Effect of Elevated CO2 on Growth and Biochemical changes in Catharanthus roseus - An Valuable Medicinal Herb
Автор: S. Saravanan
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 3 т.17, 2021 года.
Бесплатный доступ
Increasing atmospheric CO2 concentration is generally expected to enhance plant growth, allocation and chemical composition of alkaloids in medicinal plants. The response to the elevated CO2 concentrations of various medicinal plants were studied with reference to growth and biochemical changes. Catharanthus roseus is an important medicinal plant which is being cultivated commercially in India for different purposes. It has gained interest from the pharmaceutical industry; the alkaloids vincristine and vinblastine from its sap have been shown to be an effective treatment for leukemia and lymphoma. It has a great importance in studying different parameters of C. roseus. The present study was carried out for growth and bio-chemical changes of C. roseus in different elevated CO2 levels. Open top chambers (OTCs, 3.0 m diameter, 3.0 m in height) were used to expose plants to ambient and elevated CO2 concentration (600 and 900 ppm).The experiment was conducted for five months. Carbon-dioxide enrichment studies in special open top chambers help us in understanding the changes at individual Biochemical changes and plant growth. The bio-chemical analysis revealed that the highest phenol, flavonoid, carbohydrate and tannin were recorded at 600 ppm+rh, and alkaloid was at 900 ppm. In ambient condition the highest protein was recorded in C. roseus. The plant growth revealed that the maximum fresh weight, shoot length and number of leaves were observed in 900 ppm. The maximum number of roots was observed in 600 ppm and the highest root length observed in 600+Rh. In the ambient condition, the above said characters were found to be in the lowest level.
Catharanthus roseus, Elevated CO2, Bio chemical changes, Growth parameters
Короткий адрес: https://sciup.org/143173904
IDR: 143173904
Текст научной статьи Effect of Elevated CO2 on Growth and Biochemical changes in Catharanthus roseus - An Valuable Medicinal Herb
The present scenario the term “Alternative Medicine” became very common in western culture, it focus on the idea of using the plants for medicinal purpose. But the current belief that medicines which come in capsules or pills are the only medicines that we can trust and use. Even so most of these pills and capsules we take and use during our daily life came from plants. Medicinal plants frequently used as raw materials for extraction of active ingredients which used in the synthesis of different drugs. Like in case of laxatives, blood thinners, antibiotics and antimalarial medications, contain ingredients from plants. Moreover the active ingredients of Taxol, vincristine, and morphine isolated from foxglove, periwinkle, yew, and opium poppy, respectively.
Catharanthus roseus, an annual perennial herb native to Madagascar, that were formerly included in the genus Vinca. t has gained interest from the pharmaceutical industry; the alkaloids vincristine and vinblastine from its sap have been shown to be an effective treatment for leukemia and lymphoma. Although the sap is poisonous if injected, some 70 useful alkaloids have been identified from it .The extracts are not having side effects which include hair loss. The fresh or dried flowers and leaves of plants are applied as a paste on wounds in some rural communities. The fresh juice from the flowers of C. roseus made into a tea has been used by Ayurvedic physicians in ndia for external use to treat skin problems, dermatitis, eczema and acne. To the best of our knowledge, the effect of elevated CO2 on the physiology of this plant in near natural condition has not been assessed. The present study has been conducted to study the effect of elevated CO2 on growth, productivity and biochemical changes.
MATERIALS AND METHODS
Enrichment of CO 2
The present study was conducted at the nstitute of Forest Genetics and Tree Breeding, Coimbatore, Tamil Nadu where the selected medicinal plants were grown inside the open top chambers (OTCs) of 3 m diameter and 10 m height lined with transparent PVC sheets (0.125 mm thickness) with a CO 2 levels of 600 mol mol-1. Pure CO 2 gas was used for the enrichment. Similarly OTCs were maintained at elevated temperatures (Ambient +4ºC) under ambient CO 2 (380 mol mol-1). Controls were maintained in open field outside OTCs, with ambient CO 2 (380 mol mol-1). CO 2 was provided throughout the day and night (24 h period). The experiments were laid in a Complete Randomized Design. The period of CO 2 enrichment was 180 days. A software facility called Supervisory Control and Data Acquisition (SCADA) was used to continuously control record and display the actual and desired CO 2 level, relative humidity and temperature in each OTC by feedback control loop passing through Programmable Logical Controllers (PLC) (Buvaneswaran et al., 2010). The set that was maintained in the open served as the control under ambient conditions while the set maintained inside the chamber under ambient CO 2 conditions was used to eliminate the effects of the chamber on the response of the plants. We have selected five concentrations viz., ambient, control, 600 ppm, 600+RH ppm and 900 ppm. n each concentration five plants were taken at one month intervals.
Bio-chemical analysis
The samples were air dried for about one week and ground into fine powder.150 mg of each of the powder were weighed separately and dissolved in 3.0 ml of methanol and water. For the water extracts, the solution was heated to 100°C and maintained this temperature for 15minutes. They were covered, mixed and kept for 8 hours with intermittent shaking for every 30 minutes and then allowed to stand for 48 hours for extraction. The solutions were subsequently shaken and filtered using Whatman filter paper. The filtrates were allowed to evaporate for dryness. The residue was dissolved in 5
ml of 90% methanol and water for the organic and aqueous solvent samples. These were stored at 15°C and then used for biochemical screening using the standard procedure described by Trease and Evans (1983) and Kokate (1994). The presence of alkaloids and flavonoids were further confirmed by Thin Layer Chromatography (TLC). Protein and carbohydrate were determined by Lowry’s method and Anthrone method respectively. The secondary metabolites such as phenol, tannic acid and flavonoids were quantitatively determined by Folin-Ciocalteau reagent method, FolinDenis method and Aluminium chloride colorimetric method respectively. Carbonic anhydrase was estimated by Wilbur and Anderson method (1948) and Chlorophyll by Arnon method (1949).
Statistical Analysis
The data were subjected to analysis of variance for completely randomized design with five replications. A full-factorial multivariate general linear model (GLM) analysiswas conducted using SPSS to determine whether there was significant variation in the different gas exchange and biochemical characteristics between different CO 2 conditions within the plants. Post hoc range tests using Waller Duncan t-test was performed to group the significantly different plants.
RESULTS AND DISCUSSION
Growth characters
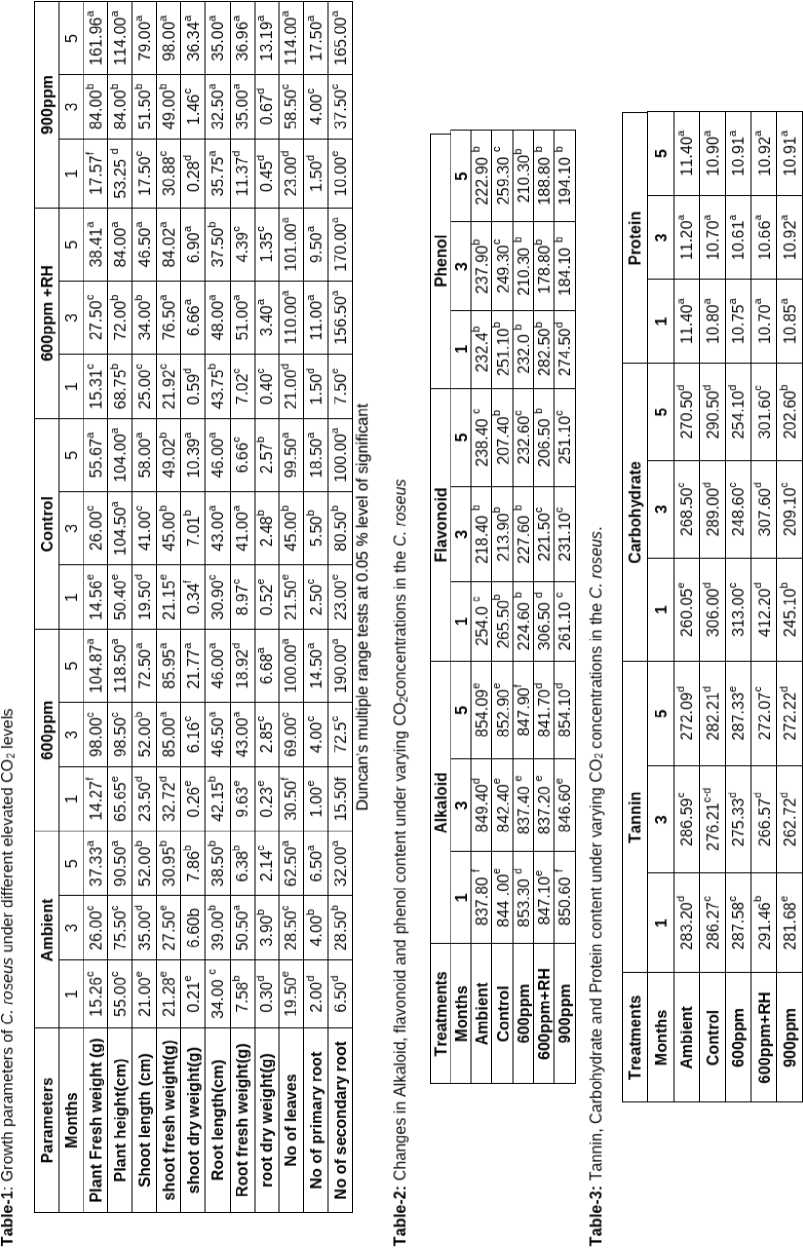
With reference to growth parameters, the maximum fresh weight (in g) and plant height (in cm) was observed in higher the concentration of elevated CO 2 levels (900 and 600ppm).The similar result was reported by (Ghasemzadeh and Jaafar, 2011) in two Zingiber officinale varieties ( Halia bentong and Halia bara ) were exposed to different CO 2 concentrations (400 and 800 ppm) resulted in increasing total plant biomass.
Highest Root length observed in 600+RH (48.0 cm), followed by 600 ppm (46.50 cm). Under the control and ambient conditions, lowest root length was observed. The similar result was reported by Dilustro et al., (2002) that fine roots were strongly stimulated by elevated atmospheric CO2. Also, he found that the root length density was significantly stimulated by CO2 and was greater in the upper 50 cm of the soil profile. The maximum number of leaves was observed in 900 ppm (114 Nos.) followed by 600+Rh (110 Nos.).The similar result was noticed by Rogers et al. (1983); Sionit et al., (1981) and Cure et al., (1989) and higher leaf area production has been reported under the higher concentration of elevated CO2 levels. Elevated atmospheric CO2 concentration in the environment has also been reported to enhance the photosynthesis and growth of many plant species (Kimball, 1983; Cure and Acock, 1986). t is also generally suspected that the studies on short term exposure of elevated CO2 over estimate the relative enhancements in CO2 assimilation rates in plants as compared to those under the long term exposures (Sage et al. 1989). Nonetheless, short term studies serve a key role in providing first approximation and indication of plants behaviour under future environmental conditions (Joshi, 2006).
The elevated CO 2 concentration increases the medicinal plant total height, biomass, etc. compared to the ambient and control (Table-1).
Bio-chemical analysis
The details of effects of elevated CO 2 on the bio chemical changes viz., alkaloids, flavonoids, phenols, tannins, carbohydrate and protein in C. roseus is shown in the Table-2 and Table-3.
n bio-chemical analysis, the plant responds positively to elevated CO 2 with reference to the production ofalkaloids, Flavonoid, phenols, tannins, carbohydrates and proteins. n alkaloids highest values was observed in elevated CO 2 condition.
Total Alkaloid content
The highest concentration of alkaloids was recorded under 900 ppm (854.10mg/ml) followed under 600 ppm (853.30 mg/ml).The lowest level of the total alkaloids was registered under ambient (837.80mg/ml). This result is in tune with many authors and revealed that, alkaloid content of wild poppy, ( Papaver setigerum ) investigated in the experimental CO 2 values (300, 400, 500 and 600 ppm) correspond roughly to the concentrations that existed during the middle of the twentieth century, the current concentration, and near and long-term projections for the current century(2050 and 2090), respectively.
Elevated carbon dioxide resulted in significant increases in leaf area and aboveground biomass. Elevated CO 2 increased the number of capsules, weight and latex production. Theamount of all alkaloids morphine, codeine, papaverine and noscapine increased significantly on a per plant basis, with the greatest relative increase occurring with recent increases in atmospheric carbon dioxide (e.g.from 300 to 400 ppm). They concluded that as atmospheric CO 2 continues to increase, significant effects on theproduction of secondary plant compounds of pharmacological interest could be expected (Ziska et al ., 2008).
Flavonoid content
The highest flavonoid was observed in 600 ppm+rh (306.50 mg ml-1) followed by control (265.50 mg ml-1). This results indicates that, the total content of bioactive flavonoid can be increased using by CO 2 enrichment. Further, the composition of flavonoid is also affected. There are indications that the composition would affect bio availability and bioactivity of the flavonoid (Lai et al., 2003).
Phenol and tannin content
With reference to the total phenol content, the highest production was observed in 600 ppm+Rh (282.50 mg ml-1) followed by 900 ppm (274.50 mg ml-1) and the least was observed in 600 ppm (210.30 mg ml-1). n the case of tannin content, the highest level was noticed in 600 ppm+Rh (291.46 mg ml-1) followed by 600 ppm (287.58 mg ml-1). The lowest tannin content was observed in ambient (272.09 mg ml-1). But, the variation was not significantly varied among the treatments. At early growth stage, the total content of leaf flavone under elevated CO 2 and their combination was lower than the control, but at maturing stage, it was increased. This is due to secondary metabolites such as flavonoid and tannins are synthesized from phenols. Similar result was reported by Goncalves et al ., (2009) in wheat that elevated CO 2 increases the content of total phenols in wheat leaves and had the greatest effect.
Carbohydrate content
The highest carbohydrate rate was observed in 600ppm+RH (412.20 mg ml-1), followed by 600 ppm
(313.0 mg ml-1) and the lowest level of carbohydrate was recorded in 900 ppm (202.60 mg ml-1). Similar results was reported by Chaitanya et al., (2002), and explained that under heat stress, carbohydrate synthesis is greatly influenced as observed from reduced activities of sucrose phosphate synthase, ADP glucose pyrophosphorylase and invertase (Vu et al ., 2001). Lilley et al., (2001) reported that elevated CO 2 conditions produced an average increase in total non-structural carbohydrate contents of 28% for clover and 16% for phalaris.
Protein content
The highest protein content was observed in ambient, followed by 900 ppm (11.40 mg ml-1). The lowest protein content was observed in 600 ppm (10.61 mg ml-1). Crop concentrations of nutritionally important minerals including calcium, magnesium and phosphorus may also be decreased under elevated CO 2 (Loladze, 2002; Taub and Wang, 2008). Similar results was proposed in FACE experiments, protein concentrations in grains of wheat, rice and barley, and in potato tubers, are decreased by 5–14% under elevated CO 2 (Taub et al., 2008).
The results of the dso et al., (2000) study showed that 75% increase in the air's CO2 concentration produced a 56% increase in the spider lily's belowground bulb biomass, where the disease-fighting substances are found. n addition, for these specific substances, they observed a 6% increase in the concentration of a two-constituent (1:1) mixture of 7-deoxynarciclasine and 7-deoxy-trans- dihydronarciclasine, an 8% increase in pancratistatin, an 8% increase in trans-dihydronarciclasine, and a 28% increase in narciclasine. Averaged together and combined with the 56% increase in bulb biomass, these percentage concentration increases resulted in a total mean active-ingredient increase of 75% for the plants grown in air containing 75% more CO2.
Ali et al . (2005) revealed that, after 45 days of ultra-high CO 2 concentrations treatment in Ginseng plant, the total root phenolic concentrations were 58% higher at 10,000 ppm CO 2 than at ambient CO 2 , 153% higher at 25,000 ppm CO 2 and 105% higher at 50,000 ppm CO 2 , as best as can be determined from the bar graphs of
their results. Likewise, total root flavonoid concentrations were enhanced by 228%, 383% and 232%, respectively, at the same ultra-high CO 2
concentrations, while total protein contents rose by 14%, 22% and 30%, non-protein thiol contents by 12%, 43% and 62%, and cysteine contents by 27%, 65% and
100% under the identical respective set of conditions. Zobayed and Saxena (2004) worked with Hypericum perforatum , a perennial herb and reported that, the extra 640 ppm of CO 2 in the high CO 2 treatment increased plant concentrations of hypericin and pseudohypericin by just over 100%. Consequently, the 180% increase in the air's CO 2 content more than doubled the dry mass produced by the well-watered and fertilized H. perforatum plants, while it also more than doubled the concentrations of hypericin and pseudohypericen found in their tissues, which means that the CO 2 increase more than quadrupled the total production of these two health-promoting substances.
Sukenik et al., (1994) reported that the maximum EPA production was obtained when 20,000 ppm CO2 was supplied 12 hours prior to the end of the exponential growth, and that the total EPA production during 4-day cultivation was about twice that obtained with ambient air. They also report that other researchers have obtained similar results, noting that EPA is mainly contained in thylakoid membranes (Sukenik et al., 1989) and that prior experiments have shown that "the amount of stroma thylakoid membrane increased in several plants under elevated CO2 concentrations (Hodgson et al., 1991). n addition, they say that in Synechococcus lividus, reduction and synthesis of thylakoid membrane occurred by CO2 deprivation and elevation, respectively (Miller and Holt, 1977) and that in Chlorella vulgaris, altering the ambient CO2 concentration varied fatty acid composition (Tsuzuki et al., 1990). Last of all, they say that the effect of CO2 on fatty acid composition and/or fatty acid content was reported in algae and higher plants (Tsuzuki et al., 1990), and that increased EPA production caused by elevated CO2 concentration was reported in P. tricornutum (Yongmanitchai and Ward, 1991). Consequently, as the atmosphere's CO2 concentration continues to rise, concentrations of omega-3 fatty acids should be widely enhanced in both aquatic and terrestrial plants, there by benefiting much of the animal life of the planet. Ghasemzadeh and Jaafar (2011) reported that, two Zingiber officinale varieties (Halia bentong and Halia bara) were exposed to different CO2 concentrations (400 and 800 ppm) and found that under the elevated CO2 concentration the total flavonoid, total phenolics, total soluble carbohydrates and starch under elevated CO2.
CONCLUSION
This present study reveals that, the medicinal plant C. roseus responds positively to the elevated CO 2 in morphological and biochemical aspects. The present study confirms that highest growth rate was noticed under the elevated CO 2 levels and the bio-chemical parameters were also showed the increasing trend under the elevated CO 2 , compared to the ambient and control.
CONFLICTS OF INTEREST
All authors have declared that they do not have any conflict of interest for publishing this research.
Список литературы Effect of Elevated CO2 on Growth and Biochemical changes in Catharanthus roseus - An Valuable Medicinal Herb
- Ali, M.B., Hahn, E.J. and Paek, K.Y. (2005). CO2-induced total phenolics in suspension cultures of Panax ginseng C.A. Mayer roots: role of antioxidants and enzymes. Plant Physiology and Biochemistry, 43: 449-457.
- Anderson, J.M., Chow, W.S. and Park, Y. I. (1995). The grand design of photosynthesis: acclimationofthe photosynthetic apparatus to environmental cues. Photosynthesis Research, 46: 129–139.
- Arnon, D. I. (1949). Copper enzymes in isolated chloroplasts. Polyphenol oxidase in Beta vulgaris. Plant Physiol., 24:1-15.
- Buvaneswaran, C., E, Edvinraj, Warrier, R.R. and Jayaraj, R.S.C. (2010).Scope and Opportunities of research on elevated carbon dioxide and plant response in tropical tree Species. ENVIS Forestry Bulletin,10 (2):10-16.
- Chaitanya, K.V, Sundar, D., Masilamani, S. and Ramachandra Reddy, A. (2002). Variation in Heat stress-induced antioxidant enzyme activities among three mulberry cultivars. Plant Growth Regul., 36: 175–180.
- Cure, J.D. and Acock, B. (1986). Crop response to carbon dioxide doubling: a literature survey. Agric For Meteorol, Vol.38:127–145. doi: 10.1016/0168-1923(86)90054-7.
- Cure, J.D,Rufty T.W Jr and Isreal D.W. (1989). Alterations in soybean leaf developmentand Photosynthesis in a CO2 enriched atmosphere. Bot. Gaz, 150: 337-345.
- Dilustro, J.J., Day, F.P., Drake, B.G., Hinkle, C.R., (2002). Abundance, production and mortality of fine roots under elevated atmospheric carbon dioxide in an oak scrub ecosystem. Environ. Exp. Bot., 48: 149–159.
- Ghasemzadeh, A. and H.Z.E.Jaafar. (2011). Effect of CO2 Enrichment on Synthesis of Some Primary and Secondary Metabolites in Ginger (Zingiber officinale).Int. J. Mol. Sci., 12: 1101-1114.
- Goncalves,S, Ferraz, M, Romano, A. (2009). Phyto toxic properties of Drosophyllum lusitanicum leaf extracts and its main compound Plumbagin. Sci. Hortic., 122: 96-101.
- Hodgson, P.A., Henderson, R.J., Sargent, J.R. and Leftley, J.W. (1991). Patterns of variation in the lipid class and fatty acid composition of Nannochloropsis oculata (Eustigmatophyceae) during batch culture. I. The growth cycle. Journal of Applied Phycology, 3: 169-181.
- Idso, S.B., Kimball, B.A., Pettit III, G.R., Garner, L.C., Pettit, G.R. and Backhaus, R.A. (2000). Effects of atmospheric CO2 enrichment on the growth and development of Hymenocallis littoralis (Amaryllidaceae) and the concentrations of several antineoplastic and antiviral constituents of its bulbs. American Journal of Botany, 87: 769-773.
- Joshi, S.C. (2006). Photosynthetic response of Thysanolaena maxima (Roxb.) Kuntze, A multipurpose and monotype plant, to different levels of CO2. Physiol Mol Biol Plants, 6 (12): 241–245.
- Kimball, B.A. (1983). Carbon dioxide and agricultural yield: an assemblage and analysis of 430 prior observations. Agron J.; 75:779–788. doi: 0.2134/agronj1983.00021962007500050014x.
- Kokate, C.K. (1994). Practical Pharmacognosy.Vallabh Prakashan, New Delhi, pp 107-113.
- Lai, MY., S.L. Hsiu, S.Y. Tsai, Y.C. Hou, and P.D.L. Chao. (2003). Comparison of metabolic Pharmaco kinetics of baicalin and baicaleinin rats. J. Pharm. Pharrnocol, 55:205-209.
- Lilley,J.M, Bolger T.P, Gifford R.M. (2001). Productivity of Trifolium subterraneum and Phalaris aquatic under warmer high CO2 conditions. New Phytologist, 150: 371–383.
- Loladze, I. (2002). Rising atmospheric CO2 and human nutrition: toward globally imbalanced plant stoichiometry? Trends in Ecology and Evolution, 17: 457-461.
- Lowry, O.H, Rosebrough, N.J, Farr, A.L, and Randall, R.J. (1951). Protein measurement with the Folin-Phenolreagents. J. Biol. Chem., 193:265-275.
- Miller, L.S. and Holt, S.C. (1977). Effect of carbon dioxide on pigment and membrane content in Synechococcus lividus. Archives Microbiologie, 115: 185-198.
- Rogers, H., Bingham, G. E, Cure, J. D, Smith, J. M and Surano, K. A. (1983). Responses of selected Plant species to elevated CO2 in the field. J. Environ. Qlty., 12: 569.
- Sage, R.F., Sharkey, T.D., and Seemann, J.R. (1989). Acclimation of photosynthesis to elevated CO2 in five C3 species. Plant Physiol.:89:590–596. doi: 10.1104/pp.89.2.590.
- Sionit, N., Stran, B. R and Bestord, R. A. (1981). Environmental control on growth and yield of okra I. Effect of temperature and CO2enrichment at cool temperature: Crop Sci, 21: 885-888.
- Sukenik, A., Cameli, Y. and Berner, T. (1989). Regulation of fatty acid composition by irradiance level in the eustigmatophyte Nannochloropsis sp. Journal of Phycology, 25: 686-692.
- Sukenik, A., Takahashi, H. and Mokady, S. (1994). Dietary lipids from marine unicellular algae enhance the amount of liver and blood omega-3 fatty acids in rats. Annals of Nutrition and Metabolism, 38: 85-96.
- Taub, D., Miller, B. and Allen, H. (2008). Effects of elevated CO2 on the protein concentration of food crops: a meta-analysis. Global Change Biology, 14: 565-575.
- Taub, D. R. and Wang, X. Z. (2008). Why are nitrogen concentrations in plant tissue lower Under elevated CO2? A critical examination of the hypotheses. Journal of Integrative Plant Biology, 50, 1365-1374.
- Trease, G. E and Evans, M. C. (1983). Textbook of Pharmacognosy. 12th edition: 343-383.
- Tsuzuki, M., Ohnuma, E., Sato, N., Takaku, T. And Kawaguchi, A. (1990). Effects of CO2 concentration during growth on fatty acid composition in microalgae. Plant Physiology, 93: 851-856.
- Vu, J.C.V., Gesch R.W., Pennanen A.H., Allen, L.H.J. Boote, K.J, and Bowes, G. (2001). Soybean photosynthesis, Rubisco and carbohydrate enzymes function at supra-optimal temperatures in elevated CO2. J. Plant Physiol, 158: 295–307.
- Ward JK, Strain BR. (1999). Elevated CO2 studies: past, present and future. Tree Physiology, 19: 211–220.
- Wilbur, K. and Anderson.N. (1948).Electrometric and colorimetric determination of carbonic anhydrase. J of Biol. Chem, 176:147-154.
- Yongmanitchai, W. and Ward, O.P. (1991). Growth of and omega-3 fatty acid production by Phaeodactylum tricornutum under different culture conditions. Applied Environmental Microbiology, 57: 419-425
- Ziska, L.H., S. Panicker and H.L. Wojno, (2008). Recent and projected increases in Atmospheric carbondioxide and the potential impacts on growth and alkaloid production in wild poppy (Papaver setigerum DC.).Earth and Environmental Science, Climatic Change, 91: 395-403.
- Zobayed, S. and Saxena, P.K. (2004). Production of St. John's Wort plants under controlled environment for maximizing biomass and secondary metabolites. In Vitro Cellular and Developmental Biology - Plant 40: 108-114.


