days treatment, plants were harvested. After drying at 70°C for 2 days and determining plants dry weight, samples were ashed in a muffle furnace at 550°C for 8h and concentration of Cu and Mn were determined by atomic absorption spectrophotometry (Shimadzu, AA 6500).
For determination of chlorophyll concentration, third leaves (pair of leaves in sunflower) immediately after harvest were used to extract of chlorophyll by N,N dimethylformamide according to Moran (1982). For determination of root length, fresh roots were used according to the method of Tennant (1975).
Another group of plants were used for measurement of gas exchange parameters and chlorophyll fluorescence.
Determination of gas exchange parameters
CO 2 assimilation and transpiration rates of attached leaves were measured with a calibrated portable gas exchange system (LCA-4, ADC Bioscientific Ltd., UK) always between 9:00 A.M. and 15:00 P.M. Measurements were carried out on one mature, fully expanded and attached leaf from 4 plants per treatment illuminated with the treatment specific light intensity (LL, IL or HL). With the exception of PPFD, no microenvironmental variable inside the chamber was controlled.
The net photosynthesis rate by unit of leaf area ( A ) and the stomatal conductance to water vapor ( g s ) were calculated using the values of CO 2 and humidity variation inside the chamber, both measured by the infrared gas analyzer of the portable photosynthesis system. Other parameters calculated were the ratio of intercellular air space and atmospheric CO2 molar fraction ( Ci/Ca ) and photosynthetic water use efficiency ( WUE ) was determined by the ratio of net photosynthesis rate ( A ) to transpiration rate ( E ) ( WUE = A/E ).
Determination of chlorophyll fluorescence
Chlorophyll fluorescence parameters were recorded in parallel for gas exchange measurements in the same leaf, using a portable fluorometer (FIM, ADC Bioscientific Ltd., UK). Leaves were acclimated to dark for 30 min before measurements were taken. Initial ( F 0 ), maximum ( F m ), variable ( F v =F m -F 0 ) and the F v :F m ratio were recorded.
Experiments were under taken in complete randomized blocj design with 4 replications. Statistical analyses were carried out using sigma stat (3.02) with Tukey test ( p <0.05).
RESULTS
Effect of metal toxicity and various light intensities on plants growth
Cu toxicity: Shoot and root dry weight decreased in response to Cu toxicity. Shoot growth of rice was inhibited up to 60-73%, while growth of sunflower diminished only 11-49%. Reduction of root growth was also different in Cu-treated rice and sunflower plants, in rice the reduction was 58-82% and in sunflower was 35-64%. A clear effect of light conditions on the extent of growth inhibition by Cu toxicity was observed. Growth under higher light intensity caused an increased susceptibility of plants to Cu toxicity. This effect was more prominent in sunflower, in which both of shoot and root growth responded much more negatively to Cu toxicity when grown under higher light intensity e.g. up to 49% reduction in HL compared to only 11% reduction in LL plants. However, dry matter of shoot and root in plants grown at higher light intensity was higher than plant at lower light conditions at similar Cu treatments (Table 1).
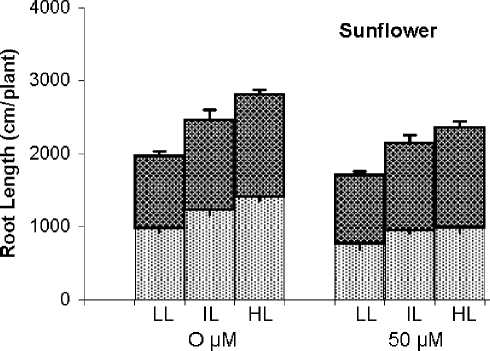
Root length was also affected by both light conditions of growth and Cu toxicity. Higher light intensity per se increased significantly root length and, in contrast to its effect on shoot and root growth, when applied together with Cu toxicity alleviated partly the inhibitory effect of Cu toxicity. Reduction of root length for LL plants by 100 µM Cu was 40% and 51%, but for HL plants the corresponding values were only 30% and 42% for rice and sunflower respectively. Another difference between response of dry matter and length of root was higher susceptibility of sunflower than rice to Cu toxicity when root growth was expressed on length basis (Fig. 1).
Mn toxicity: Mn toxicity inhibited shoot and root growth with different extent than Cu toxicity, and the reverse effect of light intensity on the expression of Mn toxicity was observed. The response of shoot growth was different from 63% reduction for LL plants to 10% inhibition in HL plants for rice and from 57% reduction in LL to 79% increase in HL sunflower plants. Similar trend was observed for root growth, with the exception of no growth improvement in response to Mn toxicity in HL plants. Such as Cu, susceptibility to Mn toxicity was higher in rice than sunflower, regarding both shoot and root growth (Table 1).
Similar with dry matter production, root length responded positively to light intensity, and Mn treatments affected root length differently depending on plant species e.g. reduction in rice and stimulation in sunflower. Therefore, sunflower plants treated by 100 µM Mn and grown under HL conditions, have the highest root length (Fig. 1).
Shoot and root concentration of Cu and Mn
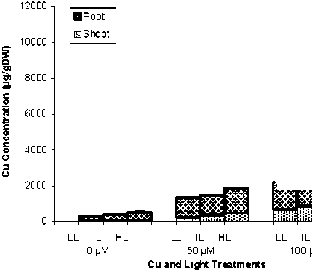
As it is obvious from Fig. 2, majority of Cu in rice was accumulated in roots while in sunflower shoot and root accumulated Cu with similar extent.
In contrast to Cu, rice plants accumulated high amounts of Mn in shoots. Copper concentration of shoot and particularly root was increased in rice plants treated by higher light intensity but in sunflower HL plants have lower Cu concentration in shoot. The effect of higher light intensity on Mn concentration was similar between rice and sunflower, HL conditions reduced drastically Mn accumulation in shoot and root.
Chlorophyll concentration
In general, leaf chlorophyll a and b concentration decreased under high light conditions, but Cu and Mn treatment did not reduce it in both studied plants. In some treatment combinations rather an increase in chlorophyll a and b concentration was observed. However, chlorophyll b content responded differently in rice plants. Chlorophyll b content of Mn treated rice plants did not change significantly in response to higher light intensity (data not shown). The ratio of chlorophyll a/b in plants treated by toxic concentrations of Cu and Mn, was influenced by both light conditions and the level of Cu and Mn in the medium. Higher light intensity reduced chlorophyll a/b ratio in control and Mn treated rice plants, but did not change it in Cu treated rice, which was the result of concomitant reduction of chlorophyll a and b (data not shown). In sunflower, in contrast to rice, chlorophyll a/b ratio did not change significantly in response to higher light intensity and Mn treatment did not change the response of chlorophyll a/b ratio to light intensity. Additionally this ratio increased in response to Cu treatment in sunflower in contrast to rice (Fig. 3).
The sum of chlorophyll a and b content (chlorophyll a+b) was influenced in response to higher light intensity in rice and sunflower and under control as well as Cu and Mn treated plants. However, the effect of metal toxicity was different depending on plant species. Manganese treatment did not affect chlorophyll a+b content in rice, but induced an increase in sunflower. The chlorophyll a+b content of rice plants treated by 50 µM Cu increased compared to control plants but decreased with increasing Cu treatment at 100 µM. In contrast, chlorophyll a+b content was continuously increased in response to Cu treatment up to 100 µM in sunflower. Generally, higher light conditions caused a reduction of chlorophyll a+b content, this effect was similar at Cu- and Mn-treatments and observed in both rice and sunflower plants (Fig. 3).
Gas exchange parameters
A significant effect of light intensity on the net photosynthesis rate ( A ) was observed only in control plants.
Cu toxicity: Assimilation of CO2 (A) was inhibited by 44% in LL and 58% in HL rice plants. Reduction of assimilation rate due to Cu toxicity in sunflower was 57% and 80% in LL and HL plants respectively. Transpiration rate (E) did not change significantly in response to Cu treatment either in rice or sunflower, but as expected, higher light intensity increased significantly the water lost via transpiration. The ratio of Ci/Ca did not change in response to Cu treatment, but decreased in response to higher light conditions and that was observed only in rice. Stomatal conductance (gs) decreased in response to Cu treatment particularly in rice plants, however, such reduction was not statistically significant. As expected, stomatal conductance increased significantly in IL and HL plants compared to LL plants. Though an increased assimilation rate (A) in HL compared to LL plants, the water use efficiency (WUE) diminished significantly in the latter than former plants (statistical analysis among values for light treatments was not shown) because of concomitant induction of stomatal conductance (gs) and increase in transpiration rate (E) (Table 2).
Mn toxicity: Mn treatment reduced slightly the net assimilation rate ( A ), which was more prominent in sunflower than rice. This reduction was similar (50-56%) in LL than HL plants. Similar with Cu treatment, transpiration rate ( E ) was affected only slightly by Mn treatment, but light intensity increased stomatal conductance ( g s ) significantly. The ratio of Ci/Ca decreased slightly in response to higher light intensity and that was observed only in rice. Water use efficiency ( WUE ) changed in response to Mn treatment particularly in sunflower, it was diminished up to 55% in LL and 40% in HL sunflower plants. This ratio decreased as the result of more prominent reduction of CO2 assimilation ( A ) than that of transpiration ( E ) in Mn treated plants (Table 3).
Chlorophyll fluorescence
Photosynthetic activity was also characterized in terms of photochemical efficiency using measurements of chlorophyll fluorescence. For each treatment combination, chlorophyll fluorescence was measured in dark-adapted tissues. F 0 value of dark adapted tissues (fluorescence emissions when all reaction centers are open for photochemistry) was influenced by light conditions. In rice IL and HL intensities decreased F 0 value, but in sunflower, only HL caused such reduction. In response to Cu treatment, F 0 values increased in rice but not in sunflower. The F m values of dark adapted tissues (fluorescence emissions when all the reaction centers are closed) increased continuously in response to increasing light intensity in both species. Fm values increased in Cu treated plants in comparison to control plants, in sunflower, however, F m values decreased significantly in sunflower under similar treatments. Higher light intensity results in increase of F v values in both species, it was reduced in response to Cu treatment, which was significant only in sunflower. T m values decreased in response to Cu treatment in both studied species mainly in plants grown under higher light intensities. In HL rice and sunflower, Tm values diminished significantly up to
18%, but the reduction for LL plants was in tendency and as low as 5% (Table 4).
In contrast to Cu, Mn treatment did not alter significantly the F 0 and F m values in rice. In sunflower, similar with Cu treated sunflower plants and in contrast to rice, F 0 and F m values decreased in response to Mn treatment slightly or significant. T m values decreased in both studied plants, but in contrast to Cu treated plants, reductions were significant only in LL and IL Mn treated plants (Table 5).
Growing under higher light intensity, increased the optimal quantum efficiency of PSII in dark-adapted chloroplasts ( F v /F m ratio), but Cu and Mn treatment did not change F v /F m significantly. In contrast, F v /F 0 was influenced by both light intensity and Cu treatment, and with a less extent by Mn treatment. Reduction of F v /F 0 ratio in response to Cu treatment was lower in LL than HL plants in both species, and the reduction was higher in rice compared to sunflower, e.g. 22% and 10 % in LL rice and sunflower compared to 39% and 19% in HL rice and sunflower respectively (Table 4 and 5).
4000 -,
Rice
BMn Treatment
■E re
3000 -
в Cu Treatment
E о
2000 -
CT c
Ф
^ш ^№ |Ш№ sr@8r $R®« &Ж§ &;Ж &Ж 8^0 w® ш^ SW $M W8 &&ж :&&& '.IB .'
о о
ОС
1000 -

^ЛУф^ж^ж-
№i№w $№3 &&$i $$^ 8^ es№ й%У:9 ' ^ ......
^B:
$W
-ЖЙФ
$№
-^ Me
■I M
Wt® ФЖФН
■S ЦЦ
S;S;8S ЙОТЙ i^O "*w
1 LL IL HL О pM
IL HL
50 pM
LL IL HL
100 pM
Cu/Mn and Light Treatments
Cu/Mn and Light Treatments
BMn Treatment EH Cu Treatment
Е^^^ ййЖ йЖ$
^^ ^М ЙЙЙЯЙЙ
^м ^Ж адда»
*■>?*•* ТТТ7:
row :Г:;гГ<^>::;г^;
LL IL HL
100 рМ
Fig. 1. Root length (mg plant-1) of rice and sunflower treated with Cu or Mn and grown under three light conditions.
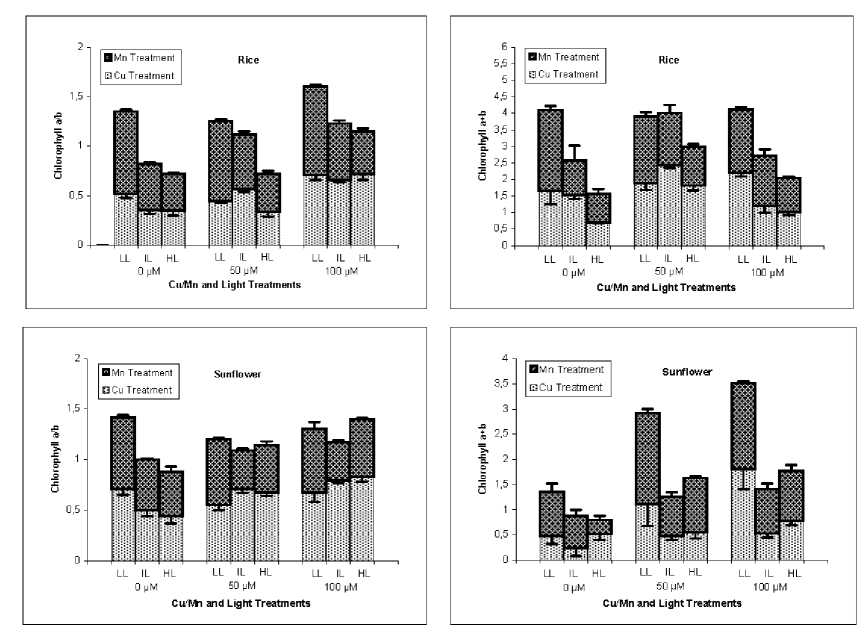
Fig. 2 . Changes in the leaf chlorophyll a/b ratio and chlorophyll a+b amounts in rice and sunflower treated with Cu or Mn and grown under three light conditions.
ш
®i® Si® к»:
рта н M :;: й?й SM »:» у»; я»; St® ®SS t®S St® ®SS Si® »:4№>t»t >ж
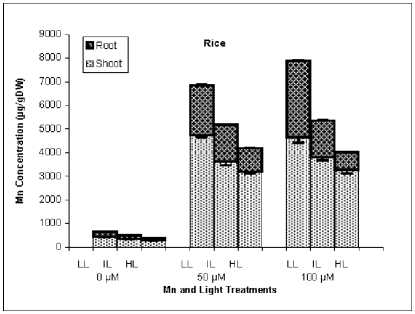
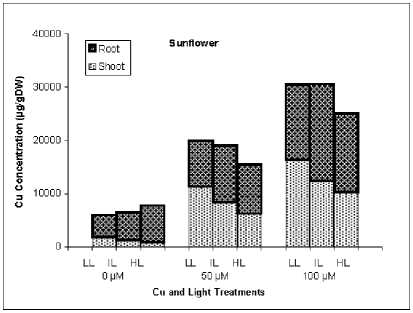
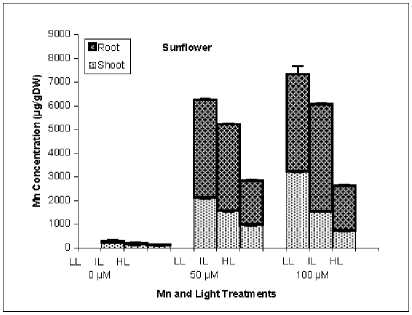
Fig. 3. Shoot and root concentration of Cu and Mn in rice and sunflower treated with Cu or Mn and grown under three light conditions
Table 1. Shoot and root dry weight (mg plant-1) of rice and sunflower treated with 50 and 100 µM of Cu or Mn and grown under three light conditions including low (LL=100 µmol m-2s-1 PPFD), intermediate (IL=500 µmol m-2s-1 PPFD) or high (HL=800 µmol m-2s-1 PPFD) intensity for 14 days in hydroponic medium. Data in each column within each plant species followed by the same letter are not significantly different (p<0.05).
|
Plant Species |
Cu (pM) |
Shoot DW (mg plant'1) |
Root DW (mg plant"1) |
||||
|
LL |
IL |
HL |
LL |
IL |
HL |
||
|
Rice |
0 |
80±2 a |
109±8a |
385123a |
1212 a |
2412 a |
7616 a |
|
50 |
75±6 a |
65±2b |
20714b |
1411 a |
1611 b |
6317 b |
|
|
100 |
32±2 b |
43±6C |
10318c |
512 b |
711 c |
1414 е |
|
|
Sunflower |
0 |
201.16a |
479±123 |
887175a |
2312 a |
6812 a |
19913a |
|
50 |
176±20a |
377117b |
650157b |
1512 b |
5017 b |
137±21b |
|
|
100 |
179±19a |
320±8c |
44816c |
1513 b |
3314 c |
72110е |
|
|
Plant |
Mn |
Shoot DW (mg plant"1) |
Root DW (mg plant"1) |
||||
|
Species |
(pM) |
LL |
IL |
HL |
LL |
IL |
HL |
|
Rice |
0 |
8(H2a |
109±8a |
3851238 |
1212 a |
2412 a |
7616 b |
|
50 |
40±8b |
98±18a |
3531358 |
511 b |
911 b |
10015a |
|
|
100 |
30±8b |
119115 1 |
348132a |
612 b |
1012 b |
7817 b |
|
|
Sunflower |
0 |
201±6a |
479±12b |
887175c |
2312 a |
6812 b |
199113b |
|
50 |
111±7 b |
639±82a |
1739151a |
1411 b |
8612 a |
284111a |
|
|
100 |
86±6C |
465 122b |
1548148b |
912 c |
4311 c |
187119b |
|
Table 2. Gas exchange parameters including net photosynthetic rate ( A ), transpiration ( E ), the ratio of intercellular air space and atmospheric CO2 molar fractions ( Ci/Ca ), stomatal conductance to water vapor ( gs ) and instantaneous water use efficiency ( WUE ) in attached leaves of rice and sunflower treated with 50 and 100 µM of Cu and grown under three light conditions. Data in each column within each measured parameters followed by the same letter are not significantly different (p<0.05).
|
Cu (pM) |
Rice |
Sunflower |
|||||
|
LL |
IL |
HL |
LL |
IL |
HL |
||
|
A |
0 |
18+6“ |
19±6a |
24+6 = |
42+9“ |
78+15“ |
82+18= |
|
(цшо1 111 2 S4) |
50 |
12+6“ |
14±7‘ |
15+7 ab |
16+5 b |
20+10b |
21+12b |
|
too |
10+3“ |
9±5 * |
10+4 b |
18+7 b |
21+9 b |
16+8 b |
|
|
E |
0 |
0.25+0.10“ |
041±0.01 = |
0.71+0.12“ |
0.47+0.1 “ |
0.95+0.5 “ |
1.3+0.6“ |
|
(mmol tn2 s4) |
50 |
0.20+0.05 = |
0.32±0.01 = |
0.58+0.31 “ |
0.49+0.2 “ |
0.95+0.6“ |
1.0+0.4“ |
|
100 |
0.19+0.03 “ |
0.30±0.10a |
0.71+0.21 “ |
0.49+0.1 “ |
0.75+0. 5 8 |
1.2+0.4“ |
|
|
Ci/Ca |
0 |
4.2+1.7 “ |
2.8+0.9 ’ |
2.2+0.9 ’ |
0.6+0.3 “ |
0.6+0.2 1 |
0.7+0.3 “ |
|
50 |
4.8+1.9 * |
2.9+1.8 * |
2.3+1.3 * |
0.5+0.2 * |
0.6+0.2 = |
0.8+0.3 “ |
|
|
100 |
4.8+0.5 “ |
2.9±1.4 1 |
2.4+1.1 * |
0.5±0.2 a |
0.6+0.3 “ |
0.7+0.4 “ |
|
|
gs |
0 |
17+9“ |
30+15’ |
51+16‘ |
38+16= |
40+13‘ |
60+12° |
|
(mol m2 s4) |
50 |
11+8“ |
20+13a |
37+181 |
31+10' |
35+19“ |
53+14“ |
|
100 |
15±9a |
17±9‘ |
51+16 ’ |
30+17' |
33+10“ |
55+19“ |
|
|
WUE |
0 |
72±8" |
46+7“ |
34+5“ |
89+5 “ |
82+4“ |
63+15“ |
|
(|imol mol4) |
50 |
60±7" |
44+9“ |
26+6“ |
33+9 6 |
21+6 b |
21+8 b |
|
100 |
63±8" |
40+8“ |
34+8“ |
37+8 b |
28+8 b |
16+6 ъ |
|
Table 3. Gas exchange parameters including net photosynthetic rate ( A ), transpiration ( E ), the ratio of intercellular air space and atmospheric CO2 molar fractions ( Ci/Ca ), stomatal conductance to water vapor ( gs ) and instantaneous water use efficiency ( WUE ) in attached leaves of rice and sunflower treated with 50 and 100 µM of Mn and grown under three light conditions. Data in each column within each measured parameters followed by the same letter are not significantly different (p<0.05).
|
Mn (pM) |
Rice |
Sunflower |
|||||
|
LL |
IL |
HL |
LL |
IL |
HL |
||
|
л |
0 |
18+6" |
19+6" |
24+6" |
42+98 |
78+15" |
82+18’ |
|
(pmol m2 s') |
50 |
12+48 |
16+9" |
20+9 ab |
20+13b |
30+15” |
32+16” |
|
100 |
10+3 8 |
1®8* |
10+4 ” |
21+8 ” |
28+11” |
36+13” |
|
|
E |
0 |
0.25+0.01 " |
0.41+0.01 1 |
0.71+0.2" |
0.47+0.1 8 |
0.95+0.5 ” |
1.3+0.6 8 |
|
(mmol m2 s *) |
50 |
0.221:0.10 8 |
0.35+0.01 1 |
0.63+0.3 8 |
0.48+0.58 |
0.73+0.5 * |
1.1+0.5 " |
|
100 |
0.20+0.00 1 |
0.30+0.1 1 |
0.60+0.5 1 |
0.53+0.1 3 |
0.65+0.2 Я |
1.0+0.6 8 |
|
|
Ci/Ca |
0 |
4.2+1.7 8 |
2.8+1.2 1 |
2.2+1.1 * |
0.6+0.2" |
0.6+0.1 * |
0.7+0.1 * |
|
50 |
5.2+2.48 |
3.0+1.3 8 |
2.4+1.5 1 |
0.5+0.2 8 |
0.4+0.1 3 |
0.7+0.1 1 |
|
|
100 |
5.4+2.5 8 |
3.2+1.8 1 |
2.5+1.0" |
0.5+0.2 8 |
0.4+0.2 3 |
0.7+03" |
|
|
gs |
0 |
17+9 8 |
30+15" |
51+16" |
38+16" |
40+13 8 |
60+12’ |
|
(mol m2 s1) |
50 |
12+8 8 |
18+7" |
41+15" |
36+10" |
40+198 |
51+11 " |
|
100 |
10+7 * |
22+8 ‘ |
35+15* |
3H11* |
46+17* |
53+16" |
|
|
ИГЕ |
0 |
72+8 8 |
46+7" |
34+138 |
89+5 8 |
82+4" |
63+4 8 |
|
(p.mol mol1) |
50 |
55+9 b |
46+8 8 |
32+14" |
42+7 ” |
41+8 ” |
29+5 ” |
|
100 |
60+7 ab |
50+8 ‘ |
32+108 |
40+9” |
43+6 ” |
38+8 ” |
|
Table 4. Chlorophyll fluorescence parameters including F 0 , F m , F v and T m and calculated F v /F m and F v /F 0 in attached leaves of rice and sunflower treated with 50 and 100 µM of Cu and grown under three light conditions. Data in each column within each measured parameters followed by the same letter are not significantly different (p<0.05).
|
Cu (цМ) ■ |
Rice |
Sunflower |
|||||
|
LL |
IL |
HL |
LL |
IL |
HL |
||
|
Fo |
0 |
655+18b |
479+25b |
296+43b |
659+898 |
661+658 |
379+268 |
|
50 |
814+251 |
610+65 * |
451+63 8 |
514+66” |
556+54 8b |
409+418 |
|
|
100 |
818+15 1 |
634+49 * |
472+33" |
524+43"” |
535+39” |
403+338 |
|
|
Fm |
0 |
2185+25b |
2229+28b |
2283+25b |
2110+57" |
2570+128 |
2910+428 |
|
50 |
2294+378 |
2330+298 |
2401+34" |
1511+25” |
1871+22” |
2570+29 ” |
|
|
100 |
2305+223 |
2349=198 |
2411+49" |
1554+38” |
1811+19= |
2577+58” |
|
|
Fv |
0 |
1.530+39" |
1750+57 1 |
1987+30" |
1451+458 |
1909+16" |
2531+37" |
|
50 |
1480+208 |
1720+28 ‘ |
1950+22" |
997+21 ” |
1315+26” |
2161+45” |
|
|
100 |
1487+128 |
1715+628 |
1939+34" |
1030+27” |
1276+35” |
2174+63” |
|
|
T |
0 |
683+451 |
750+128 |
750+15" |
703+558 |
777+568 |
779+218 |
|
50 |
675+24 * |
732+208 |
633+15b |
697+288 |
750+448 |
653+16” |
|
|
100 |
652+83 * |
700+15 b |
617+21 ” |
670+208 |
723+29a |
637+15 ” |
|
|
Fv/Fm |
0 |
0.70+0.03 8 |
0.79+0.03 8 |
0.87+0.04 a |
0.69+0.07 8 |
0.74+0.01 * |
0.87+0.03 8 |
|
50 |
0.65+0.04 8 |
0.78+0.04 8 |
0.81+0.03 8 |
0.66+0.05 " |
0.74+0.02 1 |
0.84+0.04 8 |
|
|
100 |
0.65+0.02 8 |
0.73+0.03 8 |
0.80+0.02 8 |
0.66+0.04 8 |
0.71+0.02 8 |
0.84+0.04" |
|
|
Fv/F0 |
0 |
2.34+0.22 8 |
3.65+0.23 8 |
6.71+0.078 |
2.20+0.05 8 |
2.89+0.02 8 |
6.68+0.14" |
|
50 |
1.82+0.08 b |
2.82+0.04” |
4.32+0.03 b |
1.94+0.03” |
2.37+0.05” |
5.28+0.11 ” |
|
|
100 |
1.82+0.08 b |
2.71+0.13 1 |
4.11+0.10” |
1.97+0.06 ” |
2.39+0.09” |
5.39+0.19” |
|
Table 5. Chlorophyll fluorescence parameters including F0 , Fm , Fv and Tm and calculated Fv/Fm and Fv/F0 in attached leaves of rice and sunflower treated with 50 and 100 µM of Mn and grown under three light conditions. Data in each column within each measured parameters followed by the same letter are not significantly different (p<0.05).
|
Mn (цМ) |
Rice |
Sunflower |
|||||
|
LL |
IL |
HL |
LL |
IL |
HL |
||
|
Fo |
0 |
655446° |
479457b |
296453 ‘ |
659449 ° |
661436" |
379453 ” |
|
50 |
706444 ‘ |
652462 * |
352475" |
631435 " |
6054291 |
371435 * |
|
|
100 |
726436° |
689454 ” |
376460 ‘ |
621428" |
619436 ‘ |
407449 ” |
|
|
F |
0 |
2185422* |
2229452b |
2283429” |
2110445" |
2570439 " |
2910469= |
|
50 |
2221432 ” |
2620428 1 |
2332450” |
1927427b |
2263456b |
2707434b |
|
|
100 |
2234435 ‘ |
2651435 ‘ |
2351456” |
1934452b |
2267447b |
2711436b |
|
|
Fv |
0 |
1530436” |
1750424b |
1987434” |
1451453 = |
1909476 " |
2531437 " |
|
50 |
1515444” |
1968466” |
1980439” |
1296434b |
1658476b |
23364411 |
|
|
100 |
1508448’ |
1962444" |
1975430” |
1313448b |
1648467b |
2304421b |
|
|
T |
0 |
683423 = |
750488 ” |
750496 " |
703462 ” |
777=44 ‘ |
779478 1 |
|
50 |
6204376 |
635476 ”b |
640465 " |
644455= |
657456b |
660490 ” |
|
|
100 |
605419b |
608442b |
629436 * |
623486= |
629470b |
653475 " |
|
|
Fv/Fm |
0 |
0.7140.05 1 |
0.7940.05 3 |
0.8740.04 ” |
0.7140.03” |
0.7740.04" |
0.8940.04" |
|
50 |
0.6940.06” |
0.7540.05 * |
0.8540.05 ” |
0.7140.04" |
0.7540.03 ” |
0.8840.03 ” |
|
|
100 |
0.6840.05 ” |
0.7540.04 " |
0.8540.03 " |
0.7040.04 " |
0.7540.03 ” |
0.8840.05 " |
|
|
Fv/Fo |
0 |
2.3440.08 1 |
3.6540.04 3 |
6.7140.06” |
2.2040.11 ” |
2.8940.21 " |
6.6840.07 = |
|
50 |
2.1540.10 ^ |
3.0240.11 b |
5.6340.05 b |
2.0540.10 = |
2.7440.26 ” |
6.3040.12 b |
|
|
100 |
2.0840.13 b |
2.8540.08 ■ |
5.2540.05= |
2.1140.17” |
2.6640.19 " |
5.6640.04 = |
|
DISCUSSION
Effect of Cu and Mn toxicity at different light intensities on plants growth and metal accumulation
Cu toxicity inhibited growth of plants much more than similar concentrations of Mn in the medium. A high susceptibility of plants to Cu toxicity compared to other heavy metals such as Mn and Zn was reported by other authors (Marschner, 1995). Rice expressed higher susceptibility to both Cu and Mn toxicity than sunflower independent from light conditions. At all heavy metals treatments, light conditions influenced drastically growth and response of plants to Cu and Mn toxicity. In both rice and sunflower, growth was significantly improved due to higher light intensity, indicated that even at HL intensity the light conditions was not higher than photosynthetic demand of plants and could not be considered as a stress factor. Interestingly, though stimulation of growth by higher light intensity in both Cu and Mn treated plants, the inhibitory effect of Cu and Mn toxicity was affected differently. Higher light intensity strengthened the effect of toxicity of Cu while ameliorated that of Mn. Sunflower plants grown under HL conditions demonstrated even a significant growth stimulation of shoot and root by Mn concentrations as high as 100 µM in the medium which was reached for shoot to 94% at 50 µM and 74% at 100µM Mn.
A significant growth stimulation by Mn concentration up to 100 µM in HL sunflower plants implies that sunflower could be used as a model plant for study of mechanisms for Mn tolerance.
Growth stimulation by Mn treatments could be most likely attributed to an improved water balance. Transpiration of plants increased due to higher light intensity, but with different extent in control and Mn-treated plants. Increase of transpiration in HL compared to LL conditions was 2.76 folds in control plants, while it was 2.29 in plants treated with 50 µM Mn and was only 1.89 fold in the presence of 100 µM Mn. Such an effect of Mn treatment on reduction of transpiration rate was not observed in rice, the transpiration rate of HL control rice plants was 2.84 folds higher than LL plants, and for 50 µM and 100 µM Mn treatments were 2.86 and 3.0 folds. Positive effect of Mn treatment on water balance of sunflower plants was observed neither in Cu treated rice nor sunflower. Differential effect of Mn treatment on transpiration in rice and sunflower could be attributed to broader leaves and larger transpiration surface in sunflower than in rice.
Surprisingly, lower transpiration of sunflower plants treated by Mn and grown under higher light intensities was not associated by lower stomatal conductance. The latter parameter increased with similar extent e.g. 1.57 fold in control and 1.60 fold in Mn (100 µM) treated plants when grown under higher light intensity. It implies that lower transpiration was not due to stomatal closure, but to a lower evaporation from leaf cells surfaces and lower water vapor pressure in stomatal chamber most likely because of a lower water potential of leaf cells. The possible effect of Mn on water potential of leaf cells should be further investigated.
The most important effect of light intensity was observed on the Cu and Mn concentration of shoot and root. Interestingly, in rice high light intensity decreased Mn concentration of shoot and root but increased that of Cu. In sunflower, high light intensity decreased concentration of both heavy metals. Therefore, effect of light on alleviation of growth inhibition by Mn treatment at least in part was due to Mn dilution. Our previous work demonstrated that light intensity alters Cu and Mn accumulation via affecting Cu uptake and transport (Hajiboland and Boniadi, 2005). However, the exact mechanism of the effect of light intensity on uptake and transport of Mn is not clear but most likely could be explained as a consequence of lower transpiration of Mn treated leaves. Manganese accumulation in leaves of common bean increased by higher light intensity (Gonzalez et al., 1998) but remained unchanged in rice because of no change in the transpiration rate (Lidon, 2001)
Cu and Mn distribution between shoot and root was different particularly in rice, roots accumulated high amounts of Cu than shoot, in contrast, Mn accumulated mainly in shoot. Our previous study showed that, root apoplasm is the main compartment of Cu accumulation particularly in rice (Hajiboland and Boniadi, 2005). For Mn, different compartmentation in shoots of rice and sunflower plants is the cause of higher Mn sensitivity in rice than sunflower. In rice, majority of Mn in shoot is found in readily re-translocable, symplasmic compartment, while in sunflower, Mn accumulates in the bases of leaf trichoms and thus remained out of symplasm and therefore caused no damage to the cells in shoot (Hajiboland and Boniadi, 2005).
Effect of Cu and Mn toxicity at different light intensities on chlorophyll and photosynthesis
Chlorophyll: The light-induced reduction of chlorophyll a+b concentration under all treatment combinations could be well explained by the induction of chlorophyll degradation by active oxygen species produced under higher light intensity in leaves (Asada, 1999, Behera and Choudhury, 2002). In contrast, chlorophyll a+b concentration of leaves was not substantially influenced by Cu toxicity likely because of reduction of leaf growth simultaneous with chlorophyll degradation. However, a significant increase in chlorophyll concentration of Mn treated sunflower plants, which was not associated by reduction of leaf dry weight and area is not clear and should be studied further.
Separation of total chlorophyll into chlorophyll a and b and a comparison of the ratio of chlorophyll a with chlorophyll b reveals a preferential protection against higher light intensity and a higher sensitivity to Cu compared to Mn treatment for chlorophyll b. Therefore, the relative abundance of this pigment increases as light intensity rises and fells as Cu concentration in the medium increases.
On the other hand, Mn treatment makes chlorophyll b concentration in rice much resistance to the high light intensity, therefore chlorophyll b in Mn treated rice plants did not change in IL and HL compared to LL plants. In contrast, the sensitivity of chlorophyll a and b concentration in sunflower to higher light intensity did not change by exposure of plants to toxic concentrations of either Cu or Mn.
Leaves particularly under IL and HL conditions showed an increasing trend for chlorophyll a/b concentration in response to Cu treatment, which implies a differential effect of Cu on chlorophyll a and b in both studied plants. An increase in the chlorophyll a/b ratio, resulting from faster degradation of chlorophyll b, indicated a preferential decrease in light harvesting chlorophyll a/b-binding proteins (LHC) associated with PSII (LHCII) to transfer excitation energy to the PSII core complex (Xu et al., 1995).
Gas exchange
A concomitant reduction of A and g s under Cu and Mn toxicity indicated that inhibition of CO 2 assimilation observed in both studied plants was partly attributable to stomatal limitation. The limitations to CO 2 assimilation imposed by stomatal closure may promote an imbalance between photochemical activity at photosystem II (PSII) and the electron requirement for photosynthesis, leading to an overexcitation and subsequent photoinhibitory damage of PSII reaction centers (Souza et al., 2004).
The inhibitory effect of heavy metal treatments on stomatal opening was frequently reported. Micromolar concentrations of Hg, Pb and Zn have been shown to inhibit stomatal movements in broad bean because of blocking water channels in guard cell membranes (Yang et al., 2004). The inhibitory effect of Cd on stomatal opening was attributed to the entrance of Cd via Ca channels and affecting guard cell regulation in an ABA-independent manner (Perfus-Barbeoch et al., 2002).
However, in this work reduction of stomatal conductance was mainly in tendency and not significant. Therefore, it could not be the sole reason for a lower assimilation rate of Cu and Mn treated leaves. It implies that non-stomatal limitations involve also in inhibition of photosynthesis. Moreover, no significant variations of Ci in response to heavy metals particularly in HL plants ( Ci data were not shown) suggested the existence of a non-stomatal limitation of photosynthesis in the two stressed plants.
In contrast to the effect of Cu and Mn treatment, a significant reduction of Ci/Ca was observed in response to light intensity in rice. In addition, Ci/Ca values were much higher in rice than sunflower under all three light conditions. It is known that, at the steady state favorable environmental conditions, the Ci/Ca ratio holds constant for a great number of plant species (Ehleringer and Cerling, 1995). The actual value varies according to the terrestrial biome type, with a tendency to be higher in rainforest tropical species than xerophytic or tropical dry forest tree species (Lloyd and Farghuhar, 1994). The Ci/Ca ratio is considered to be an appropriate indicator for the stomatal limitation of photosynthesis (Farghuhar and Sharkey, 1982). The smaller the Ci/Ca ratio, the greater is the stomatal limitation of photosynthesis and the more conservative the plant species is in relation to water use. In the present experiment, high amounts of Ci/Ca ratios particularly in LL rice plants, indicate that rice plants has a tendency to be nonconservative in relation to water use, in a similar manner shown for many other tropical rainforest species (Ishida et al., 1996). Difference between rice and sunflower in water conservation strategy could be well explained regarding that rice particularly used cultivar in this work, is a lowland crop species of tropical origin.
Chlorophyll fluorescence
The biophysical basis underlying changes in the photosynthetic characteristics of studied plants was assessed using chlorophyll fluorescence. Measurement of chlorophyll a fluorescence is a non-invasive, powerful and reliable method, to assess the PSII function of Cu and Mn treated leaves in this work.
The initial chlorophyll fluorescence yield, F 0 reflects the minimal fluorescence yield when all Q A are in the oxidized state. According to our results, the increase of F0 recorded under Cu and Mn toxicity in rice, can be interpreted as a reduction of the rate of energy trapping by PSII centers. However, in sunflower, Cu and Mn treatment did not change the rate of energy trapping by PSII, which was reflected in increasing values of F 0 . Reduction of energy trapping could be the result of a physical dissociation of light harvesting complex from PSII core, as it has been observed under other stresses such as heat (Armond et al., 1980). In addition, Cu and Mn treated sunflower plants had a significant smaller F m value compared to the control, which in relation to the slightly reduced Tm , indicates that an increasing fraction of reaction centers (RCs) becomes inactivated. It is well known that when F m is attained, all PQ molecules are reduced. Thus, we can suggest that F m depression may reflect also a decreased size of antennas and/or a diminished pool of PQ (Ouzounidou et al., 1998).
Copper and Mn toxicity reduced the F v value in both rice and sunflower, indicating that a structural and functional disorder of the photosynthetic apparatus and damage to the PSII had occurred (Osmond, 1994, Pereira et al., 2000, Murkowski, 2001). Rice had higher reduction of F v and F v /F 0 ratios under Cu and Mn stress than sunflower, indicating that the photosynthetic apparatus in rice was more susceptible to heavy metal stress than in sunflower. The reduction of F v could be attributed to a decrease in the rate constant for photochemistry of PSII and, to a greater extent, to an increase in the non-radiative dissipation rate constant (Ouzounidou et al., 1997).
The negative effect of Cu and Mn toxicity for both rice and sunflower focused mainly in the decreased proportion of active chlorophyll associated with the reaction center of PSII (decreased Fv/F0). Also, it might be possible that especially under Cu toxicity inactivation of the ferredoxin, an electron transmitter due to changes in its chemical structure and reduction of the activity of NADP+ photoreduction occurred (Ouzounidou et al., 2003). The changes in the ratio of Fv/F0 were coincided on differential growth response of plants concerning Cu and Mn treatment as well as plant species. This finding also demonstrates that the measurement of Fv and Fv/F0 could be used as an efficient, nondestructive method for assaying Cu and Mn toxicity.
The direct effect of increasing F 0 , is the slight decrease of F v /F m ratio. The maximal quantum yield of PSII ( F v /F m ) ratio declined less than 10% between the control and heavy metal treated leaves. The preservation of this parameter under Cu and Mn toxicity, as indicated by very small changes, probably is the consequence of a modification of the Q A to Q B electron transfer (Ouzounidou et al., 1998).
Light intensity altered the fluorescence rise time ( T m ) differently depending on heavy metal species. Reduction of T m was more pronounced in LL and IL than HL plants when treated by toxic concentrations of Cu. In contrast, in Mn treated plants, the fluorescence rise time decreased significantly in LL and IL but not in HL plants. The fluorescence rise time has been shown to be correlated with the size of the photosynthetic units (PSU). Its higher reduction in HL plants in response to Cu and in LL plants under Mn toxicity provides evidence that an appreciable change has occurred in the size of PSU under these treatment combinations. Interestingly, the toxic effect of Cu on plants growth was more pronounced in HL compared with LL plants and for Mn treated plants the opposite was observed.
The increasing amount of F v /F m in response to increasing light intensity and the high amounts of 0.87-0.80 at HL conditions showed that an enough photochemistry occurred only in HL plants in growth chamber. Therefore, It is expected no photochemical damage occurred even at HL conditions in control plants.
CONCLUSION
We propose that, Cu and Mn toxicity depresses the leaf photosynthetic capacity primarily by causing a significant alteration of the composition and functional competence of the PSUs. Most likely, heavy metal toxicity did not reduce the number of PSUs per unit leaf area. Any drastic change in chlorophyll content in contrast to reduction of photosynthetic rates is consistent with the view that the chlorophyll remaining in Cu and Mn treated leaves is not associated with active reaction centers, integrating functional PSUs. It means that the residual chlorophyll were associated with disabled PSUs. The observations that the fluorescence rise times are lower for the Cu and Mn treatments and changes in the Tm are coincided on the different effect of light intensity on the expression of Cu and Mn toxicity, confirm also this hypothesis.
However, the extent of reduction of CO 2 assimilation rate was much higher in sunflower than in rice at both Cu and Mn treatment. This effect was not reflected in growth response of plants, rice responded more negatively than sunflower to both Cu and Mn stress. It could be concluded that growth and dry matter production is not determined only by the assimilation rate but other factors e.g. oxidative damage and metabolic disturbances are from another important components of plants response to heavy metals.
Список литературы Effect of Cu and Mn toxicity on chlorophyll fluorescence and gas exchange in rice and sunflower under different light intensities
- Armond, P.A., Bjorkman, O. and Staehlin, L.A. (1980) Dissociation of supra-molecular complexes in chloroplast membranes. A manifestation of heat damage to the photosynthetic apparatus. Biochem. Biophys. Acta, 601, 433-442.
- Asada, K. (1999) The water-water cycle in chloroplasts: scavenging of active oxygen and dissipation of excess photons. Ann. Rev. Plant Physiol. PlantMol. Biol., 50, 601-639.
- Behera, R.K. and Choudhury, N.K. (2002) High irradiance induced pigment degradation and loss of photochemical activity of wheat chloroplasts. Biol. Plant, 45 (1), 45-49.
- Dannel, F., Pfeffer, H. and Marschner, H. (1995) Isolation of apoplasmic fluid from sunflower leaves and its use for studies on influence of nitrogen supply on apoplasmic pH. J. Plant Physiol., 146, 273-278.
- De Vos, C.H.R. and Schat, H. (1991) Free radicals and heavy metal tolerance. In Rozeman, J. and Verkleij, J.A. C. (eds.), Ecological Responses to Environmental Stresses Kluwer Academic Publishers, Dordrecht, The Netherlands, pp. 22-30.
- Ehleringer, J.R. and Cerling, Т.Е. (1995) Atmospheric CO2 and the ratio of intercellular to ambient CO2 concentrations in plants. Tree Physiol, 15, 105-111.
- Farghuhar, G.D. and Sharkey, T.D. (1982) Stomatal conductance and photosynthesis. Ann. Rev. Plant Physiol., 33, 317-345.
- Fernandes, J.C. and Henriques, F.S. (1991) Biochemical, physiological and structural effects of excess copper in plants. Bot. Rev., 57,246-273.
- Foy, C.D., Chaney, R.L. and White, M.C. (1978) The physiology of metal toxicity in plants. Ann. Rev. Plant Physiol, 29, 511-566.
- Gonzalez, A., Steffen, K.L. and Lynch, P.J. (1998) Light and excess manganese. Implication for oxidative stress in common bean. Plant Physiol., 18, 493-504.
- Gounaries, K., Barber, J. and Harwood, J.L. (1986) The thylakoid membranes of higher plant chloroplasts. Biochem. J., 237, 313-326.
- Hajiboland, R. and Boniadi, H. (2005) Accumulation of copper on root apoplasm and retranslocation to young leaves in rice, maize and sunflower with different toxicity tolerance. Pat J. Biol. Sci., 8 (11), 1599-1609
- Horiguchi, T. (1988) Mechanism of manganese toxicity and tolerance of plants. VII. Effect of light intensity on manganese-induced chlorosis. J. PlantNutr., 11, 235-245.
- Ishida, A., Toma, T. and Matsumoto, Y. (1996) Diurnal changes in leaf gas exchange characteristics in the uppermost canopy of a rain forest tree Dryobalanops aromatica Gaerth. F. Tree Physiol., 16, 779-785.
- Kautsky, H., Appel, W. and Amann, H. (1960) Chlorophyllfluorescenzund kohlensaureassimilation.Biochemishe Zeitschrift, 322, 277-292.
- Kyle, D.J. (1987) The biochemical basis for photoinhibition of photosystem II. In Kyle, D.J., Osmond, C.B. and Arntzen, C.J. (eds.), Photoinhibition, Elsevier, Amsterdam, pp. 197-226.
- Lidon, F.C. (2001) Tolerance of rice to excess manganese in the early stages of vegetative growth. Characterization of manganese accumulation. J. Plant Physiol., 158, 1341-1348.
- Lloyd, J. and Farghuhar, G.D. (1994) 13C determination during CO2 assimilation by the terrestrial biosphere. Oecologia, 99, 201-215.
- Marschner, H. (1995) Mineral Nutrition of Higher Plants. 2nd Edition, Academic Press, UK. Mc Cain, D.C. and Markley, J.L. (1989) More manganese accumulates in maple sun leaves than in shade leaves. Plant Physiol., 90, 1417-1421.
- Maxwell, K. and Johnson, G.N. (2000) Chlorophyll fluorescence-a practical guide. J. Exp. Bot, 51(345), 659-668.
- Moran, R. (1982) Formulae for determination of chlorophyllous pigments extracted with N,N-Dimethylforamide. Plant Physiol., 69, 1376-1381.
- Murkowski, A. (2001) Heat stress and spermidine, effect on chlorophyll fluorescence in tomato plants. Biol. Plant, 44, 53-57.
- Nable, R.O., Houtz, R.L. and Cheniae, G.M. (1988) Early inhibition of photosynthesis during development of Mn toxicity in tobacco. Plant Physiol., 86, 1136-1142.
- Osmond, C.B. (1994) What is photoinhibition? Some insights from comparisons of shade and sun plants. In Baker, N.R. and Bowyer, J.R. (eds.), Photoinhibition of Photosynthesis, from Molecular Mechanism to the Filed. Bios Scientific Publisher, Oxford, UK, pp. 1-24.
- Ouzounidou, G., Moustakas, M. and Strasser, R. (1997) Sites of action of copper in the photosynthetic apparatus of maize leaves: kinetic analysis of chlorophyll fluorescence oxygen evolution, absorption changes and thermal dissipation as monitored by photoacoustic signals. Aus. J. Plant Physiol, 24, 81-90.
- Ouzounidou, G., Ilias, I., Tranopoulou, H. and Karatagalis, S. (1998) Amelioration of copper toxicity by iron on spinach physiology. J. Plant Nutr., 21,2089-2101.
- Ouzounidou, G., Ilias, I. Kabataidid, M. and Chatzimichail, A. (2003) Comparative study of nutrient deficiencies on growth and photochemistry of tobacco. J. Plant Nutr., 26, 1605-1616.
- Patsikka, E., Aro, E-M. and Tyystjarvi, E. (1998) Increase in the quantum yield of photoinhibition contributes to copper toxicity in vivo. Plant Physiol, 117, 619-627.
- Patsikka, E., Aro, E-M. and Tyystjarvi, E. (2001) Mechanismofcopper-enhanced photoinhibition in thylakoid membranes. Physiol. Plant, 113, 142-150.
- Patsikka E., Kairavuo, M., Sersen, F., Aro, E-M. and Tyystjarvi, E. (2002) Excess copper predisposes photosystem II to photoinhibition in vivo by outcompeting iron and causing decrease in leaf chlorophyll. Plant Physiol., 129, 1359-1367.
- Pereira, W.E., de Siqueira, D.L., Martynez, C.A. and Puiatti, M. (2000) Gas exchange and chlorophyll fluorescence in four citrus rootstocks under aluminium stress. J. Plant Physiol., 157,513-520.
- Perfus-Barbeoch, L., Leonhardt, N., Vavasseur, A. and Forestier, С (2002) Heavy metal toxicity: cadmium permeates through calcium channels and disturbs the water status. The Plant J., 32 (4), 539-548.
- Quartacci, M.F., Pinzino, C, Sgherri, C.L.M., Dalla Vecchia, F., and Navari-Izzo, F. (2000) Growth in excess copper induces changes in the lipid composition and fluidity of PSII-enriched membranes in wheat. Physiol. Plant, 108, 87-93.
- Souza, R.P., Machadoa, E.C. Silva, J.A.B., Lagoa, A.M.M.A. and Silveria, J.A.G. (2004) Photosynthetic gas exchange, chlorophyll fluorescence and some associated metabolic changes in cowpea (Vigna unguiculata) during water stress and recovery. Environ. Exp. Bot, 51, 45-56.
- Stiborova, M., Doubravova, M. Brezinova, A. and Friedrich, A. (1986) Effect of heavy metal ions on growth and biochemical characteristics of maize (Zea mays L.). Biologia, 20, 418-425.
- Tennant, D. (1975) A test of modified line intersect method of estimating root length. J. Ecology, 63, 995-1001.
- Vichnevetskaia, K.D. and Roy, D.N. (1999) Oxidative stress and antioxidative defense with an emphasis on plants antioxidants. Environ. Rev., 7,31-51.
- Weckx, J.E.J., and Clijsters, H.M.M. (1997) Zinc phytotoxicity induces oxidative stress in primary leaves of Phaseolus vulgaris. Plant Physiol. Biochem., 35, 405-410.
- Wissemeier, A.H. and Horst, W.J. (1992) Effect of light intensity on manganese toxicity symptoms and callose formation in cowpea (Vigna unguiculata L. Walp.). Plant Sci., 143, 299-309.
- Woolhouse, H.W. (1983) Toxicity and tolerance in the responses of plants to metals. In Lange O.L., Nobel, P.S., Osmond, C.B. and Ziegler, H. (Eds.). Physiological Plant Ecology III: Responses to the chemical and biological environment. Springer, Berlin, Germany, pp. 245-300.
- Xu, Q., Paulsen, A.Q. Guikema, J.A. and Paulsen, G.M. (1995) Functional and ultrastractural injury to photosynthesis in wheat by high temperature during maturation. Env. Exp. Bot, 35, 43-54.
- Yang, H.M., Zhang, X.Y. and Wang, G.X. (2004) Effects of heavy metals on stomatal movements in broad bean leaves. Russ. J. Plant Physiol., 51 (4), 464-468.
- Ymela, I., Gatzen, G., Picorel, R., Holzwarth, A.R. (1996) Cu (Il)-inhibitory effect on photosystem II from higher plants: a picosecond time-resolved fluorescence study. Biochemistry, 35, 9469-9474.
- Yoshida, S., Forno, D.A., Cock, J. H. and Gomez, K. (1972) Routine methods of solution culture for rice. In Laboratory Manual for Physiological Studies of Rice. 2nd Edition, The International Rice Research Institute, Philippines, pp. 53-57


