Effect of drought and salinity stress on calcium oxalate crystals of Portulacaria afra. (L.) Jacq
Автор: Javkar Ruchira, Avhad Anil
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 1 т.19, 2023 года.
Бесплатный доступ
Oxalic acid (C2H2O4) and Calcium (Ca2+) react to form the salt Calcium oxalate (CaOx), which crystallises into a variety of topologically diverse crystals. CaOx crystals have been found in at least 215 plant groups, which corresponds to numerous species. Crystals can be found in vascular, epidermal, ground, and other tissues in addition to roots, stems, leaves, flowers, fruits, and seeds. They develop in crystal idioblasts, specialised cells, in their vacuoles. According to recent studies, CaOx crystals are in fact useful tools that are crucial, especially in stressful conditions. As plants lack an excretory system, the Ca component regulates the cytosolic concentration levels and immobilises excess amounts of this element. Oxalates operate as a dynamic carbon store and set off an alert during photosynthesis, which results in the production of CO2. The article aims to provide readers with a greater understanding of Portulacaria afra's CaOx crystals and the projected crystal disintegration that would liberate carbon and supply the photosynthetic cycles with it as defence against salinity and drought stress.
Alarm photosynthesis, calcium oxalate crystals, energy dispersive x-ray spectroscopy (eds), environmental scanning electron microscopy (esem), portulacaria afra. (l.) jacq
Короткий адрес: https://sciup.org/143179372
IDR: 143179372
Текст научной статьи Effect of drought and salinity stress on calcium oxalate crystals of Portulacaria afra. (L.) Jacq
Calcium oxalate (CaOx) is a salt of oxalic acid (C 2 H 2 O 4 ) and calcium (Ca2+) that forms insoluble crystals of diverse morphology ( rasad R and Shivay Y. S 2017). CaOx crystals have been found in at least 215 families, which is a plethora of plants (Raman V et al. , 2014) (Franceschi, V., 2001). Crystals can be found in vascular, epidermal, ground, and other tissues as well as in roots, stems, leaves, flowers, fruits, and seeds (Dickison, W.C.,2000). They are formed in the vacuoles of specialised cells called crystal idioblasts, which differ from the neighbouring cells in terms of structure and composition. Crystals idioblasts are frequently found in the mesophyll or bundle sheath extensions of leaves (Karabourniotis G et al ., 2020). The enormous variation in organ, tissue, and cell distribution between plant species raises the possibility that crystals have independent origins of formation and diverse roles (Horner, H et al ., 2020) (Franceschi, V.R and Nakata, A. 2005).
Recent studies confirmed that CaOx crystals are in fact multifunctional tools that are crucial, particularly under stressful situations (Horner, H and Wagner B. L 2020). They are flexible storage systems that deliver calcium and oxalate ions as needed. These two component inclusions, each perform important duties Given that plants lack an excretory system, the Ca component regulates the cytosolic concentration levels and immobilises excess amounts of this element. In contrast to oxalate in the leaves, which can serve as a dynamic carbon reservoir and produce CO 2 in a process known as alarm photosynthesis. Oxalate in the root can participate in nutrient acquisition, metal detoxification, mineral weathering, and the selection of beneficial bacterial populations. Moreover, oxalate of all organ and tissues can take part in defence reactions upon pathogen and/or herbivore attack (Nakata, . A. 2003).
CaOx crystals embedded in mesophyll serve as dynamic carbon storage during alarm photosynthesis When plants are exposed to CO2 limiting conditions, such as whole or partial stomata closure during drought, crystal breakdown releases CO2, which is then utilised for photosynthesis. However, the use of CaOx as a source of CO2 for photosynthesis appears to be limited to some plant species or circumstances associated with adverse environments, particularly water stress conditions (Webb, M. A. 1999).
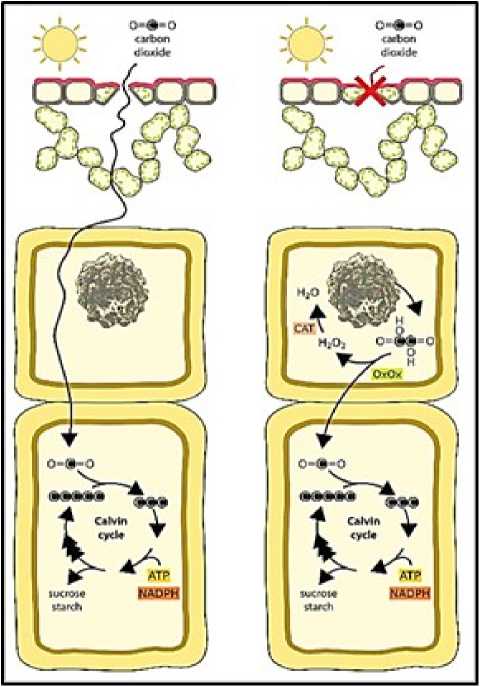
Figure. 1 Schematic representation of Biochemical reactions involved in alarm photosynthesis (Georgia Tooulakou et al.; 2016 a)
Tooulakou et al., (2016 a) indicated that leaf CaOx crystals function as dynamic carbon pools, providing CO2 for photosynthesis when stomata remain totally or partially closed (e.g., under drought conditions) (McDowell N et al., 2008). Under these conditions, gas exchange is limited and CO2 starvation conditions may be created within the mesophyll. The carbon starvation hypothesis (McDowell et al. 2008, McDowell and Sevanto 2010, McDowell 2011) predicts that reduced carbon assimilation via photosynthesis as a result of stomatal closure leads to an imbalance between carbon availability (gain) and loss as a result of metabolic demands (e.g., growth and maintenance). Over time, if drought persists, such negative balance can lead to an exhaustion of carbon reserves and, ultimately, to carbon starvation and plant death. Moreover, closed stomata lead not only to carbon starvation, but also to an imbalance between incident energy and available intercellular CO2, with the rate of reducing power production overpassing the rate of its use by the Calvin cycle.
Salinity stress is a significant limiting factor that leads to a reduction in photosynthesis due to the disruption in the photosynthetic pigment system (Sarker, U and Oba S. 2019). Stomata close more frequently in response to salinity stress, which reduces CO 2 uptake through the stomatal pore and, as a result, photosynthesis. The rate of photosynthesis per unit area dropped because there were fewer stomata. Of the two pigment systems, hotosystem II ( S II) is more crucial for carrying out photosynthesis. It is most susceptible to salt stress, and it has been demonstrated that its efficacy decreases (Kolomeichuk, L.V et al ., 2020).
Alarm photosynthesis appears to play a relatively minor contribution in terms of photosynthetic activity's overall efficiency. With the intention of safeguarding and preserving the photosynthetic apparatus rather than generating a sizable amount of carbon dioxide, it promotes a low rate of photosynthetic activity. The purpose of the paper was to obtain a deeper understanding of the presence of CaOx crystals in P. afra and the anticipated crystal disintegration that would release carbon, which would then be fed to the photosynthetic cycles as a defence against drought and salinity stress.
MATERIALS AND METHODS
Cultivating Portulacaria afra
Portulacaria afra (L.) Jacq. saplings were brought from a local nursery. The saplings were potted in pots (6" x 6" dimensions) containing a 50:50 garden soil -sandy soil mixture and grown in natural conditions under direct sunlight and temperature varying from 30-35°C in the day and 20-25°C night temperature, 65% of relative humidity, 14h/16h of light and 10h/8h dark photoperiods respectively. The saplings were watered every third day to maintain tissue water potential and were allowed to acclimatize and grow for 120 days. Natural fertilizer was added to the pots monthly. Clones were obtained from the horizontal branches growing from the main trunk of the now well grown plant saplings termed as the mother plants. These branches were cut by giving a slant cut just below the nodes. The end of the vegetative cuttings was coated with a rooting powder and kept exposed to air for 24 hours. Next day the cuttings were planted for its vegetative propagation in other pots. The mix in these pots consist of 50 % organic matter 50 % sandy soil The rest of the conditions were all kept similar. The clones continued to propagate vegetatively for 2 months At the end of two months drought and salt treatments were started.
Establishing the stress conditions.
lants were segregated in different groups such as Control, Short Term Drought, Long Term Drought, Short Term Salinity Stress and Long-Term Salinity Stress Each group were allotted five plants each. The plants in short term drought were watered once at the end of every week while the long-term drought plants were watered after every 15 days. The longer stress experiment was conducted to ascertain any changes during a more extensive drought period. (Brigitte B. et al., 1993). The Salt treatment began with checking threshold salt concentration. Varying concentrations of saline water was applied to plants such as 100 mM, 300 mM and 500 mM NaCl for both short-term and long-term salinity stress. This treatment was given for a total of 30 days and 60 days for short term and long term respectively. The plants were harvested before every rewatering. Leaves were plucked and stored at -20°C until they were used for further research.
Measurements of Crystal Density and Size of Crystals
One-gram leaves from control and each group of treated plants were collected and surface cleaned under running tap water and patted dry. 2% sodium hypochlorite solution was added to the petridishes for bleaching the leaves for next 72 hours. The now white/ transparent leaves devoid of any pigment were surface cleaned with distilled water. Thin sections were taken to observe the crystals under microscope. Different area of visions was observed for control, drought and salinity Images of crystals in chlorine-bleached leaves were obtained using a Trinocular LCD microscope ( ulse Life science) equipped with a microscope digital camera (MICA S), and length of crystals were denoted by digital image analysis (MICA S 3.7). Fiji Software was used as an image analysis tool for measurement of overall crystal dimensions. The experiment was performed in triplicates.
ESEM and EDS Analysis.
For Environment scanning electron microscopy (ESEM), small pieces of leaves were fixed in 3% glutaraldehyde in 0.1 M phosphate buffer, pH 7.2, for 36 hours at 4°C, dehydrated with a 30 to 100% acetone gradient, critical point dried (Tooulakou et al ., 2016) Further ESEM and EDS analysis was carried out in SAIF IIT Bombay. The leaf sections were mounted on stubs with self-adhesive double-sided carbon discs and sputter coated with platinum and digital micrographs were obtained. Difference in crystal elements were measured with Energy dispersive X-ray Spectroscopy (EDS) analysis.
RESULTS
The length of the crystals was compared, through MICA S 3.7 software. The length in control group can be seen to be ranging from a minimum of 2.63 mm to 4.50 mm (Figure 3.d ). In the short-term drought, a sharp decline in the length of the crystals is observed ranging from a minimum of 1.97 mm to 3.86 mm (Figure 4.d). As the drought conditions become severe with the longterm stress setting in, complete disintegration of CaOx crystals is seen quite evidently in Figure 5.d. In salinity stress, the crystals in the leaves of short-term stress with a salinity of 100 mM were from 2.06 mm to 4.74 mm (Figure 6.d) whereas of 300 mM were from 1.98 mm to a maximum of 4.03 mm (Figure 7.d). Figure 8.d shows the starting of disintegration of CaOx crystals with increasing salinity (500 mM). Similarly, the crystals in long term stress leaves with a salinity of 100 mM were from 2.56 mm to 3.32 mm (Figure 9.d) whereas of 300 mM were from 1.66 mm to a maximum of 3.14 mm (Figure 10.d). Figure 11.d shows complete disintegration of CaOx crystals with 500 mM of salinity for a period of long- term stress.
Measurement of overall crystal dimensions were carried out through the Fiji software. Figure 3.c and 3.d, shows that maximum number of Calcium oxalate crystals are present in the Control group, whereas they are least in the long-term drought group as seen in Figure 5.c and 5. d. The area of vision for Short-term drought showed 53.5 average crystals while long-term drought showed 42 average crystals, which is a marginal difference. The same trend is seen with the crystal lengths in figure 4.c and 5.c. The quantity of crystals for treated reduces as the salt concentration rises. 100 mM of short-term salinity revealed 87.5 crystals, while 300 mM exhibited 55 crystals. The longterm salinity stress follows a similar pattern, with 100 mM showing 67.25 average crystals and 300 mM exhibiting just 52 average crystals.
When the average crystal area across all biological replicates from the groups of controls and short- and long-term drought were compared, the area reveals a significant difference, measuring 143.75 µm2 in the control group and 61.15 µm2 and 44.2 µm2 in the short-and long-term drought groups, respectively. In salinity, the area occupied by crystals likewise continues to decline along with the number of crystals ( Figure 6.d, 7.d, 8.d, 9.d, 10.d, 11.d) . In the short-term salinity, the area falls from 51 µm2 to 43.65 µm2 from 100 mM to 300 mM, while in the long-term salinity, the area drops from 72.6 µm2 to 69.4 µm2 from 100 mM to 300 mM. It was also noted that although there were virtually the same numbers of crystals in the control and 100 mM shortterm salinity, the area of the crystals decreased from 143.75 µm2 of control to 51 µm2 of salinity. All these observations testify to a general reduction in the amount of Calcium oxalate crystals (Figure 12).
The EDS reports clearly shows the decline in weight percentage of Carbon from 60.6 % to 29.7 % and atomic percentage from 68.1 % to 36.1 % when compared to crystal spotted in control to a crystal spotted in leaf of a drought affected plant (Treated). Figure 13
The Figure 14.a and 14.b shows Scanning Electron Microscope images at the magnification of 8000x. The reduction in overall size and density of the crystal is observed in drought stress in comparison to the control one. A disintegrated type of crystal, losing its characteristic shape of CaOx druse was captured in long term salinity stress (Figure 14.c).

Figure 2 Portulacaria afra (L.) Jacq. experimental setup
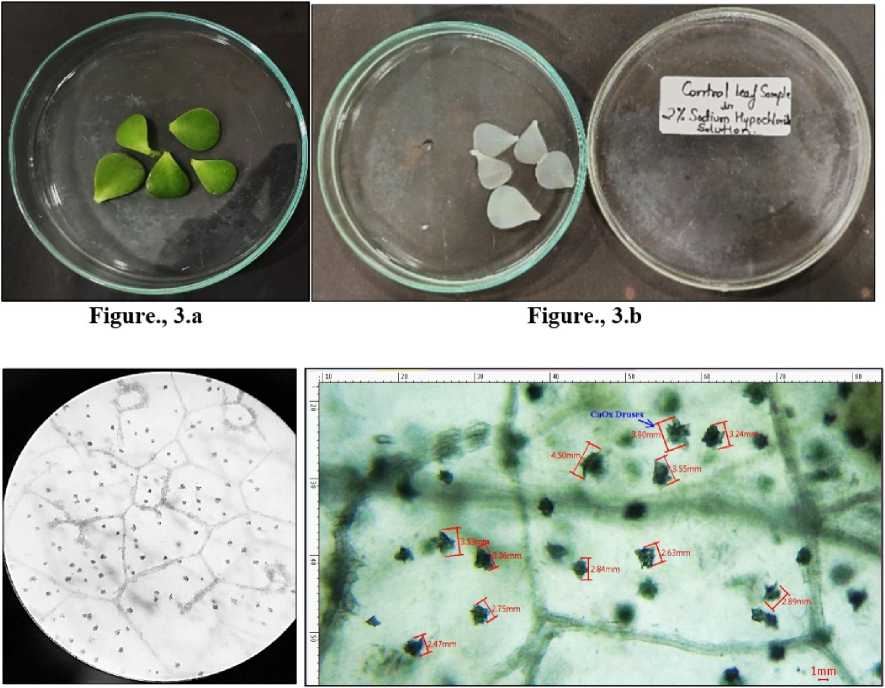
Figure., З.с Figure., 3.d
Figure 3 L eaves collected from Portulacaria afra grown under control conditions. 3.a) Before bleaching. 3.b)
Depigmented leaves after bleaching in sodium hypochlorite solution. 3.c) CaOx crystals under light microscope -10X. 3.d) Crystals of CaOx druses measured inMICA Ssoftware

Figure 4 Leaves collected from Portulacaria afra grown under short term drought conditions. 4.a) Before bleaching. 4.b) Depigmented leaves after bleaching in sodium hypochlorite solution. 4.c) CaOx crystals under light microscope -10X. 4.d) Crystals of CaOx druses measured in MICA S software.

Figure 5 Leaves collected from Portulacaria afra grown under long term drought conditions. 5.a) Before bleaching. 5.b) Depigmented leaves after bleaching in sodium hypochlorite solution. 5.c) CaOx crystals under light microscope -10X. 5.d) Complete disintegration of the druses of CaOx crystals denoted with blue arrows in MICA S software.
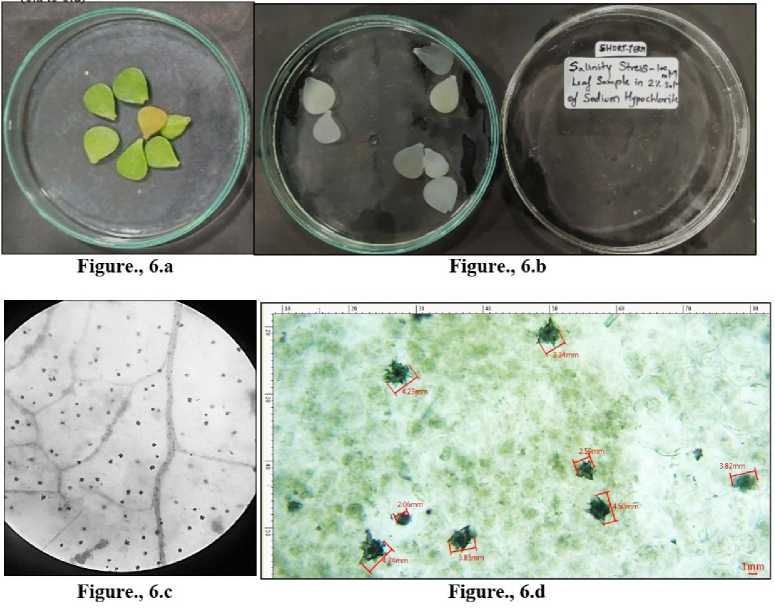
Figure 6 L eaves collected from Portulacaria afra grown under short term salinity-100mM. 6.a) Before bleaching. 6.b) Depigmented leaves after bleaching in sodium hypochlorite solution. 6.c) CaOx crystals under light microscope -10X. 6.d) Crystals of CaOx druses measured in MICA S software.
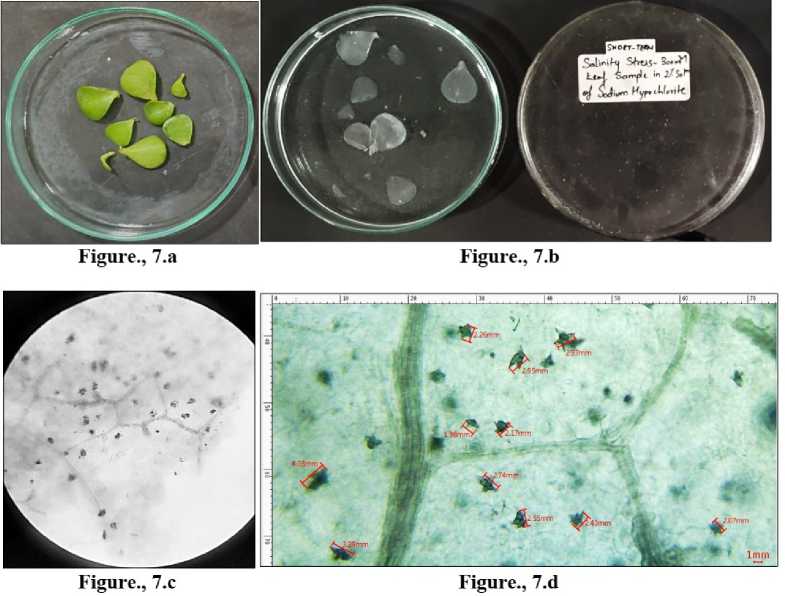
Figure 7 L eaves collected from Portulacaria afra grown under short term salinity-300mM. 7.a) Before bleaching. 7.b) Depigmented leaves after bleaching in sodium hypochlorite solution. 7.c) CaOx crystals under light microscope -10X. 7.d) Crystals of CaOx druses measured in MICA S software.
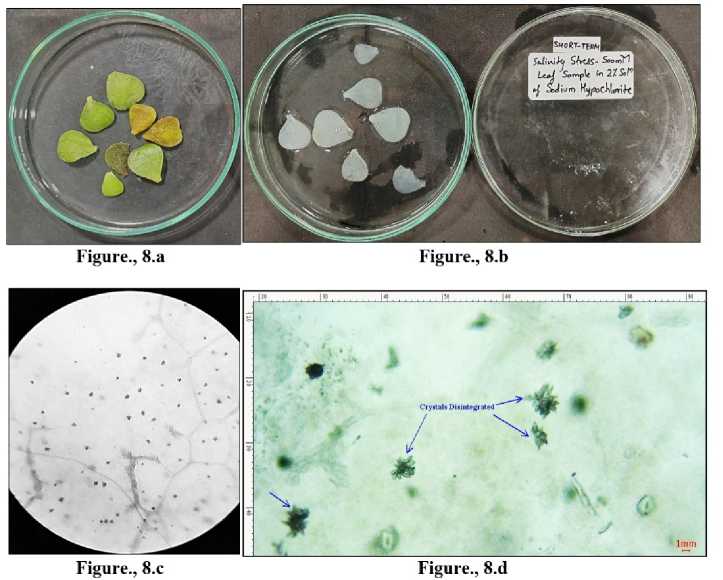
Figure 8 Leaves collected from Portulacaria afra grown under short term salinity-500 mM. 8.a) Before bleaching. 8.b) Depigmented leaves after bleaching in sodium hypochlorite solution. 8.c) CaOx crystals under light microscope -10X. 8.d) Beginning of disintegration of the druses of CaOx crystals denoted with blue arrows in MICA S software.

Figure 9 Leaves collected from Portulacaria afra grown under long term salinity-100 mM. 9.a) Before bleaching. 9.b) Depigmented leaves after bleaching in sodium hypochlorite solution. 9.c) CaOx crystals under light microscope -10X. 9.d) Crystals of CaOx druses measured in MICA S software.
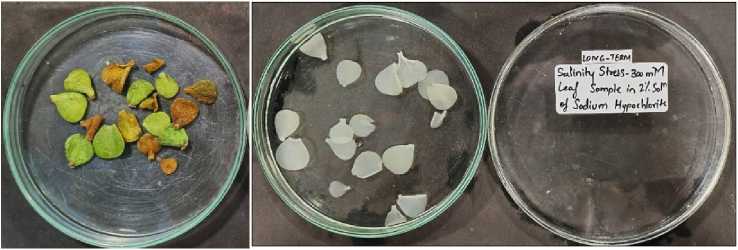
Figure., lO.a
Figure., lO.b
Figure., lO.c
Figure., 10.d
Figure 10 Leaves collected from Portulacaria afra grown under long term salinity-300 mM. 10.a) Before bleaching. 10.b)
Depigmented leaves after bleaching in sodium hypochlorite solution. 10 .c) CaOx crystals under light microscope -10X. 10.d) Crystals of CaOx druses measured in MICA S software.

Figure 11 Leaves collected from Portulacaria afra grown under long term salinity-500 mM. 11.a) Before bleaching. 11.b) Depigmented leaves after bleaching in sodium hypochlorite solution. 11.c) CaOx crystals under light microscope -10X. 11.d) Disintegration of the druses of CaOx crystals denoted with blue arrows in MICA S software.
Number and Area of crystals in Portulacaria afro, leaves
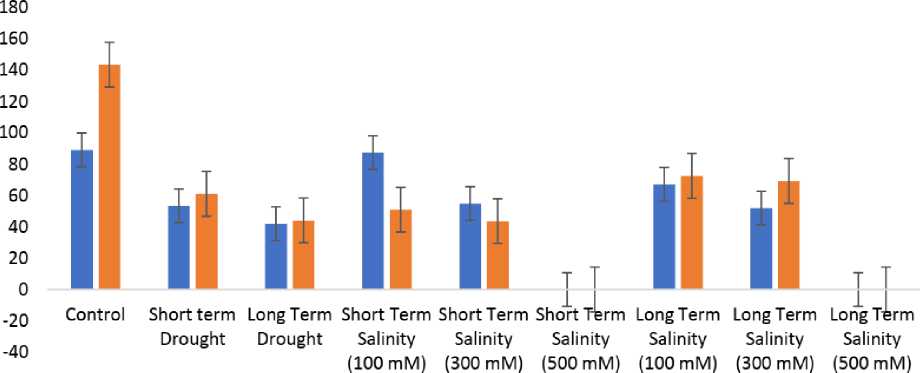
■ No. of Crystals ■ Area of crystals
Figure 12 G raphical representation of correlation between the number and area of crystals of Control, Drought and Salinity treated plants of Portulacaria afra
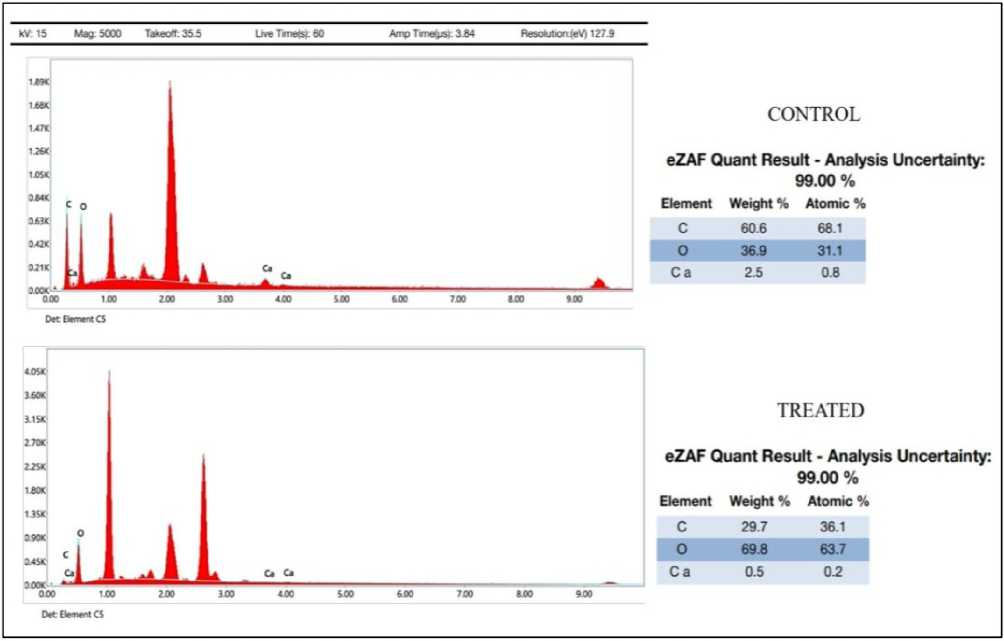
Figure 13 Energy dispersive X-ray Spectroscopy (EDS)
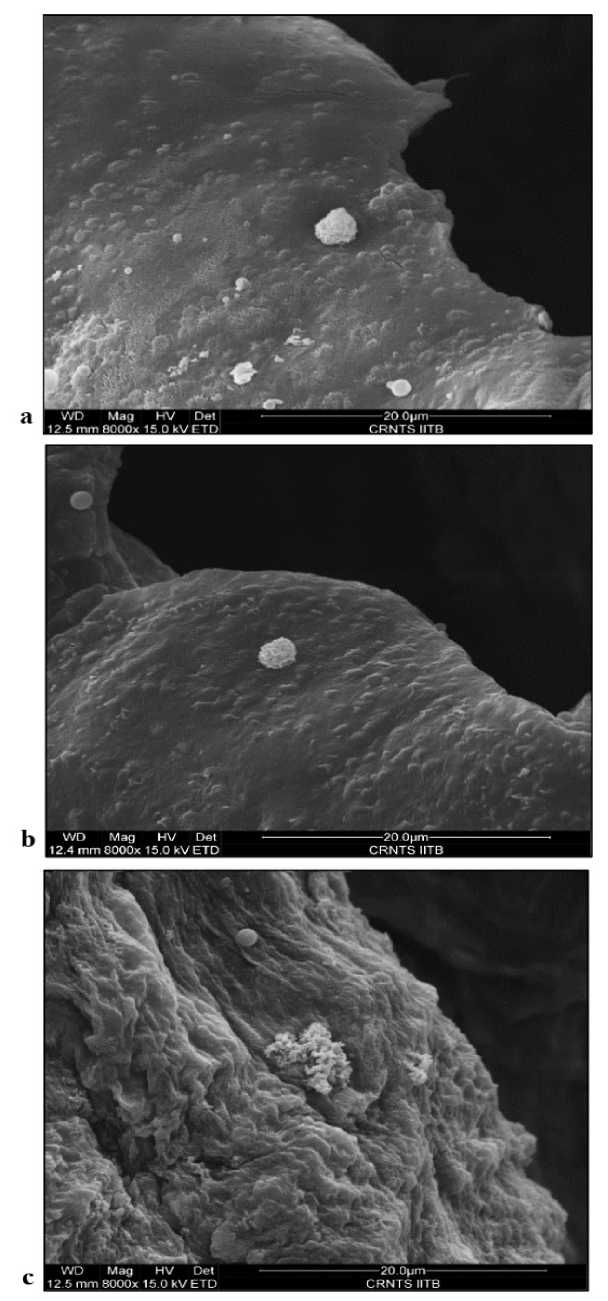
Figure 14 ESEM images showing Calcium Oxalate crystals of Portulacaria afra leaf grown under 14.a) Control 14.b) Drought stress and 14.c) Salinity stress conditions.
DISCUSSION
The findings of the current investigation support the discovery of calcium oxalate crystals in Portulacaria afra leaves. All of the biological replicas of Control contain the highest quantity and largest area of CaOx crystals compared to treated plants, proving that these crystals are not only present but also exist in their structurally intact form as "druses of CaOx" Crystals (Figure 3.d) When investigating the impact of carbon deficiency on the structure of CaOx crystals by subjecting the plants to short- and long-term drought stress and various salinity concentrations. The findings supported the link between the CaOx crystal breakdown and the CO 2 shortage circumstances. While least number of crystals are observed in plants experiencing drought, moderate number of crystals with a considerable decrease in size of the crystals were seen in salinity stress plants (Figure 4.d, 7.d and 10.d) . This is consistent with the carbon hunger hypothesis, which states that reduced photosynthesis due to stomatal closure causes an imbalance between the amount of carbon available (gain) and the amount lost due to metabolic needs (e.g., growth and maintenance). The negative balance that results from a drought lasting for such a long time can eventually cause plant death and the exhaustion of carbon storage.
Over time if drought persists, closed stomata not only leads to carbon starvation, but also to an imbalance between incident energy and available intercellular CO 2 The rate of reducing power production overpasses the rate of its use by the Calvin cycle. The carbon starvation hypothesis has been doubted by several researchers (Hummel et al ., 2010, Sala et al ., 2010) and it is now being questioned as the primary reason of plant death (Adams et al ., 2017). However, artificial carbon starvation represents a useful tool for the investigation of the metabolic events occurring under conditions, such as drought, that restrict carbon assimilation.
The results obtained from the present study confirms the presence of calcium oxalate crystals in leaves of Portulacaria afra. The abundant number of CaOx crystals with maximum area in comparison to treated plants seen in all the biological replicates of Control establishes the presence of CaOx crystals and also emphasizes on crystals being present in their structurally intact form of “druses of CaOx” Crystals When we further examined the effect of carbon starvation on the composition of CaOx crystals by means of establishing short term and long-term drought stress and by subjecting the plants to varying concentration of Salinity. The results confirmed the connection between the CO2 starvation conditions and the CaOx crystal decomposition. This was predicted by (Tooulakou et al., 2016a, 2016b) on the basis that stomatal closure (caused either by ABA or drought conditions) is accompanied by crystal decomposition in a number of plant species.
When the plant is under stress from salinity and drought, the stomata in the leaves are closed to stop excessive water loss and exosmosis. In turn, this prevents CO 2 from entering through stomata and fixing itself in the photosynthetic tissue. Portulacaria afra exhibits other physiological metabolic cycles running regularly despite the stomata being closed and CO 2 not entering the leaves, compensating for the lack of CO 2 for photosynthetic fixation and maintaining the cycles. This is only possible because the carbon from calcium oxalate crystals is taken and supplied into the cycles as part of the CO 2 replenishment process. While oxalate represents a rich source of carboxyl groups that could be converted to CO 2 by the enzyme oxalate oxidase (Tooulakou et al., 2016a). The oxalate oxidase enzyme may be responsible for this crystal deterioration Compared to plants that are well-watered, stress-affected plants leaves have an increase in the enzyme oxalate oxidase, helping the plant cope with the effects of salt stress and drought.
ACKNOWLEDGMENT
The authors are grateful to The rincipal of the institution, and all colleagues for their valuable guidance and support. Additionally, authors also thank SAIF IITB for carrying out ESEM and EDS analysis of the sample.
CONFLICTS OF INTEREST
The authors declare that they have no potential conflicts of interest.
Список литературы Effect of drought and salinity stress on calcium oxalate crystals of Portulacaria afra. (L.) Jacq
- Adams H. D., Zeppel M. J., Anderegg W. R., Hartmann H., Landhäusser S. M., Tissue D. T. and McDowell N. G. (2017) A multi-species synthesis of physiological mechanisms in drought-induced tree mortality. Nature ecology & evolution., 1(9), 1285-1291.
- Bastide B., Sipes D., Hann J. and Ting I. P. (1993) Effect of severe water stress on aspects of crassulacean acid metabolism in Xerosicyos. Plant Physiology., 103(4), 1089-1096.
- Dickison W. C. (2000) Integrative plant anatomy. Academic press.
- Franceschi V. (2001) Calcium oxalate in plants. Trends in Plant Science., 6(7), 331.
- Franceschi V. R. and Nakata P. A. (2005) Calcium oxalate in plants: formation and function. Annual review of plant biology., 56, 41.
- Horner H. T. and Wagner B. L. (2020) Calcium oxalate formation in higher plants. In Calcium oxalate in biological systems CRC press, pp. 53-72.
- Hummel I., Pantin F., Sulpice R., Piques M., Rolland G., Dauzat M. and Muller B. (2010) Arabidopsis plants acclimate to water deficit at low cost through changes of carbon usage: an integrated perspective using growth, metabolite, enzyme, and gene expression analysis. Plant physiology., 154(1), 357-372.
- Karabourniotis G., Horner H. T., Bresta P., Nikolopoulos D. and Liakopoulos G. (2020) New insights into the functions of carbon–calcium inclusions in plants. New Phytologist., 228(3), 845-854.
- Kolomeichuk L. V., Efimova M. V., Zlobin I. E., Kreslavski V. D., Murgan O. G. K., Kovtun I. S., Khripach V.A., Kuznetsov V.V. and Allakhverdiev S. I. (2020) 24-Epibrassinolide alleviates the toxic effects of NaCl on photosynthetic processes in potato plants. Photosynthesis research., 146(1), 151-163.
- McDowell N. G. (2011) Mechanisms linking drought, hydraulics, carbon metabolism, and vegetation mortality. Plant physiology., 155(3), 1051-1059.
- McDowell N. G. and Sevanto S. (2010) The mechanisms of carbon starvation: how, when, or does it even occur at all? The New Phytologist., 186(2), 264-266.
- McDowell N.G., Pockman W. T., Allen C. D., Breshears D. D., Cobb N., Kolb T., Plaut J., Sperry J., West A., Williams D.G. and Yepez E. A. (2008) Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought? New phytologist., 178(4), 719-739.
- Nakata P. A. (2003) Advances in our understanding of calcium oxalate crystal formation and function in plants. Plant Science., 164(6), 901-909.
- Prasad R and Shivay Y. S. (2017) Oxalic acid/oxalates in plants: from self-defence to phytoremediation. Current science., 1665-1667.
- Raman V., Horner H. T and Khan I. A. (2014) New and unusual forms of calcium oxalate raphide crystals in the plant kingdom. Journal of plant research., 127(6), 721-730.
- Sala A., Piper F. and Hoch G. (2010) Physiological mechanisms of drought-induced tree mortality are far from being resolved. The New Phytologist., 186(2), 274-281.
- Sarker U. and Oba S. (2019) Salinity stress enhances color parameters, bioactive leaf pigments, vitamins, polyphenols, flavonoids and antioxidant activity in selected Amaranthus leafy vegetables. Journal of the Science of Food and Agriculture., 99(5), 2275-2284.
- Tooulakou G., Giannopoulos A., Nikolopoulos D., Bresta P., Dotsika E., Orkoula M. G., Kontoyannis C.G., Fasseas C., Liakopoulos G., Klapa M.I. and Karabourniotis G. (2016) Alarm photosynthesis: calcium oxalate crystals as an internal CO2 source in plants. Plant Physiology., 171(4), 2577-2585.
- Tooulakou G., Giannopoulos A., Nikolopoulos D., Bresta P., Dotsika E., Orkoula M. G., Kontoyannis C.G., Fasseas C., Liakopoulos G., Klapa M.I. and Karabourniotis G. (2016) Reevaluation of the plant “gemstones”: Calcium oxalate crystals sustain photosynthesis under drought conditions. Plant signaling & behavior., 11(9), 00111.
- Webb M. A. (1999) Cell-mediated crystallization of calcium oxalate in plants. The plant cell., 11(4), 751-761.


