Effect of heavy metal ions and carbohydrates on the activity of cauliflower ( Brassica oleracea var. botrytis) myrosinase
Автор: Prakash Om, Rai Ajeet Kumar, Singh Jagdish, Singh P.M.
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 2 т.9, 2013 года.
Бесплатный доступ
Myrosinase is an enzyme of cruciferous vegetables, hydrolyse glucosinolates. The breakdown products are involved in plant defence against insect and also have anti-fungal property. Myrosinase has been purified to apparent homogeneity from 5 days old germinated cauliflower seedlings having a specific activity of 12.71 units/mg proteins with 54.6 % recovery, using ammonium sulfate fractionation followed by gel filtration chromatography on Sephadex G-100. Effect of some metal ions and carbohydrates on the activity of partially purified cauliflower myrosinase was studied. Sr +2 at 4 mM concentration exhibited marked activating effect on the activity up to 2.7 fold while Fe +2 significantly inhibited. However, Sn +2 and Ba +2 increased the activity to a certain extent and then suppressed. On the other hand, some metal ions [Fe +2, Fe +3, Cu +2 and Zn +2] strongly inhibited the activity even at lower concentrations. Several carbohydrates viz., glucose, fructose, sucrose, maltose and sorbitol even at comparatively higher concentrations had little detectable inhibitory effects. Activation kinetics of myrosinase in presence of Sn +2 and Sr +2 were studied between 0- 20min. The rate of reaction was almost constant till 15 min and then slight deactivation was recorded at various concentrations used.
Myrosinase, partial purification, metal ions, carbohydrates, cauliflower
Короткий адрес: https://sciup.org/14323728
IDR: 14323728
Текст научной статьи Effect of heavy metal ions and carbohydrates on the activity of cauliflower ( Brassica oleracea var. botrytis) myrosinase
Plant myrosinase (thioglucosidase glucohydrolase EC 3.2.3.147), is an interesting enzyme from cruciferous vegetables, catalyses hydrolysis of glucosinolate to form volatile and nonvolatile products, such as isothiocyanates, thiocyanates, oxazolindine-2-thiones, indoles and nitriles, depending on the reaction conditions and availability of the ferrous ions (Halkier and Du, 1997).
The myrosinase-glucosinolate system has numerous functions in the plant. The glucosinolate degradation products are involved in plant defence against insects and phytopathogens, in sulfur and nitrogen metabolism and growth regulation. The breakdown products not only contribute to the aroma and flavour of crucifers, but have also been shown to display diverse and interesting biological properties such as hepatotoxic or goitrogenic, antibacterial, anti-fungal, and/or anti-carcinogenic activities (Zhang and Talalay, 1994; Lewis and Fenwick, 1987; Fahey et al., 1997). Myrosinase and the glucosinolates are physically separated from each other in cells, and thus hydrolysis can only occur when cells are disrupted by the processes as mastication (Verkerk et al., 2001; Buchwaldt et al., 1986 ; Lönnerdal and Janson, 1973). Myrosinase has been purified from several sources, including white mustard (Sinapis alba) by Bjorkman and Janson (1972), cress (Lepidium sativum) by Durham and Poulton (1989), yellow mustard (Brassica juncea) by Ohtsuru and Hata, (1972) and rape seed (Brassica napus) by Lönnerdal and Janson (1973). The role of myrosinase mediated breakdown products of glucosinolates in reducing various types of cancers in human and animal cell models have been widely studied by Conaway and associates (2002) Talalay and Zhang, 1996; Zhang and Talalay, 1998; Gamet-Payrastre et al., 2000). In addition, some metal ions have been shown to regulate the formation of sulphoraphane from glucosinolates in broccoli (Liang et al., 2006). With these in view, present study was undertaken to explore the possible direct effects of some heavy metal ions and carbohydrates on the activity of partially purified myrosinase from cauliflower seedlings.
MATERIALS AND METHODS
Chemicals and materials
Sinigrin monohydrate and BSA were purchased from Sigma- Aldrich Corporation, USA. Sephadex G-100 was from Pharmacia Fine Chemicals, Uppsala, Sweden. All other metal chloride and carbohydrates were of Analytical Grade either from HiMedia or Sisco Research Laboratories, India. All solutions were prepared using quartz double distilled water. Seed of brassica vegetables was obtained from Seed Production Division, Indian Institute of Vegetable Research (IIVR) Varanasi, India.
Purification of myrosinaseSample Preparation
The seeds (5 gm.) were surface-sterilized by rinsing in 70% ethanol and after that the seeds were kept for 5-10 minutes in 1.0% sodium hypochlorite followed by exhaustive rinsing with sterile distilled water. These seeds were plated on moist and sterile paper towels in petri plates at a density of about 10 seeds/cm2 and allowed to grow at 25°C under white fluorescent light on a 16:8 h light/dark cycle. After 5 days, sprouts were gently collected from the surface of the paper towels and immediately homogenized in 25 ml of potassium phosphate buffer (20mM, pH 7.4). The crude extract thus obtained was filtered through properly washed and pre-chilled muslin cloth and was centrifuged at 15000g, for 15 min at 0-4oC to remove the insoluble materials.
Enzyme and protein assay
Myrosinase activity was determined by measuring the rate of decrease in absorbance at 227 nm resulting from the hydrolysis of sinigrin as per method described by Palmieri and associates (1982). Protein concentrations were determined by the method of Lowry and associates (1951) with BSA as a standard. Specific activities are expressed as units/mg of protein.
Ammonium Sulphate fractionation
The crude myrosinase was precipitated with fine crushed solid ammonium sulphate. The supernatant was saturated up to 60% with (NH 4 ) 2 SO 4 under continuous stirring and maintaining the pH 7.4 by adding 0.1M KOH drop by drop at 4oC. The suspension was centrifuged at 15000 g for 15 min. at 0-4oC. The pellet thus obtained was dissolved in extraction buffer (20mM, pH 7.4). The final volume was made up to 10.5 ml. This fraction was extensively dialyzed using thoroughly washed and pre activated dialysis tube with continuous stirring for 24 hours at 0-4 ° C against the same buffer.
Gel filtration chromatography
The dialyzed extract was further purified using gel filtration chromatography (Sephadex G-100). The Sephadex beads were activated using extraction buffer for 72 hours with washing from time to time. Activated beads were poured slowly in a glass chromatography column with the help of a glass rod to avoid trapping of air bubbles. The sample obtained from previous step was applied to the pre-equilibrated column. Eluents were collected in different fraction tubes of 1ml. The fractions containing myrosinase activity were pooled and concentrated against solid sucrose. The concentrated sample was diluted with extraction buffer according to need. The above sample was stored in a refrigerator at 0-4oC and used throughout this study.
Analytical electrophoresis
Slab gel electrophoresis was carried out at 25oC in a mini protein electrophoresis apparatus (BioRad, Hercules, CA, USA). 8% Native PAGE was carried out according to the method of Laemmli (1970). The bands were visualised by Coomasie Brilliant blue R-250 and in order to check the purity of the enzyme preparation, activity staining was done by the method of Lönnerdal and Janson (1973).
Effect of metal ions and carbohydrates on myrosinase activity
A stock solution of each of the desired reagent was prepared in double distilled water. The residual myrosinase activity of suitably diluted enzyme was determined in the presence of varying concentration of these reagents added in the standard assay mixture.
RESULTS AND DISCUSSION
Partial purification of enzymes:
7.3 fold (specific activity 26.0 units/mg protein) with 87% yield.
Effect of metal ions on cauliflower myrosinase activity:
Various metal ions such as Fe+3, Zn+2, Sn+2, Sr+2, Ba+2 , Cu+2 and Fe+2 at various concentration ranging from (1 to 40mM) were tested for activation/ inhibition effects. Sn+2, Sr+2 and Ba+2 had strong activation effects on myrosinase activity (Fig. 2). Among these three metal chlorides Sr+2 had a strongest activation effect and activity continuously increases from 0-4mM and maximum activity was recorded at 4mM (nearly 2.7 times of the control) afterward activity decreases slowly and the enzyme became completely inactive at 40mM . Tani and associates (1974) however, reported that Sr+2 ions were an inhibitor of bacterial myrosinase. This difference in the behaviour might be due to the structural difference in the myrosinase. Plant myrosinase had a rather large molecular weight as compared to the bacterial enzyme. Similarly, stannous ions had maximum activity (1.67 times of control) at 2mM concentration and beyond which there was a decrease in the activity and no activity was recorded at 6mM onwards. Similar observations of minor activation were also reported in bacterial myrosinase by Tani and associates (1974). Ba+2 showed a little activation of enzyme and maximum activation was recorded at 10mM.
A concentration dependent and time dependent inhibition in the enzyme activity was observed in case of Fe+3, Fe+2, Zn+2 and Cu+2 (observed from 140mM, Fig. 3). Fe+2 made a complete loss in the activity at 1mM while Fe+3 showed 50% residual activity as that of control at 1 mM and there was zero activity at 4 mM. Fig.3 indicated that ferrous and ferric ions were strong inhibitor of cauliflower myrosinase. In earlier study Jwanny and associates
(1995) reported the similar finding with Fe+2 at 1mM concentration. A similar trend with respect to concentration dependence was observed in case of Cu2+ where more than 2/3rd activity was lost at 1mM and only 8% of maximum activity (maximum in control) was remained at 2mM and no activity was recorded at 4mM. Among Fe+3, Fe+2, Zn+2 and Cu+2, Zinc ion had a mild inhibitory effect on myrosinase and had complete loss in activity at 6mM (Data not shown). The enzyme forms a dimer stabilized by a Zn2+ ion and is heavily glycosylated by Burmeister and associates (1997). Similar finding was also reported by Jwanny and associates (1995) and Tani and associates (1974). The inhibitory effect may be due to the blocking of the binding site of the substrate or instability of enzyme- substrate complex.
Liang and associates (2006) also reported inhibitory effect of ferrous and ferric ion on Myrosinase activity. Moreover, the inhibition by ferrous ion in the formation of sulforaphane was higher than that of ferric ion. Similar finding was also recorded with Cu2+, copper ion not only influenced the aglycone rearrangement but also inhibited the enzyme activity.
Effect of carbohydrates on myrosinase:
The effect of various carbohydrates such as glucose, fructose sucrose, maltose and sorbitol was studied in the concentrations range of 5 to 50mM (Fig. 4). The concentration of these sugars exhibiting detectable inhibition in cauliflower myrosinase was found to be 20mM suggesting that all these carbohydrates were weak inhibitor of Myrosinase. Amongst these carbohydrates, glucose was a strongest inhibitor and > 50% activity was lost at 50mM concentration while sucrose had a weakest inhibition. The trend of inhibition was recorded as glucose > fructose > sorbitol > maltose > sucrose at
50mM (Data not shown). Tani and associates (1974) also reported the inhibitory effect of various carbohydrates at 100mM. Shikita and associates (1999) reported that glucose was a weak inhibitor of myrosinase; it may be due to the binding of carbohydrates to the products.
Time –course of myrosinase action in presence of Sn+2:
Time-course of effect of Sr+2 on the activity of the myrosinase:
As described earlier (Fig. 2), Sr2+ in the concentration range of 1-20 mM had activation effect on myrosinase, and the activation was significant with 4 mM Sr2+. In order to explore the time-course of activation, three concentrations viz., 1,2 and 4mM, were selected and studied for 20 min. Fig. 6 indicated that at 4mM concentration of Sr2+, the rate of reaction was better than lower concentrations. Time dependent rate of reaction was recorded at these concentrations. Nearly till 15 min the rate of reaction was constant and further, little decrease in activity was detected (remains 80% of maximum) till 20 min.
The results reported above are suggestive of the fact that heavy metal ions at suitable concentrations are probably stabilizing the enzyme such that the myrosinase continues to act on its substrate for longer duration of time, without significant decrease in the activity.
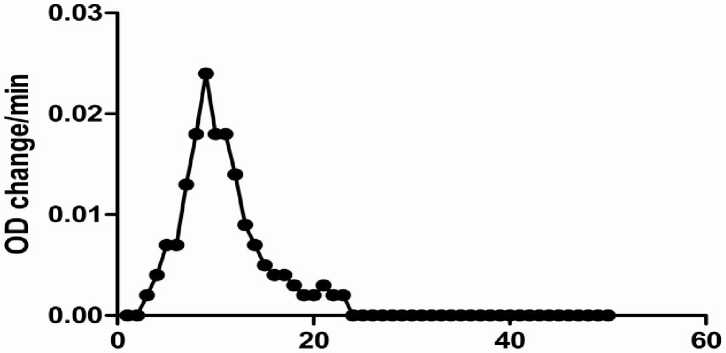
Elution Volume
Figure 1: Purification profile in cauliflower myrosinase in Sephadex G-100 gel filtration chromatography.
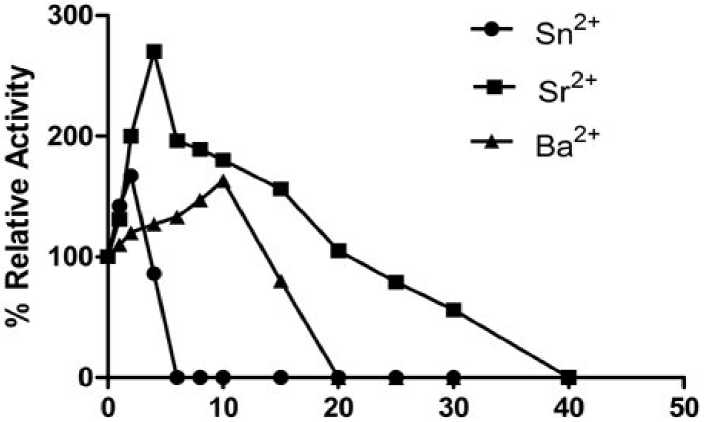
Metal ion conc.(mM)
Figure 2: Effects of metal ions on the activity of myrosinase of cauliflower seedling.
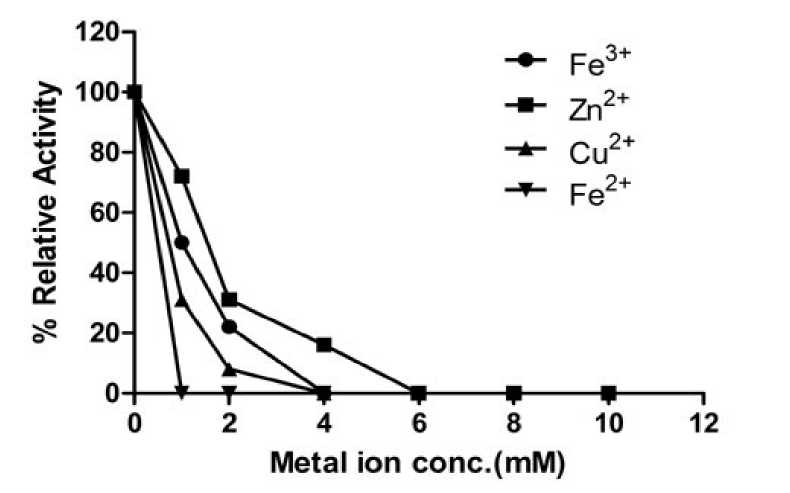
Figure 3: Effects of metal ions on the activity of myrosinase of cauliflower seedling.
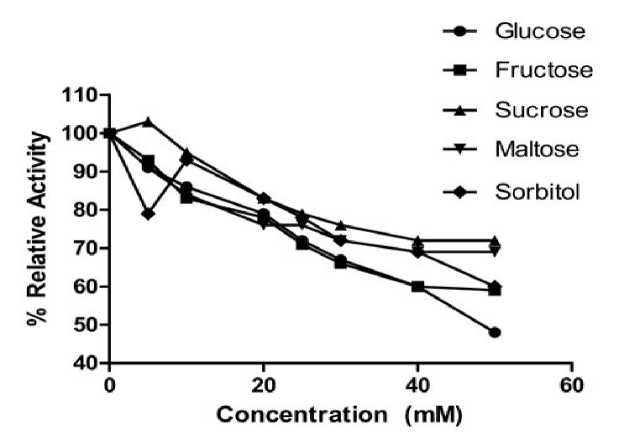
Figure 4: Effects of carbohydrates on the activity of myrosinase of cauliflower seedling.
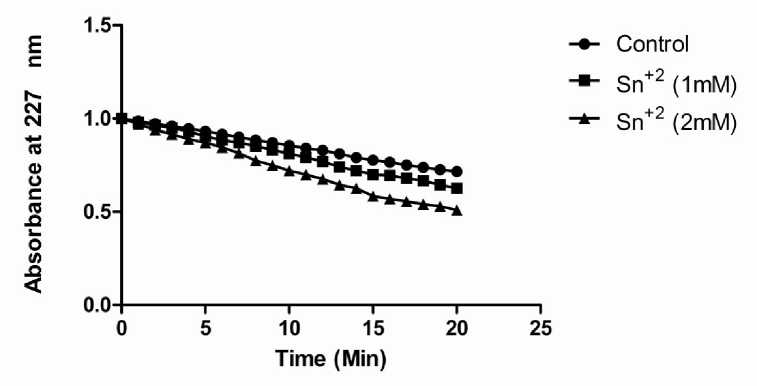
Figure 5: Rate of reaction of myrosinase at different concentration of Sn+2 with control at different time interval.
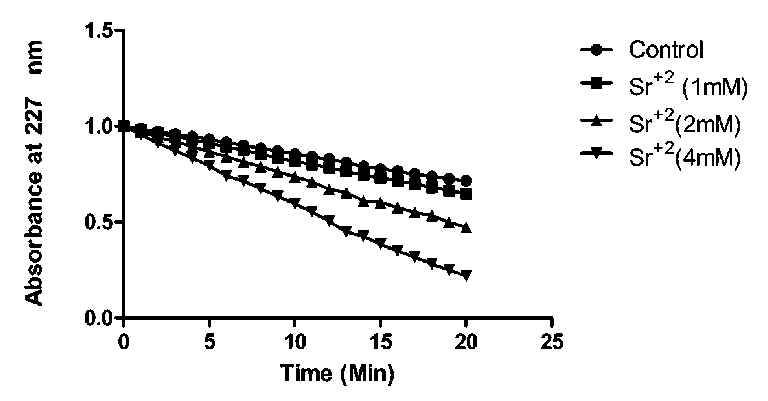
Figure 6: Rate of reaction of myrosinase at different concentration of Sr+2 with control at different time interval.
Table 1 . Purification of myrosinase from Cauliflower Seedling.
|
Sample |
Volume (ml) |
Total activity (unit) |
Total Protein (mg) |
Specific Activity (U/mg) |
Purification fold |
Yield (%) |
|
Crude |
22.5 |
12.09 |
9.54 |
1.26 |
- |
- |
|
0-60% Amm. Sulphate |
10.5 |
8.31 |
3.612 |
2.3 |
1.65 |
62.6 |
|
Dialysis |
9.0 |
8.22 |
3.50 |
2.34 |
1.66 |
62.0 |
|
Sephadex G-100 |
3.0 |
7.25 |
0.57 |
12.71 |
10.08 |
54.6 |
|
CD(5%) |
0.597 |
0.203 |
0.133 |
0.115 |
1.32 |
CONCLUSION
ACKNOWLEDGEMENTS
The author Ajeet Kumar Rai would like to acknowledge the support of Banaras Hindu University,Varanasi and Indian Institute of Vegetable Research, Varanasi.
Список литературы Effect of heavy metal ions and carbohydrates on the activity of cauliflower ( Brassica oleracea var. botrytis) myrosinase
- Bjorkman, R. and Janson, J.C. (1972) Studies on myrosinases 1. Purification and characterization of a myrosinase from white mustard seed (Sinapis alba, L.). Biochim Biophys Acta 276(2): 508-518.
- Buchwaldt, L., Larsen, L.M. and Ploger, A. (1986) Fast polymer liquid-chromatography isolation and characterization of plant myrosinase, beta-thioglucoside glucohydrolase, isoenzymes, J Chromatogr 363. 71-80.
- Burmeister, W., Cottaz, S., Driguez, H., Iori, R., Palmieri, S. and Henrissat, B.(1997) The crystal structures of Sinapis alba myrosinase and a covalent glycosyl-enzyme intermediate provide insights into the substrate recognition and active-site machinery of an S-glycosidase. Structure, 5, 663-675.
- Chiao, J.W., Chung, F.L., Kancherla, R., Ahmed, T., Mittleman, A. and Conaway, C.C., (2002) Sulforaphane and its metabolite mediate growth arrest and apoptosis in human prostrate cancer cells, Int. J. Oncol. 20, 631-636.
- Conaway, C. C., Yang, Y. M. and Chung, F. L. (2002) Isothiocyanates as cancer chemo-preventive agents: their biological activities and metabolism in rodents and humans. Curr. Drug Metab. 3, 233-255.
- Durham, P.L. and Poulton, J.E. (1989) Enzymic properties of purified myrosinase from Lepidium sativum seedlings, Z. Naturforsch. 45, 173-178.
- Fahey, J.W., Zalcmann, A.T. and Talalay, P. (1997) The chemical diversity and distribution of glucosinolates and isothiocyanates among plants, Phytochemistry 56, 5-51.
- Gamet-Payrastre, L., Lumeau, P.Li.S., Cassar, G., Dupont, M., Chevolleau, S., Gase, N., Tulliez, J.and Terce, F. (2000) Sulforaphane, a Naturally Occurring Isothiocyanate, Induces Cell Cycle Arrest and Apoptosis in HT29 Human Colon Cancer Cells, Cancer Res., 60, 1426-1433
- Halkier, B.A. and Du, L.C. (1997) The biosynthesis of glucosinolates, Trends Plant Sci. 2, 425-431.
- Jwanny, E.W., El-Sayed, S.T., Rashad, M.M., Mahmoud, A.E. and Abdallah, N.M. (1995) Myrosinase from roots of Raphanus sativus, Phytochemistry 39, 1301-1303.
- Laemmli, U.K. (1970) Cleavage of structural protein during the assembly of head of bacteriophage T4. Nature 227, 680-685.
- Lewis, J. and Fenwick, G.R. (1987) Glucosinolate content of Brassica vegetables: Analysis of twenty-four cultivars of calabres, Food Chemistry 25, 259-268.
- Liang, H., Yuan, Q. and Xiao, Q. (2006) Effect of some metal ions on myrosinase activity and the formation of sulforaphane in broccoli seed. Journal of molecular catalysis B: Enzymatic 43, 19-22.
- Lönnerdal, B. and Janson, J.C. (1973) Studies on myrosinases. II. Purification and characterization of a myrosinase from rapeseed (Brassica napus L.), Biochim. Biophys. Acta 315, 421-429.
- Lowry, O.H., Roserbrough, N.J., Farr, A.L. and Randall, R.J. (1951) Protein measurement with folin phenol reagent. J. Biol. Chem. 193, 265-275.
- Ohtsuru, M. and Hata, T. (1972) Molecular properties of multiple forms of plant myrosinase, Agric. Biol. Chem. 36, 2495-2503.
- Palmieri, S., leoni, O. and Iori, R. (1982) A steady-state study of myrosinase with direct ultraviolet spectrophotometric assay. Anal. Biochem. 123, 320-324.
- Rouzaud, G., Rabot, S., Ratcliffe, B. and Duncan, A.J. (2003) Influence of plant and bacterial myrosinase activity on the metabolic fate of glucosinolate in gnotobiotic rats, British Journal Nutrition 90, 395-404.
- Shikita, M., Fahay, J.W., Golden, T.R., Holtzclaw, W.D. and Talalay, P. (1999) An unusual case of uncompetitive activation by ascorbic acid: purification and kinetic properties of a myrosinase from Raphanus sativus seedlings. Biochem. J. 341, 725-732.
- Talalay P, Zhang Y. (1996) Chemoprotection against cancer by isothiocyanates and glucosinolates. Biochem Soc Trans 24(3): 806-810.
- Tani, N., Ohtsuru, M. and Hata, T. (1974) Isolation of myrosinase producing microorganism. Agr. Biol. Chem. 38: 1617-1622.
- Verkerk, R., Dekker, M. and Jongen, W.M.F. (2001) Post-harvest increase of indolyl glucosinolates in response to chopping and storage of Brassica vegetables, J. Sci. Food Agric. 81, 953-958.
- Xian, Li. and Kushad, M. (2005) Purification and characterization of myrosinase from horseradish (Armoracia rusticana) roots, Plant Physiology Biochemistry. 43, 503-511.
- Zhang, Y. and Talalay, P. (1994) Anticarcinogenic activities of organic isothiocyanates: chemistry and mechanisms. Cancer Res. (Suppl.) 54, 1976s-1981s.
- Zhang, Y. and Talalay, P. (1998) Mechanism of Differential Potencies of Isothiocyanates as Inducers of Anticarcinogenic Phase 2 Enzymes Cancer Res., 58, 4632 -4639
- Zhang, Y., Kensler, T.W., Cho, C.G., Posner G.H. and Talalay, P.(1994). Anticarcinogenic activities of sulforaphane and structurally related synthetic norbornyl isothiocyanates. Proc. Natl. Acad. Sci. U.S.A., 91(8): 3147-3150.


