Effect of Exogenous Trehalose on Physiological Responses of Wheat Plants Under Drought Stress
Автор: Ebtesam Ahmed Qaid
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 4 т.16, 2020 года.
Бесплатный доступ
Drought stress causes physiological changes in plant morphological growth and development. The protective role of trehalose (Tre) was investigated through exogenously applied to seedlings of wheat cv. Sakha 93 plants. Pots experiment were divided into four groups: control, Tre treatment, drought stress and drought stress with Tre treatment. Samples were collected in two stages after 7 and 14 days from drought stress to determine the fresh weight, dry weight, chlorophyll pigments, relative water content, electrolytic leakage, proline, malondialdehyde, antioxidant enzyme activities, and carbohydrates content. Drought stress reduced many growth and physiological characters. It significantly increased specific activities of guaiacol peroxidase (GPX) and ascorbate peroxidase (APX) and significantly decreased the catalase (CAT) activity. It caused significantly increased in the Tre content while reduced sucrose and starch contents. Combination exogenous applied Tre (40 mM) with drought stress improved some growth parameters and photosynthetic pigments. Application of Tre markedly decreased proline and malondialdehyde contents, GPX; APX activities whereas increased CAT activity. Tre treatment appeared increased in the internal Tre content, on the other hand; exogenous Tre maintained the sucrose and starch contents in the seedlings of wheat plants under drought stress. The results concluded that applied Tre alleviated the adverse effect of drought stress on wheat plants by enhancing the antioxidant defense system and conservation of the membrane stability.
Alleviate, Antioxidant Enzymes, Combination, Morphological, Physiological, Protective
Короткий адрес: https://sciup.org/143173860
IDR: 143173860
Текст научной статьи Effect of Exogenous Trehalose on Physiological Responses of Wheat Plants Under Drought Stress
Abbreviations: APX: Ascorbate peroxidase, CAT: catalase, GPX: guaiacol peroxidase, MDA: Malondialdehyde, ROS: Reactive oxygen species, RWC: Relative Water content
Wheat ( Triticum aestivum L.) is the most important food crop in many developing countries. Wheat grains are a concentrated source of vitamins, minerals, and protein (Rauf et al., 2007). Its productivity has noticeably affected by most of the environmental constraints.
Among abiotic stress, drought causes marked changes in molecular, biochemical, and physiological processes that affecting plant growth and development (Farooq et al ., 2010). It has an adverse effect on plant photosynthetic efficiency that due to closing stomata which limits CO 2 diffusion into leaf, or inhibits Rubisco activity and breaks down energy balance and break down the distribution during photosynthesis (Rapacz et al. , 2010).
In plants, the links between the production of reactive oxygen species (ROS) and photosynthetic metabolism are particularly important. The ROS has been shown to cause membrane integrity loss, oxidative damage biomolecules including protein, lipids, and DNA which leads to the release of malondialdehyde (MDA) and the regulation of antioxidant enzymes (Zhang et al., 2007). The plants can avoid the damage caused by drought stress through enhancement of the antioxidant defense system that scavenges ROS and compatible solutes accumulation (Alam et al., 2014). Enhanced antioxidant enzymes activities such as superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX) improve drought tolerance in many crop plants (Alam et al., 2013).
Many substances are accumulated in plants under biotic and abiotic stresses like soluble sugars and free amino acids. Among different compatible solutes, Tre is a non-reducing disaccharide that is important storage carbohydrate and stress protection metabolites in yeast, bacteria, and certain fungi (Elbein et al., 2003), (Gancedo & Flores, 2004). Only trace amounts of Tre were detected in higher plant tissues (Garg et al., 2002). Tre improves the response of plants to drought, nutrition element deficiency, or salinity stresses (Chang et al., 204). Tre is likely to function through its ability to act as a direct and indirect scavenger of ROS, conferring protection to the machinery of protein synthesis (Zhu,
Exogenously applied Tre is readily accumulated and transported by leaf or roots tissues and displays significant roles as osmoprotectants (Luo, et al., 2010) Application of Tre for improvement of environmental stress tolerance was reported in several plants such as Zea mays under salt stress (Zeid, 2009), (Li et al., 2014)]; Arabidopsis thaliana against drought stress (Stolker, 2010); rice seedlings under salt stress (Nounjam et al., 2012)]; Catharanthus roseus has grown under saline condition (Chang et al., 2014). and Brassica species under drought stress (Alam et al., 2014).
The present study investigated the role of exogenous application of Tre in reducing the adverse effect of drought on growth parameters, different antioxidant enzymes in cv. Sakha 93 plants under drought stress.
MATERIALS AND METHODS
Wheat seeds ( Triticum aestivum cv. Sakha 93) were purchased from Agriculture Research Center, Giza, Egypt.
Seeds of Sakha 93 cultivar were sterilized with ethanol (70%) and washed with distilled water then surface sterilized with 20% sodium hypochloride (v/v) for 20 min, and thoroughly rinsed with sterilized distilled water. Fifteen seeds were sown in each pot (12 cm in diameter) contained 500g of sterilized perlite and vermiculate (1: 1, w/w). The average environmental conditions were: temperature, 25 ± 3 °C; light, 5000 Lux; RH, 70% during entire period of experimentation.
Seedlings were grown up to 10 days irrigated with sterilized tap water (70% field capacity) then thinning was started so that five plants of uniform size were maintained in each pot. Pots were divided into four groups:
|
Control |
plants were irrigated with the half strength of Hoagland solution |
|
Treated Tre |
plants were irrigated with half strength of Hoagland solution supplemented with 40 mM Tre |
|
Drought |
Plants were subjected to drought by withholding irrigation for 7 and 14 days |
|
Drought +Tre |
Plants were treated with 40 mM Tre, then subjected to drought by withholding irrigation for 7 and 14 days |
Plants were subjected to drought after 20 days from planting. Samples were collected in two stages after 7 and 14 days from drought stress to determine various physiological and biochemical parameters. Each treatment was replicated three times under the same experimental conditions. To determine FW, 10 shoots were separated and weighed to determine FW (mg/ plant). To quantify DW, 10 shoots from each treatment were oven-dried at 60 °C for 3 days and weighed, then expressed as mg/plant.
RWC was measured according to Jones and Turner, (1978). Freshly excised leaves were weighed to obtain leaves samples weight (FW).Then, the samples were immediately transferred to the deionized water for 3 h under normal room light and temperature. After 3 h the samples were taken out of the water and well dried of any surface moisture quickly and lightly with filter paper and weighed to obtain fully turgid weight (TW). The samples were oven-dried at 70°C for 48 h and weighed (after being cooled) to determine the dry weight (DW). RWC was calculated by the following formula: RWC (%) = [(FW–DW)/(TW–DW)]×100
Fresh leaves were extracted with 80% acetone and supernatant were obtained by centrifuging at 5,000g. the absorbance of the supernatants was were taken with V-visible spectrophotometer at 663, 644, and 470 nm and concentrations of chlorophyll a, b, and carotenoids were calculated according to Metzner et al. (1965). The concentration of each pigment was calculated from the following formula:
Chlorophyll a = 10.3 E663 - 0.918 E644
Chlorophyll b = 19.7 E644 – 3.87 E663
Carotenoids = 4.2 E470 (0.0264 Ch a + 0.426 Ch b)
The results were expressed as µg pigment /ml extract, then calculated as mg pigment/g fresh weight. Determination of Proline Content
Proline (Pro) content in leaf tissues was determined according to the protocol of Bates et al. (1973). Fresh leaf tissue (0.5 g) was homogenized well in 10 ml of 3% sulfosalicylic acid in ice. The homogenate was centrifuged at 11,500×g for 15 min. Two ml of the filtrate was mixed with 2 ml of acid ninhydrin and 2 ml of glacial acetic acid. The mixture was placed at 100°C in the water bath for 1 h, then transferred in to test tube and kept in ice to be cooled, after it was cooled, 4 ml of toluene was added and mixed thoroughly by vortex mixture. After some time chromophore containing toluene was read spectrophotometrically at 520 nm. Purified proline with different known concentrations was used to make a standard curve by comparing with the proline content of plant samples were calculated.
Electrolyte leakage (EL) of tissue was measured according to the method described by Gilley and Fletcher, (1997), using conductance meter (Model CD – 4301, Lutron). EL was calculated as a ratio of the conductivity before and after boiling.
The level of lipid peroxidation was measured by estimating malondialdehyde (MDA), using thiobarbituric acid (TBA) according to Dhindsa and Matowe, (1981). The MDA level was calculated by using the molar extinction coefficient of 155 mM–1cm–1 and expressed as μmol of MDA g–1 fresh weight.
Soluble protein was determined according to Lowry et al. (1951) as follows:
Mixture of alkaline sodium carbonate solution (2% Na2CO3 in 0.1 N NaOH), 0.5 % CuSO4 and 1.0 potassium and sodium tartrate was prepared by the ratio of (98 : 1 : 1) (v/v/v). To 1.0 ml of the plant extract, 0.5 ml of the previous mixture was added and allowed to stand at room temperature for 10 min then; 0.5 ml of diluted folin reagent 1: 9 (v/v) was added and mixed rapidly. After 30 min the absorbance was read at 750 nm. The standard curve of protein was carried out using egg albumin as standard protein.
Leaves were ground using pre-chilled mortar and pestle, and ice-cooled 100 mM K-phosphate pH 7.8, 60 mg PVP (Polyvinyl pyrolidene), were added. The homogenate was vortexed and centrifuged at 12000 g for 15 min at 4°C. The supernatant was considered as enzyme crude extract and used to measure the activities of GPX, APX, and CAT.
The specific activity was calculated after estimation of total soluble proteins according to Lowry et al ., (1951) .
The activity of GPX was determined according to the method of Velikova et al., 2000. The reaction mixture of 3 ml contained 50 mM K-phosphate buffer PH 7, 0.2% guaiacol (w/v) and 0.04 ml enzyme extract was prepared. The absorbance at 470 nm was measured 5 min after the addition of 3 mM H 2 O 2 . The activity of GPX was calculated as mM of guaiacol reduced using the extinction coefficient of 26.6 mm-1cm-1. The specific activity was calculated as mM guaiacol reduced min-1 mg-1 protein.
For APX assay, the method described by Nakano and Asada, (1980) was used.
The reaction mixture of 3 ml contained 0.5 mM ascorbic acid, 0.1 mM EDTA (Ethylene diamine tetra acetic acid), and 0.1 ml enzyme extract. The reaction was initiated by adding 1.5 mM H 2 O 2 . The absorbance of the reaction mixture was measured at 290 nm. The specific activity of APX was calculated using the extinction coefficient of 2.8 mM-1 cm-1 and expressed as mM ascorbate oxidized min-1 mg protein.
CAT activity was estimated by 3 ml reaction mixture contained 10 mM k- phosphate buffer pH7, 0.1 ml enzymes extract, and 0.035% H2O2. The activity of CAT was calculated based on the decline in the absorbance at 240 nm as the decomposition of H2O2 and used the extinction coefficient of 40 mM-1 cm-1, and represented as mM H2O2 reduced min-1 mg-1 protein according to Velikova et al. (2000).
Extraction and Determination of Trehalose
Extraction of trehalose:
Trehalose extraction was carried out according to Ferreira et al. (1997), by boiling 100mg frozen tissue in 2 ml ethanol, evaporated at 60 ºC, the residue was dissolved in 5 ml of 5 mM H 2 SO 4 , centrifuged at 10,000 g for 10 min and filtered. Filtrate was heated in a boiling water bath for one hour to hydrolyze sucrose in the extract because the sucrose retention time is the same as that of trehalose. Then pH was adjusted to 7.0, the solution was evaporated and the residue was dissolved in distilled water.
Determination of trehalose:
Trehalose content was determined by the method described by Cizmarik et al., (2004). The determination of trehalose was carried out using HPLC (Hewlett Packard, HP 1090 liquid chromatography), using Hypersil, 100 × 3 mm, 3µm column.
The separation was carried out at 32 °C using a flow rate of 0.8 ml/ min with acetonitrile: H 2 O (85: 15) as the mobile phase. The elution was detected with a Diode Array Detector (DAD) at 192 nm.
Extraction and Determination of Sucrose :
Extraction of sucrose
Sucrose was extracted by grinding 0.1 g of tissue, mixing with 10 mg PVP, 80% ethanol. The homogenate has been incubated at 70°C for 20 min. After centrifugation at 4,000 g for 10 min, the supernatant was separated and the pellet was re-extracted twice with 80% ethanol at 70 °C for 30 min. The supernatant was pooled and evaporated. The residue was dissolved in 1 mL of deionized H 2 O.
Determination of Sucrose
The content of sucrose was determined using the previously mentioned HPLC method (Cizmarik et al., 2004).
Extraction and Determination of Starch
Extraction of starch:
Starch was extracted according to the method of
Grotelueschen and Smith, (1967). 0.1 g of fine dry powder of shoots was extracted with 5 ml of 80 % ethanol, by boiling the sample in 95 °C water bath for 10 min, centrifuged at 2,500 g for 5 min. For complete extraction, the previously mentioned steps were repeated twice. The supernatants were pooled and used for the determination of total soluble sugars especially fructan content. The remaining residues were extracted by adding 5 ml of 0.005 N H 2 SO 4 , heated in the water bath at 95 °C for one hour and centrifuged at 4,000 for 10 min.
Determination of starch:
Released glucose was determined in the supernatant by glucose oxidase peroxidase kit according to the methoddescribed by Bergmeyer and Bernt, (1974).
Results are the mean of three measurements for each treatment. All obtained data were subjected to oneway analysis of variance (ANOVA), and the mean differences were compared by Duncan's multiple range test (DMRT) using SPSS software version 10. Different letters indicate significant differences between treatments at p ≤ 0.05.
RESULTS
Plant growth parameters such as fresh weight (FW), and dry weight (DW), were determined to estimate the negative effects of drought stress on plant growth and the potential mitigation effects of Tre on the droughtstress wheat plant (cv. Sakha 93).
Data in Table 1 indicate that drought stress and trehalose treatment caused a significant effect on FW, DW, and leaves area. FW of drought-stress wheat plants decreased by 22.16%, 26.60% whereas DW decreased by 20.32% and 25.23% at days 7 and 14, respectively, compared with control. However, wheat plants subjected to drought stress that pretreated with 40 mM Tre showed significantly mitigation in FW which reaching to 13% and 15.11% and DW that reached to 10.74% and 13.84% at days 7 and 14, respectively compared with control.
Wheat plants under drought stress displayed inhibition in leaf area which reaching to 32.24% at 14
days compared with control whereas the inhibition was 19.27% in the case of plants that pretreated with 40 mM Tre.
Tre application (40 mM) did not alter RWC compared with control. RWC significantly decreased in wheat plants that suffered from drought stress which reached to the highest value (23.14%) at 14 day compared with control. Whereas pretreated with Tre caused alleviation from the adverse factor of drought stress on RWC of wheat plants which reached to 12.12% compared with control.
Data in Table 2 cleared the effect of drought stress and exogenous Tre application on chlorophyll a, b and carotenoid contents in wheat plants. Drought stress showed marked reduction in chlorophyll a, b and carotenoid contents of wheat plant cv. Sakha 93 at 7 and 14 days. The highest reduction in Chl a, b and carotenoid contents were observed at 14 days which was 24.43%, 27.74% and 45.97%, respectively. However, pretreatment with Tre (40 mM) significantly improved chlorophyll a, b and carotenoid contents under water stress which was 8.61%, 15.24%, and 25.28%, respectively compared with control.
The pattern of change in EL% in wheat plants was recorded in Table 2 . As compared to control plants, the significant increment of EL% was 59.5% at 14 days after water stress. The application of Tre reduced the negative effect of water stress on EL% which was 15.14% at 14 days compared with control.
nder drought stress, the proline content was drastically increased in cv. Sakha 93, the level was 2 and 1.8 fold than control at 7 and 14 day, respectively. Feeding with Tre significantly decreased the proline level in wheat plants which reached to 1.2 fold at the two ages, but a level still higher than the control as showing in Figure 1 .
Data in Figure 2 show that MDA level markedly increased in wheat plants, the highest value of MDA level was recorded at 14 days from drought stress compared with the control. Exogenous Tre treatment caused significant decrement in the MDA content compared to drought-stressed plants, the percentage of alleviation of drought stress was reached to 50% in the case of MDA compared to control.
Data presented in Figure 3 show that the specific activities of guaiacol peroxidase (GPX), ascorbate peroxidase (APX) and catalase (CAT) in the shoot of cv. Sakha 93.
The effect of Tre treatment (40 mM) on antioxidant enzyme activities was non-significant at the first time of the experiment compared with control. On the other hand, Tre treatment exhibited increasing in GPX, APX and CAT activities during the second period of the experiment. Drought stress markedly increased specific activities of GPX and APX and significantly decreased the CAT activity. The highest value of GPX and APX activities was 42% and 79%, respectively compared with the control after 14 day from drought stress treatment.
On the other hand, the reduction in specific activity of CAT was 77% at the same time of drought duration compared to the control.
Externally applied Tre (40 mM) significantly increased the specific activities of GPX and APX and markedly decreased in specific activity of CAT.
Results in Table 3 revealed that Tre, sucrose and starch contents in cv. Sakha 93 leaves. Tre treatment alone led to a significant increase in the content of internal Tre, which reaching to 5-folds; sucrose and starch during the two times of the experiment which reaching to 132%, 8.5% and 17%, respectively at the second time of treatment compared to control. Tre level was increased while sucrose and starch levels were reduced in leaves of cv. Sakha 93 grown under water stress. The highest value of Tre level was 3 fold compared with control after 14 day of drought stress.
Table (1): Effect of drought and exogenous Tre (40 mM) combined with drought on growth parameters; Fresh weight (mg/plant), Dry weight (mg/plant), leaves area (cm2/plant) and RWC in wheat seedlings 7 and 14 days after drought stress.
|
Duratio n |
Treatment |
Parameters |
|||
|
FreshWeight (mg/ plant) |
Dry Weight (mg/plant) |
Leaves area (cm2/ plant) |
RWC % |
||
|
7 Day |
Control |
643.67 ± 20.11 a |
67.00 ± 2.40 a |
14.20 ± 0.78 a |
90.75 ± 1.11 a |
|
Control + Tre |
635.66 ± 22.33 a |
65.95 ± 2.21 a |
14.63 ± 0.40 a |
93.26 ± 2.18 a |
|
|
Drought |
501.00 ± 17.96 c |
53.38 ± 2.19 c |
11.09 ± 0.32 c |
77.07 ± 4.28 c |
|
|
Drought+ Tre |
559.89 ± 16.16 b |
59.80 ± 1.48 b |
12.72 ± 0.56 b |
84.25 ± 0.66 b |
|
|
14 Day |
Control |
757.37 ±22.96 b |
76.80 ± 1.95 a |
18.11 ± 0.14 a |
89.67 ± 1.67 a |
|
Control + Tre |
814.70 ± 23.04 a |
79.87 ± 1.99 a |
18.87 ± 0.30 a |
92.54 ± 5.71 a |
|
|
Drought |
555.90 ± 23.56 d |
57.42 ± 2.82 c |
12.27 ± 0.37 c |
68.92 ± 0.78 c |
|
|
Drought+ Tre |
642.93 ± 25.65 c |
66.17 ± 3.15 b |
14.62 ± 0.51 b |
78.80 ± 0.95 b |
|
Mean values was calculated from three replicates for each treatment. Different letters within each column indicate a significant difference between treatments at P≤0.05.
Table (2): Effect of drought and exogenous Tre (40 mM) combined with drought on photosynthetic pigments;
Chl.a, Chl. b and carotenoids and EL% in wheat seedlings 7 and 14 days after drought stress.
|
Duratio n |
Treatment |
Photosynthetic pigments |
EL % |
||
|
Chl.a |
Chl.b |
Carotenoid |
|||
|
7 Day |
Control |
0.963 ± 0.010 b |
0.354 ± 0.014 b |
0.085 ± 0.004 b |
31.06 ± 1.31 c |
|
Control + Tre |
1.057 ± 0.040 a |
0.406 ± 0.006 a |
0.097 ± 0.001 a |
31.13 ± 0.61 c |
|
|
Drought |
0.821 ± 0.014 d |
0.277 ± 0.011 d |
0.066 ± 0.001 d |
42.43 ± 2.08 a |
|
|
Drought+ Tre |
0.912 ± 0.012 c |
0.312 ± 0.012 c |
0.072 ± 0.001 c |
34.03 ± 0.66 b |
|
|
14 Day |
Control |
0.929 ± 0.016 b |
0.328 ± 0.005 a |
0.087 ± 0.002 b |
37.12 ± 2.78 c |
|
Control + Tre |
0.998 ± 0.001 a |
0.326 ± 0.006 a |
0.103 ± 0.002 a |
35.97 ± 1.24 c |
|
|
Drought |
0.702 ± 0.013 d |
0.237 ± 0.008 c |
0.047 ± 0.003 d |
59.21 ± 1.02 a |
|
|
Drought+ Tre |
0.849 ± 0.011 c |
0.278 ± 0.014 b |
0.065 ± 0.000 c |
42.74 ± 0.59 b |
|
Mean values was calculated from three replicates for each treatment. Different letters within each column indicate a significant difference between treatments at P≤0.05.
Table (3 ) : Effect of drought and exogenous Tre (40 mM) combined with drought on trehalose, sucrose and starch contents in wheat seedlings 7 and 14 days after drought stress.
|
Duration |
Treatment |
Trehalose |
Sucrose |
starch |
|
7 Day |
Control |
0.227 ± 0.012 d |
9.48 ± 0.23 b |
8.64 ± 0.12 b |
|
Control + Tre |
0.435 ± 0.010 c |
10.72 ± 0.70 a |
9.01 ± 0.11 a |
|
|
Drought |
0.647 ± 0.033 b |
8.67 ± 0.14 c |
7.54 ± 0.08 c |
|
|
Drought+ Tre |
0.907 ± 0.037 a |
9.76 ± 0.13 b |
8.73 ± 0.03 b |
|
|
14 Day |
Control |
0.226 ± 0.010 d |
14.11 ± 0.31 b |
9.18 ± 0.06 b |
|
Control + Tre |
0.525 ± 0.009 c |
15.31 ± 0.35 a |
10.78 ± 0.19 a |
|
|
Drought |
0.942 ± 0.021 b |
12.28 ± 0.28 c |
7.73 ± 0.05 c |
|
|
Drought+ Tre |
1.140 ± 0.050 a |
14.39 ± 0.14 b |
9.23 ± 0.12 b |
|
Mean values was calculated from three replicates for each treatment. Different letters within each column indicate a significant difference between treatments at P≤0.05.
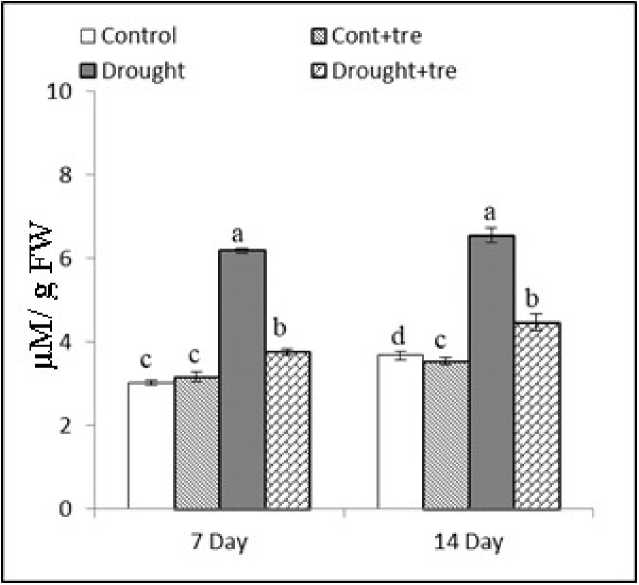
Figure (1): Effect of drought and exogenous Tre (40 mM) combined with drought on proline content in wheat seedlings 7 and 14 days after drought stress. Values are mean of three replicates ± SD. Bars with different letters are significantly different at P≤0.05.
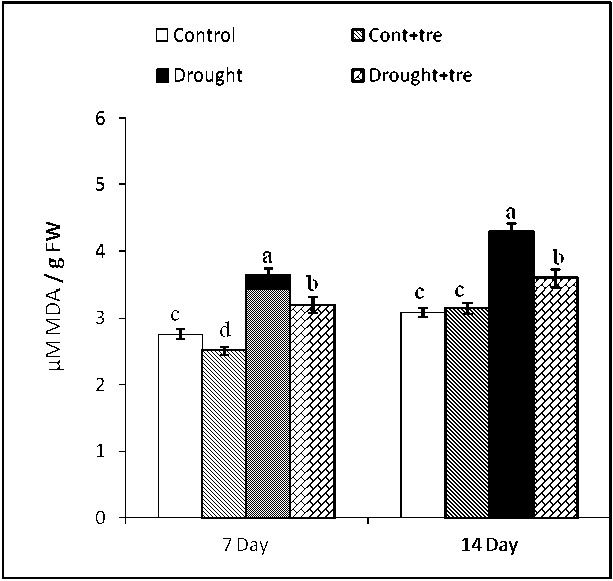
Figure (2): Effect of drought and exogenous Tre (40 mM) combined with drought on MDA content in wheat seedlings 7 and 14 days after drought stress. Values are mean of three replicates ± SD. Bars with different letters are significantly different at P≤0.05.
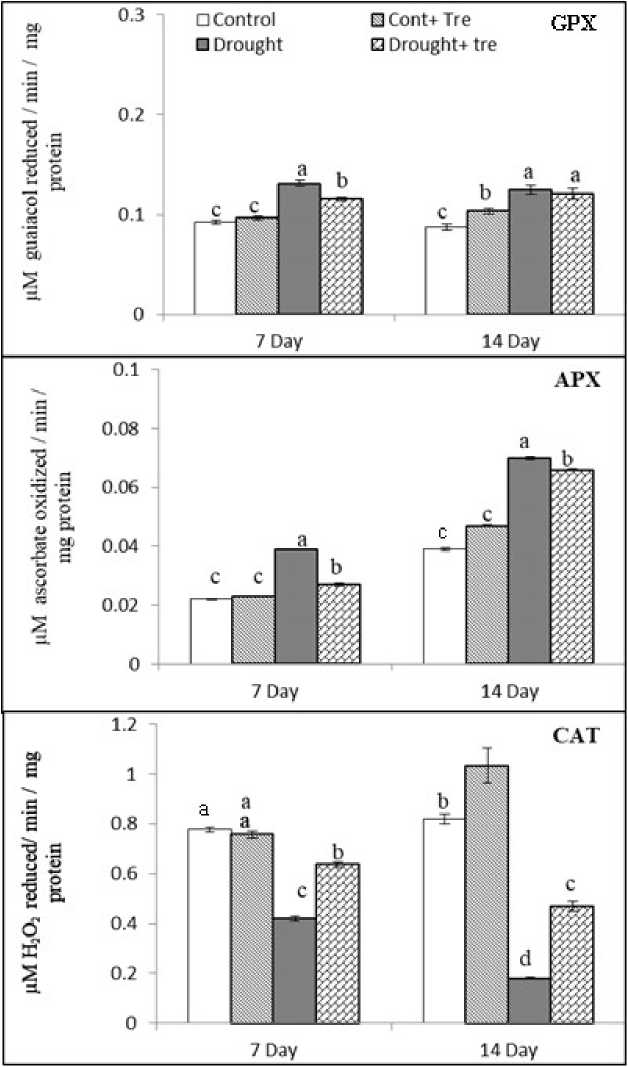
Figure (3): Effect of drought and exogenous Tre (40 mM) combined with drought on guaicol peroxidase (GPX), ascorbate peroxidase (APX) and catalase (CAT) activities in wheat seedlings 7 and 14 days after drought stress. Values are mean of three replicates ± SD. Bars with different letters are significantly different at P≤0.05.
DISCUSSION
In our results, drought stress reduced FW, DW, leaf RWC, and leaf area of cv. Sakha 93 plants. However, reduction growth parameters were restored by exogenous Tre supplementation under drought stress as achieved by ameliorating leaf RWC, fresh and dry weight. Similar findings were documented previously by Tre applied with abiotic stress (Alam et al., 2014; Ali, 201; Ali & Ashraf, 2011; Mostofa et al., 2015; Ahmed et al., 2016; Akram et al., 2016).
nder Cu-stress, plant height, fresh and dry weights, RWC significantly decreased in rice seedlings, on the other hand, Tre pretreatment could enhance tolerance of rice seedlings to Cu-stress (Mostofa et al., 2015).
Although drought stress caused reduction chlorophyll pigments in cv. Sakha 93 plants, but the interaction of Tre addition and drought stress alleviated the photosynthetic pigment contents. The same results were observed in many plants under abiotic stress: maize under salinity stress (Zeid, 2009); rice seedlings under Cu-stress (Mostofa et al., 2015); Brassica seedlings under drought stress (Alam et al. , 2014); wheat under drought stress (Ahmed et al., 2016); radish under drought stress (Akram et al., 2016). Tre has positive influence on rate photosynthesis through interaction with the sugar-signaling network (Ali & Ashraf, 2011). Drought stress has an indirect effect on stomata closure that due to a decrease in leaf CO 2 concentration. A low level of CO 2 may cause a reduction in NADP+ available to accept electrons from the photosynthetic system (PSI and PSII). Thereby initiate O 2 reduction accompanied by ROS production (Hernandez et al., 2000).
The degree of cell membrane injury induced by abiotic stress may be easily estimated through membrane leakage to cell electrolytes. Our study, EL% increased in cv. Sakha 93 plants by drought stress, meanwhile application of Tre reduced the values of EL% in stressed wheat plants. The same results were recorded in previous (Quan et al., 2004; Valentovic et al., 2014; Aldesuquy & Ghanem, 2015; Li et al., 2014).
Proline level increased in leaves of cv. Sakha 93 plant under drought stress; the similar results were recorded in several reports (Nahar et al., 2013; Alam et al., 2014; Mostofa et al., 2015; Ibrahim & Abdellatif, 2016; Ahmed et al., 2016). Many researchers reported that many of compatible solutes such as proline (Pro),
Tre and soluble sugar accumulate in higher concentrations in most plants to overcome of adverse effects of stress conditions. Pro plays a vital role in somatic adjustment, stress signal transduction.
Exogenously applied Tre with drought stress reduced Pro level in cv. Sakha 93 leaves. These results are agreement with (Nounjan et al., 2012; Alam et al., 2014; Mostofa et al., 2015;; Ahmed et al., 2016). Tre prevented wheat plants from adverse effects of drought stress through it could provide osmotic protection to cells by adjustment the accumulated Tre to optimal level so that wheat plants did not need to increase the Pro level. On the other hand, these results are disagreement with reports that recoded applied Tre with drought stress increased Pro content in wheat plants (Balla et al., 2011; Ibrahim, Abdellatif, 2016).
MDA induced by drought stress was observed in cv. Sakha 93 and other previous studies (Ali & Ashraf, 2011; Alam et al ., 2014; Aldesuquy & Ghanem, 2015; Ahmed et al ., 2016). MDA content was used as an indicator of lipid peroxidation which led to membrane fluidity and increases membrane permeability (Liu et al. , 2011). Exogenously applied Tre reduced MDA level in wheat plants. These results are agreement with (Ma et al ., 2013; Alam et al ., 2014; Aldesuquy & Ghanem, 2015). Tre was an osmoprotectant that stabilizes macromolecules, protein and lipids membranes and protects biological structures from the damage to water leakage (Garg & Manchand, 2009). In contrast, the application of Tre or maltose with drought stress remarkably increased the MDA level in wheat plants (Ibrahim & Abdellatif, 2016).
Generally, APX and CAT participated in ascorbate and glutathione cycle. Superoxide dismutase generated
H 2 O 2 that was catalyzed into H 2 O by CAT and peroxidases.
Our results for GPX and APX activities were similar with other reports such as in Brassica (Alam et al., 2014); in wheat (Aldesuquy & Ghanem, 2015; Ibrahim & Abdellatif, 2016). On the other hand, CAT activity significantly reduced in wheat plants under drought stress. Our results are supported by previous researches, (Sorial et al., 2010; Alam et al., 2014).
The same results was observed in some reports in Lemna gibba under Cd stress (Duman et al., 2011); in Oryza stativa under salt stress (Nounjan et al., 2012); in Brassica juncea under drought stress (Alam et al., 2014) in Triticum aestivum under drought stress [(Aldesuquy & Ghanem, 2015; Ibrahim & Abdellatif, 2016; Ahmed et al., 2016). Tre acts as a signaling molecule under abiotic stress which induced ROS production in plants, consequently, ROS sends signals to activate enzymatic antioxidants which have a vital role in scavenging of ROS due to alleviate oxidative stress (Fernandez et al., 2010).
Exogenously applied Tre before exposing the plants to drought stress had a fluctuant effect on internal Tre, sucrose and starch content. The level of Tre was increased into 4 fold whereas levels of sucrose and starch were similar to control.
This result was supported by previous reports; Tre level increased into 145-fold in seedlings of Arbidopsis thaliana that pretreated by Tre (30 mM) (Bae et al., 2005); Tre accumulation up to 385.25 µg/ g FW in Capparis ovate exposed to drought stress (IIhan et al., 2014). Endogenous Tre increased internal Tre to 2-folds in Oryza sativa seedlings that were pretreated with Tre before exposure to Cu-stress. On the other hand, Tre application maintained the levels of sucrose and starch at the same level as control.
In contrast, some reports disagreed with our results. Tre application increased accumulation of sucrose in some plants under abiotic stress: 3-folds in seedlings of
Arbidopsis thaliana (Bae et al., 2005)]; in Catharanthus roseus under salt stress [10], in internodes of sugarcanes that grown in Tre treatment (Glassop et al., 2007).
The vital function of Tre in plants is the metabolic regulation, control of metabolization of sucrose to several pathways important for metabolism, structural and storage functions of plant cells (Ahmed et al., 2013). The addition of Tre increased accumulation of starch which is due to increase the activity of AD-glucose pyrophosphorylase (AGPase), a major enzyme controlling starch synthesis (Wingler et al., 2000) .
CONCLUSION
In conclusion, our results indicate that the drought had a negative influence on membrane characteristics, enzymatic antioxidant and growth parameters of wheat plants. On the other hand, exogenous application of Tre combined with drought appeared to ameliorate the effect of drought by decreasing membrane damage. Tre pretreatment induced growth improvement in wheat plants under drought stress that could be associated with a significant increase in Tre level that regulating carbohydrates.
ACKNOWLEDGEMENT
Список литературы Effect of Exogenous Trehalose on Physiological Responses of Wheat Plants Under Drought Stress
- Ahmed, H. E., Kord, M. A., Youssef, H. A., Qaid, E. A., (2016). Exogenous application of trehalose improves the physiological status of wheat cv. Giza 168 grown under stress. Egypt. J. Bot. 56(3), 627-646.
- Ahmed, H.E., Youssef, E.A., Kord, M.A., Qaid, E.A., (2013). Trehalose accumulation in wheat plant promotes sucrose and starch biosynthesis. Jordan J Biol Sci. 6, 143–150
- Alam, M.M., Hasanuzzaman, M. Nahar, K. Fujita, M., 2013. Exogenous salicylic acid ameliorates short-term drought stress in mustard (Brassica juncea L.) seedlings by up-regulating the antioxidant defense and glyoxalase system. Aust J Crop Sci. 7, 1053-1063.
- Alam, M.M., Nahar, K., Hasanuzzaman, M., Fujita, M., (2014). Trehalose-induced drought stress tolerance: A comparative study among different Brassica species. Plant Omics J. 7, 271-283.
- Aldesuquy, H., Ghanem, H., (2015). Exogenous salicylic acid and trehalose ameliorate short term drought stress in wheat cultivars by upregulating membrane characteristics and antioxidant defense system. J. Hortic. 2, 2–10.
- Ali, Q. (2011). Exogenous use of some potential organic osmolytes in enhancing drought tolerance in maize (Zea mays L.). http://core.kmi.open.ac.uk/display/12115108.
- Ali, Q., Ashraf, M., (2011). Induction of drought tolerance in maize (Zea mays L.) due to exogenous application of trehalose: growth, photosynthesis, water relations and oxidative defense mechanism. J. of Agronomy and Crop Sci. 197, 258-271.
- Bae, H., Herman, E. Sicher, R., (2005). Exogenous trehalose promotes non-structural carbohydrate accumulation and induced chemical detoxification and stress response proteins in Arabidopsis thaliana grown in liquid culture. Plant Sci. 168, 1293-1301.
- Balla, K., Rakszegi, M., Li, Z., Bakes, F., Bencze, S., Veisz, O., (2011). Quality of water winter wheat in relation to heat and drought shock after anthesis. Czech J. Food Sci. 29 (2), 117–128.
- Bates, L. S., Waldren, R. P., Teare, I. D., (1973). Rapid determination of free proline for water-stress studies. Plant and Soil. 39, 205-207.
- Bergmeyer, H. U., Bernt, E. M. (1974). “Methods of Enzymatic Analysis”. Bergmeyer HU. (Ed) New York, Academic Press, 2nd Edition: 1205-1212
- Candan, N. and Tarhan, L. (2003). The correlation between antioxidant enzyme activities and lipid peroxidation levels in Mentha pulegium organs grown in Ca2+, Mg2+, Cu2+, Zn2+ and Mn2+ stress conditions. Plant Science. 163: 769-779.
- Chang, B., Yang, L., Cong, W., Zu, Y., Tang, Z. (2014). The improved resistance to high salinity induced by trehalose isassociated with ionic regulation andosmotic adjustment in Catharanthus roseus. Plant Physiol. Biochem. 77, 140–148
- Cižmárik, J., Hrobonová, K., Lehotay, J., (2004). Determination of monosaccharides and disaccharides in honey by ion-exchange high performance chromatography. Acata Facultatis Pharmaceuticae Universitatis Comeniavae. 51, 73-78
- Dhindsa, A. R. S., Matowe, W., (1981). Drought tolerance in two mosses: correlated with enzymatic defense against lipid peroxidation. J. Exp. Bot. 32, 79-91.
- Duman, F., Aksoy, A., Aydin, Z., Temizgul, R., (2011). Effects of exogenous glycinebetaine and trehalose on cadmium accumulation and biological responses of an aquatic plant (Lemna gibba L.). Water, Air and Soil Pollution. 217, 545-556.
- Elbein, A. D., Pan, Y. T., Pastuszak, I., Carroll, D. (2003). New insights on trehalose: a multifunctional molecule. Glycobiology, 13, 17R-27R.
- Farooq, M., Wahid, A., Lee, D. J., Cheema, S. A., Aziz, T., (2010). Comparative time course action of the foliar applied glycinebetaine, salicylic acid, nitrous oxide, brassinosteroids and spermine in improving drought resistance of rice. J. Agron. Crop Sci. 196, 336– 345.
- Fernandez, J. M. G., Mellet, C. O., Blanco, J. L. J., Mota, J. F., Gadelle, A., Sarguet, A.C., Defaye, J., (2010). Isothiocyanates and cyclic thiocarbamates of α,α′- trehalose, sucrose, and cyclo malto oligosaccharides. Carbohydrate research, 268(1), 57-71.
- Ferreira, J. C., Paschoalin, V. M. F., Panek, A. D. and Trugo, L. C (1997). Comparison of three different methods for trehalose determination in yeast extract. Food Chem. 60: 251- 254
- Gancedo, C., Flores, C. L. (2004). The importance of a functional trehalose biosynthetic pathway for the life of yeasts and fungi. FEMS Yeast Res. 4, 351–359
- Garg, A. K., Kim, J. K., Owens, T. G., Ranwala, A. P., Do Choi, Y., Kochian, L.V., Wu, R. J., (2002). Trehalose accumulation in rice plants confers high tolerance levels to different abiotic stresses. Proceedings of National Academy of Sci. 99, 15898-15903.
- Garg, N., Manchanda, G., (2009). ROS generation in plants: boon or bane? Plant Biosyst. 143, 8–96.
- Gilley, A., Fletcher, R. A. (1997). Relative efficacy of paclobutrazol, propiconazole and tetraconazole as stress protectants in wheat seedlings. J. of Plant Growth Regul. 21, 169-175.
- Glassop, D., Roessner, U., Bacic, A., Bonnett, G.D., (2007). Changes in the Sugarcane metabolome with stem development: are they related to sucrose accumulation? Plant Cell Physiol. 48, 573–584
- Grotelueschen, R. D. and Smith, D. (1967). Determination and identification of nonstructural carbohydrates removed from grass and legume tissue by various sulfuric acid concentrations, takadiastase and water, J. Agr. Food Chem. 15; 1048-1051
- Gupta, A. K., Kaur, N., (2005). Sugar signalling and gene expression in relation to carbohydrate metabolism under abiotic stresses in plants. J. Biosci. 30, 761-776.
- Hernandez, J. A., Mullineaux, P., Sevilla, F. (2000). Tolerance of pea (Pisum sativum L.) to long term stress is associated with induction of antioxidant defenses. Plant cell & Environment 23: 853-862
- Ibrahim, H. A., Abdellatif, Y. M. R., (2016). Effect of maltose and trehalose on growth, yield and some physiological components of wheat plant under water stress. Annal of Agricultural Science, 61(2), 267-274.
- Ilhan, S., Ozdemir, F., Bor, M. (2014). Contribution of trehalose biosynthetic pathway to drought stress tolerance of Capparis ovata Desf. Plant Biol. 17, 402–407
- Jones, M. M., Turner, N. C., (1978). Osmotic adjustment in leaves of sorghum in response to water deficit. Plant Physiol. 61, 122–126.
- Li, Z. G., Luo, L. J., Zhu, L. P., (2014). Involvement of trehalose in hydrogen sulphide donor sodium hydrosulfide-induced the acquisition of heat tolerance in maize (Zea mays L.) seedlings. Botanical Studies. 55, 20-31.
- Liu, Y. L., Guo, K., Fan, D., Li, G., (2011). Effect of drought on pigments, osmotic adjustment and antioxidant enzymes in six woody plant species in Karst habitats of southwestern China. J. Environ. Exp. Bot. 71, 174–183.
- Lowry, O. H., Rosenbrugh, N. J., Farr, A. L. Randall, R. J., (1951). Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193, 265-275.
- Luo, Y., Li, F., Wang, G. P., Yang, X. H. and Wang, W., (2010). Exogenously-supplied trehalose protects thylakoid membranes of winter wheat from heat-induced damage. Biol. Plant. 54, 495–501.
- Ma, C., Wang, Z., Kong, B., Lin, T. (2013). Exogenous trehalose differentially modulate antioxidant defense system in wheat callus during water deficit and subsequent recovery. Plant Growth Regul. 70, 275–285.
- Metzner, H., Rau, H., Senger, H., (1965). Untersuchungen Zur Synchronisierbarkeit einzelner pigment-mangel mutanten von chlorella. Planta. 65, 186-194.
- Mostofa, M. G., Hossain, M. A., Fujita, M., Tran, L. P. (2005). Physiological and biochemical mechanisms associated with trehalose-induced copper-stress tolerance in rice. Scientific reports, 5, 11433..
- Nahar, K., Hasanuzzaman, M., Alam, M. M., Fujita, M. (2013). Exogenous glutathione-induced drought stress tolerance in Vigna radiate seedlings through enhanced antioxidant defense and methylglyoxal system. Interdrought IV Conference September 02- September 09, 2013, Perth, Australia
- Nakano, Y., Asada, K., (1980). Spinach chloroplasts scavenge hydrogen peroxide on illumination. Plant Cell Physiol. 21, 1295-1307.
- Nounjan, N., Nghia, P. T., Theerakulpisut, P., (2012). Exogenous proline and trehalose promote recovery of rice seedlings from salt-stress and differentially modulate antioxidant enzymes and expression of related genes. J. Plant Physiol. 169, 596-604
- Quan, R., Shang, M., Zhang, H., Zhao, Y., Zhang, J. (2004). Engineering of enhanced glycine betaine synthesis improves drought tolerance in maize. Plant Biotechnol. J. 2: 477- 486
- Ranwala, A. P., Miller, W. B., (2009). Comparison of the dynamics of non-structural carbohydrate pools in cut tulip stems supplied with sucrose or trehalose. Postharvest Biology and Technol. 52, 91–96.
- Rapacz, M., Kos´cielniak, J., Jurczyk, B., Adamska,A., Wo´ jcik, M., (2010). Different patterns of physiological and molecular response to drought in seedlings of malt- and feed-type barleys (Hordeum vulgare). J. Agron. Crop Sci. 196, 9–19.
- Ranwala, A. P., Miller, W. B., (2009). Comparison of the dynamics of non-structural carbohydrate pools in cut tulip stems supplied with sucrose or trehalose. Postharvest Biology and Technol. 52, 91–96.
- Rauf, M., Munir, M., Hassan, M., Ahmad, M., Afzal, M., (2007). Performance of wheat genotypes under osmotic stress at germination and early seedling growth stage. Afr J Agric Res. 6, 971-975.
- Sorial, M. E., El-Gamal, S. M., Gendy, A. A. (2010). Response of sweet basil to jasmonic acid application in relation to different water supplies. Biosci Res. 7, 39-47.
- Stolker, R. (2010). Combating abiotic stress using trehalose. M.Sc. Thesis, Wageningen University and Research Centre.
- Valentovic, P., Luxova, M., Kolarovic, L., Gasparikova, O. (2006). Effect of osmotic stress on compatible solutes content, membrane stability and water relation in two maize cultivars. Plant Soil & Environment 52: 186-191
- Velikova, V., Yordanov, I., Edreva, A., (2000). Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective roles of exogenous polyamines. Plant Sci. 151, 59-66.
- Wingler, A., Fritzius, T., Wiemken, A., Boller, T. and Aeschbacher, R.A. (2000). Trehalose induces the ADP-glucosepyrophosphorylase gene APL3, and starch synthesis in Arabidopsis. Plant Physiol. 124, 105-114.
- Zeid, I. M., (2009). Trehalose as osmoprotectant for maize under salinity-induced stress. Research J. of Agri. and Biol. Sci. 5, 613-622.
- Zhang, L. X., Li, S. X., Zhang, H., Liang, Z. S., (2007). Nitrogen rates and water stress effects on production, lipid peroxidation and antioxidative enzyme activities in two maize (Zea mays L.) genotypes. J. Agron. Crop Sci. 193, 387–397.
- Zhu, J. K., (2002) Salt and drought stress signal transduction in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 53, 247– 273.


