Effect of leaf cold damage after chilling temperature treatment on growth and reproductive parameters of Chilli pepper plants
Автор: Rajametov Sherzod Nigmatullayevich, Yang Eun-Young, Cho Myeong-Cheoul, Jeong Hyo-Bong
Журнал: Овощи России @vegetables
Рубрика: Селекция и семеноводство сельскохозяйственных растений
Статья в выпуске: 1 (63), 2022 года.
Бесплатный доступ
Relevance. Pepper is sensitive to chilling temperatures in all growth stages and low temperatures are main factors affecting plant growth, fruit growth and development and productivity. Evaluation and identification low temperature (LT) tolerant pepper genotypes at different growth stages is actual in breeding program for developing new cultivars. In present study we investigated the effect of the leaf cold damage within 25% (LCD) after chilling treatment in seedling stage on vegetative and reproductive traits of pepper accessions with different cold tolerance. Material and methods. In this study two pepper accessions “PE-J-2” and “Neokgwang” selected as chilling tolerant and susceptible in juvenile stage (3-4 true leaf stage), were used respectively. The seedlings of the selected pepper accessions with 25% visual cold damages of leaf green part (become lightly yellowed-whited or desiccated-dried) and control non-treated (NT) were grown in a glasshouse condition (D/N 30-32/22-24°C) for 10 weeks to evaluate the effect of LCD on pepper accessions vegetative and reproductive parameters after chilling pre-treatment. In pepper plants the vegetative parameters such as plant height (PH), leaf length (Ll) and width (LW), number of internodes (NI), length of main axis (LMA), plants fresh weight (PFW) and roots fresh weight (RFW), and reproductive the number of flowers (NFL) and fruits (Nfr), fruit set ratio (FS), fruit length (FL) and diameter (FD), total yield per plant (TY) were measured. The experimental design of this study was completely randomized. Statistical analysis was performed using the SAS Enterprise Guide 7.1 (SAS Institute Inc., Cary, North Carolina, USA). Results. According to the research result several accessions were identified: the accessions screened in juvenile stage as cold tolerant cannot always manifest good some agronomical traits performance at growth stages and it may range depending on the genotype specific features. The seedlings with LCD within 25% may significantly affect the vegetative and reproductive parameters of pepper plants. The phenomenon was recorded more distinctive in the correlations between some vegetative and reproductive parameters among NT and LCD plants.
Pepper, temperature, seedling, plant, root, leaf, flower, fruit set, fruit, correlation
Короткий адрес: https://sciup.org/140290727
IDR: 140290727 | УДК: 633.842:581.16.043 | DOI: 10.18619/2072-9146-2022-1-5-11
Текст научной статьи Effect of leaf cold damage after chilling temperature treatment on growth and reproductive parameters of Chilli pepper plants
emperature is one of the main environmental factors affecting vegetative and reproductive growth of plants [1-3]. Low temperature stress is a major environmental factor in temperate zone and limits plant growth and productivity [4,5], and plants have different abilities to deal with low temperatures, where LT significantly exhibits reduction in photosynthesis, delays the formation of leaf, truss appearance, number of flowers, fruit set and fruit growth [2, 6-9].
Under such conditions, plants try to maintain homeostasis to acquire freezing tolerance and this involves extensive reprogramming of gene expression and metabolism [10-12], and the changes in physiological and biochemical processes under LT conditions are related to the transcript levels of a number of cold-regulated genes [13-15]. Although several hypotheses have been proposed to explain tolerance or sensitivity to chilling in tomato plants, where the physiological mechanisms responsible for cold tolerance remain unclear [16].
LT stress in plants can be divided into two categories [15]: chilling stress (0–20°C) and freezing stress (<0°C). Chillingsensitive plants such as vegetable crops pepper and tomato may suffer from cold injury and reduce productivity within temperature 0–15°C [17,18].
The plants may develop several mechanisms to minimize potential damage during LT stress and subsequent recovery periods, where the plants response is a highly complex process that involves physiological and biochemical modifications [9,19].
However, in literature scares information is available on the influence of the degree of cold damages on the agronomical traits at recovery among pepper exposed to LT in seedlings stage, which makes it difficult to assess cold tolerance potential of each genotype. Recently, multiple methods to screen and understand the mechanism for cold-tolerant genotypes among the Solanaseous crops were validated at different growth stages under low temperature stress condition [18,2024]. Moreover, several methods to screen cold tolerant genotypes among the tomato and pepper crops have been developed in early growth stages [11,13,19,25,26].
Meanwhile, recently the findings in evaluation of the tomato genotypes heat tolerance in juvenile stage revealed that the heat tolerance rate in the seedling stage might not always be associated with reproductive parameters [27]. Therefore, in present study our target was to evaluate the effect of pre-cold treatment on pepper plants agronomical traits distinguished by tolerance rates to low temperature and identifies the any linkage of cold damage in juvenile stage with subsequent flowering and reproductive growth periods. It is a continuation of our research work which has been recently done on identification of the early screening methods of pepper ( Capsicum L.) seedlings tolerance to low temperature [28].
Materials and MethodsPlant materials and growth condition
Twenty-one pepper accessions seedlings were sown in 31 st January of 2020 in plastic trays (52 x 26 cm in size, 6 x 6 cm cells with pot volume 5 liter) containing 1:1 ratio of sand and commercial bed soil (Bio Sangto, Seoul, Korea) containing coco peat (47.2%), peat moss (35%), zeolite (7%), vermiculite (10.0%), dolomite (0.6%), humectant (0.006%) and fertilizers (0.194%). A liter of water was provided to each tray daily, and the trays were placed in a glasshouse under normal condition
(26/18 °C in day/night with relative humidity within 60-70%) in National Institute of Horticultural and Herbal Science (NIHHS). Hereinafter, the seedlings 38 days old with 3-4 true leaf stage were transferred into growth chamber on 9th March 2020.
The pepper seedlings were maintained at 5°C, light intensity 800 µmol m-2s-1 (D/N 12/12h) and relatively humidity was within 65-70% and were treated under low temperature condition for 25 days [28]. All seedlings after low temperature treatment were transferred into normal condition as described above for recovery during 7 days. Then, accessions “PE-J-2” and “Neokgwang” were selected as cold tolerant and susceptible according to screening results of pepper seedlings on chilling tolerance among twenty-one accessions, where LCD in two accessions were 30.0 and 72.2% after chilling treatment, respectively.
Methods
The experiment was grouped as control (NT) and plants within 25% LCD among two accessions. To assay effects of LCD on agronomical traits the seedlings of both pepper were transplanted into pots on 6th April 2020 after 7 days of recovery. Soil in the pots were prepared as described above and regularly watered to avoid drought and fertilized weekly (Mulpure, Daeyu Co. Ltd., Gyeongsan, South Korea), and plants were cultivated for 10 weeks in a glasshouse condition (D/N 30-32/22-24°C, RH 40-60%).
Plant height (PH) was measured with interval 7 days for 70 days after transplanting (DAT). Days to flowering after transplanting of plants were measured from second internode flowers. Leaf length (LL) and width (LW), fruit length (FL) and diameter (FD) were measured from leaves located on 3rd internodes of plants, using 5 replicates. The reproductive parameters number of flowers (NFL) and fruits (NFR), fruit set ratio (FS), total yield per plant (TY) were counted from first to fourth internodes of each plant. Number of internodes (NI), length of main axis (LMA), TY and plants fresh weight (PFW) and roots fresh weight (RFW) were measured on 70 DAT.
Fruit set (FS, %) was calculated as follows:
Fruit set (%) = T---h--e----n---u--m----b---e---r---o--f---f--r-u---i-t-s------- x100
The number of flowers
Statistical analysis
The experimental design of this study was completely randomized. Statistical analysis was performed using the SAS Enterprise Guide 7.1 (SAS Institute Inc., Cary, North Carolina, USA), and mean values were compared with a significance level of 5% using Duncan’s multiple range test or the Student’s t-test at the p<0.05, p<0.01, and p<0.001 levels, respectively. Correlation coefficient analyses were done with EXCEL 2016 (Microsoft, WA, USA).
ResultsEffect of leaf cold damage on vegetative parameters
Leaf cold damage affect differently growth rate of plant height among pepper accessions (Fig. 1). So, the biggest differences (p≤0.001) in growth rate in the percentage ratio between NT and LCD plants within first week after transplanting into pots were observed. Pepper accession “PE-J-2” showed the highest index differences in plant growth than “Neokgwang” (Fig. 1A), but the index was lower in both accessions on 59.0 and 44.0% than in NT plants, respectively.
The highest intensity growth rate was determined in both accessions plants in NT within 28 and 35 DAT, then, hereinafter the intensity growth in LCD plants of “Neokgwang”
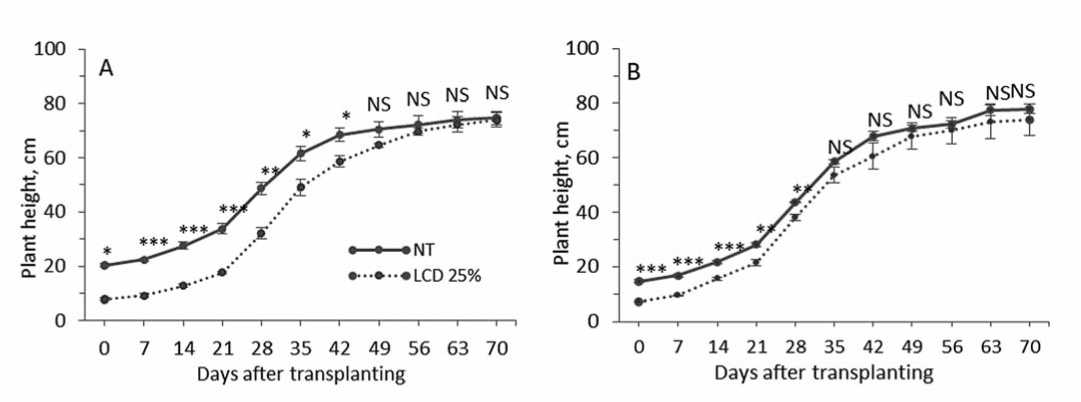
Figure 1.Effectof leaf cold damage on pepper accessions growth rate- “PE-J-2” (A) and “Neokgwang” (B).Verticalbars representSE (n=5)and significant differences were evaluated with Student’s t-test (p ≤ 0.05,p ≤ 0.01,and p ≤ 0.001) and denoted by *,**,and ***, respectively and NS indicates notsignificant
increased slightly on a par with NT plants and within 35 DAT showed no differences (Fig. 1B). On 50 DAT no significant differences in growth rate between NT and LCD plants in both accessions were identified; the index reduced in “Neokgwang” and “PE-J-2” on 4.0 and 8.3%, respectively and reached almost the same value of plants in NT condition.
The effect LCD within 25% on vegetative and reproductive traits among pepper accessions was different. So, in both accessions regardless of cold tolerance degree significant increase of the PFW in LCD plants comparedto NT were observed (Fig. 2A), while contrary result, decreasing of the LMA in LCD plants were identified (Fig. 2B). Only, in LCD plants of “PE-J-2” higher index of the LL than in NT plants were observed (Fig. 2C), whereas in
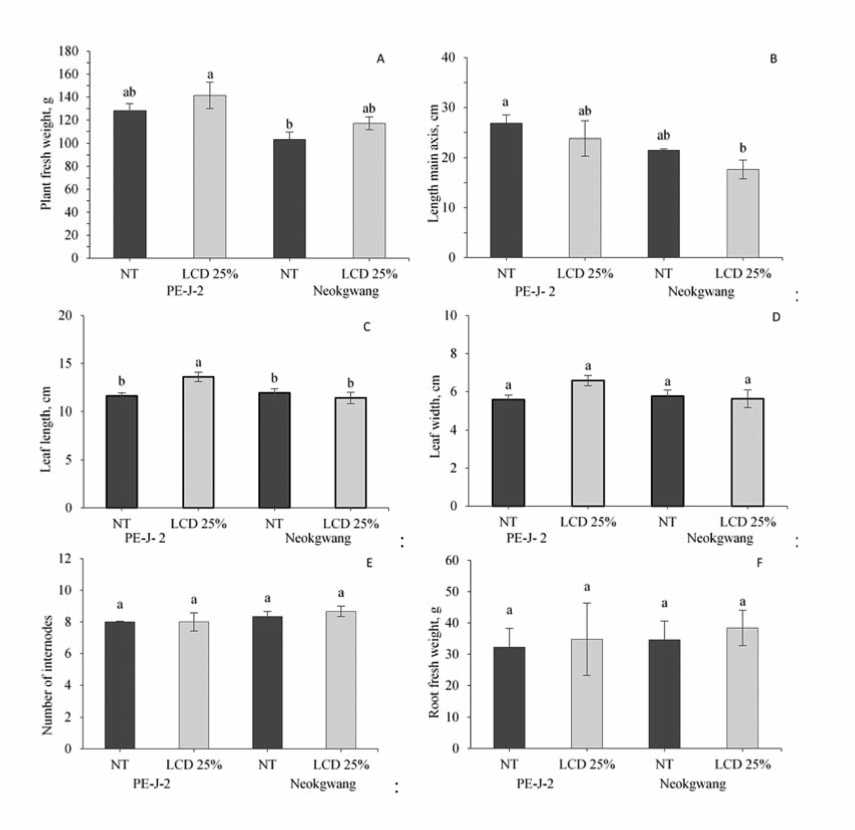
Figure 2.Effectof leaf cold damage (LCD)on pepper accessions vegetative parameters.The dataindicate the means ± SE (n=5). Differentletters above bars indicate significant difference based on Duncan’s multiple range test (p ≤ 0.05)among accessions plants from control(NT)and leaf cold damage (LCD)on 70 days aftertransplanting
measurement of the LW no identified difference between LCD and NT plants in both accessions was registered (Fig. 2D). Also, LCD 25% no critical effect on NI and RFW traits, where significant differences between NT and LCD plants in both pepper accessions were observed (Fig. 2E and F).
Effect of leaf cold damage on reproductive parameters
Days to flowering among NT and LCD plants after transplanting showed that NT plants of accessions “PE-J-2” and “Neokgwang” started to flowering within 18.3 and 25.7 DAT, respectively. Hereinafter, LCD plants started to flowering within 30.3 and 29.3 DAT, respectively, where significant difference in days were identified in “PE-J-2” about 12 days, whereas for LCD plants of “Neokgwang” needed the shortest 3.7 days to begin flowering (Table 1).
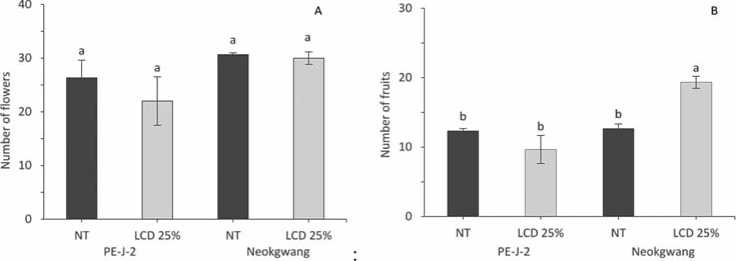
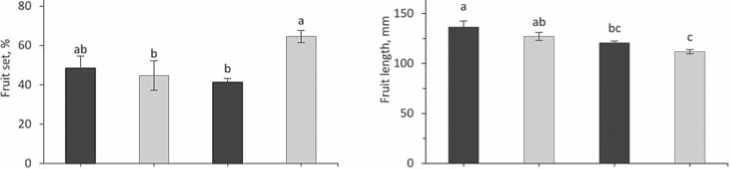
Development of flowers was not significantly ranged among NT and LCD plants of both accessions (Fig. 3A), but the quantity of fruits in “Neokwang” was significantly increased in LCD plants compared to NT (Fig. 3B), whereas in “PE-J-2” contrary results were observed, that is decreases in LCD plants. Moreover, essentially, such a pattern manifested itself in the study of fruit set (Fig. 3C). Assay of the FL showed that LCD decreased the FL in both accessions plants compared to NT (Fig. 3D), but in the measurement of the FD no difference in values between NT and LCD plants in both accessions were found.
In evaluation of the index of the total yield in NT and LCD plants of the both accessions were found that crop yield on the 70 DAT increased in LCD plants of “Neokwang”, whereas in “PE-J-2” it was decreased compared to NT plants (Fig. 3F).
Table 1. Flowering period among control (NT) and leaf cold damaged (LCD) pepper plants
Table 2. Correlation coefficients (r values) among agronomical traits from control (A) and LCD (B) plants
|
Traits |
PH |
LMA NI |
PFW |
RFW |
NFL NFR FS LL LW FL |
FD |
TY |
||||||
|
PH |
1.0 |
||||||||||||
|
LMA |
-0.488 |
1.0 |
A |
||||||||||
|
NI |
0.539 |
-0.346 |
1.0 |
||||||||||
|
PFW |
0.007 |
0.514 |
-0.194 |
1.0 |
|||||||||
|
RFW |
0.052 |
-0.395 |
0.159 |
-0.004 |
1.0 |
||||||||
|
NFL |
-0.236 |
-0.165 |
0.316 |
-0.803* |
0.273 |
1.0 |
|||||||
|
NFR |
-0.193 |
0.102 |
-0.327 |
-0.578 |
-0.734 |
0.249 |
1.0 |
||||||
|
FS |
0.175 |
0.164 |
-0.412 |
0.622 |
-0.521 |
-0.948** |
0.066 |
1.0 |
|||||
|
LL |
-0.099 |
0.016 |
0.763* |
-0.054 |
0.284 |
0.453 |
-0.441 |
-0.587 |
1.0 |
||||
|
LW |
-0.493 |
0.340 |
0.323 |
-0.203 |
-0.429 |
0.386 |
0.280 |
-0.281 |
0.600 |
1.0 |
|||
|
FL |
-0.095 |
0.646 |
-0.200 |
0.938** |
-0.325 |
-0.796* |
-0.324 |
0.702 |
-0.326 |
-0.069 |
1.0 |
||
|
FD |
-0.296 |
0.807* |
-0.232 |
0.849* |
-0.398 |
-0.623 |
-0.199 |
0.562 |
-0.216 |
-0.024 |
0.869* |
1.0 |
|
|
TY |
-0.696 |
0.867* |
-0.371 |
0.585 |
-0.365 |
-0.287 |
-0.013 |
0.282 |
0.139 |
0.504 |
0.740 |
0.884** |
1.0 |
|
PH |
1.000 |
||||||||||||
|
LMA |
-0.145 |
1.000 |
B |
||||||||||
|
NI |
0.696 |
-0.804* |
1.000 |
||||||||||
|
PFW |
-0.448 |
0.930** |
-0.934** |
1.000 |
|||||||||
|
RFW |
-0.616 |
-0.334 |
-0.116 |
-0.167 |
1.000 |
||||||||
|
NFL |
-0.106 |
0.003 |
-0.109 |
-0.128 |
0.415 |
1.000 |
|||||||
|
NFR |
-0.015 |
-0.255 |
0.168 |
-0.394 |
0.293 |
0.823* |
1.000 |
||||||
|
FS |
-0.037 |
-0.272 |
0.201 |
-0.348 |
0.017 |
0.378 |
0.823* |
1.000 |
|||||
|
LL |
-0.253 |
0.934** |
-0.804* |
0.937** |
-0.151 |
-0.183 |
-0.470 |
-0.474 |
1.000 |
||||
|
LW |
-0.140 |
0.862* |
-0.652 |
0.772* |
-0.127 |
-0.049 |
-0.135 |
-0.082 |
0.905** |
1.000 |
|||
|
FL |
-0.185 |
0.311 |
-0.342 |
0.509 |
-0.443 |
-0.790* |
-0.866* |
-0.550 |
0.468 |
0.192 |
1.000 |
||
|
FD |
-0.035 |
0.877** |
-0.654 |
0.875** |
-0.518 |
-0.412 |
-0.665 |
-0.559 |
0.572 |
0.264 |
0.703 |
1.000 |
|
|
TY |
-0.233 |
0.764* |
-0.724 |
0.688 |
-0.290 |
0.437 |
0.262 |
0.187 |
0.517 |
0.503 |
0.033 |
0.500 |
1.000 |
Significant levels for correlations: * P<0.05, **P<0.01. PH – plant height; LMA – length of main axis, NI – number of internodes, PFW- plants fresh weight, RFW – root fresh weight, NFL – number of flowers, NFR – number of fruits, FS – fruit set, LL – Leaf length, LW – leaf width, FL – fruit length, FD – fruit diameter, and TY – total yield per plant
100 с 200
NT LCD 25% NT ICO 25% NT ICO 25% NT ICO 25%
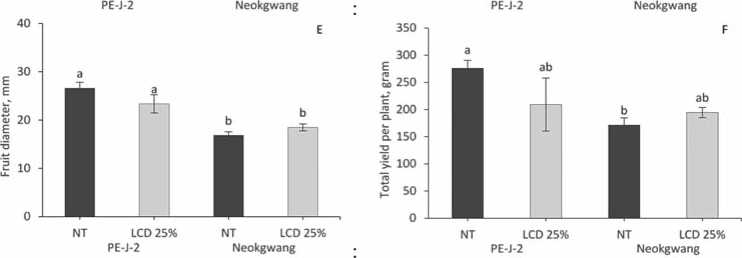
Figure 3.Effectof leaf cold damage on pepper accessions reproductive parameters.Values are means ± SE (n=5).Differentletters above bars indicate significant difference based on Duncan’s multiple range test (p ≤ 0.05) among accessions plants from control(NT and leaf cold damage (LCD)on 70 days after transplanting
Correlation between agronomical traits
The analyses of correlation coefficients among NT and LCD plants of pepper accessions (Table 2) showed that LMA had positive significant correlation with FD and TY in NT (r=0.8 07* and 0.867*, respectively) and those in LCD (r=0.877** and 0.764*, respectively). Also, PFW positively correlated with FD (r=0.849* and r=0.875**, respectively), while NFL showed negative significant correlation with FL in both NT and LCD plants (r=-0.796* and r=-0.790*). In NT condition, NI positively correlated with LL but in LCD these correlations showed somewhat different negative correlation coefficient (r=0.763* and r=-0.804*, respectively).
Discussion
Effect of LCD within 25% in seedling stage on the subsequent growth stages of agronomical traits showed that it might be critical for recovery of pepper plants and significantly affect the development of some vegetative and reproductive parameters. It is well known that, LT significantly reduces the growth rate of plants [2,8,23,24,29], which was detected in plants with LCD in the present study, where lag behind in growth rate of PH in the percentage ratio than NT plants within 50 D AT was determined. Furthermore, a decrease of value of the differences in development was observed but the ranges depended on accession. D espite this, within 4-5 weeks equal the growth intensity in NT and LCD plants was observed, but the difference in growth rate was persistent among accessions.
LCD contributed to increase the index of PFW, but decreased the LMA in both pepper accessions, whereas the index of LMA in pepper accessions might be varied depending on the genotypes under LT condition [24]. M eanwhile, the index of LL was increased only in LCD plants of “PE-J-2”, but no differences between LCD and NT plants in both accessions in measurement of the LW were recorded. There no critical effects of the LCD 25% on NI and RFW traits in both pepper accessions plants were detected.
In particular, the effect of the abiotic stress on the reproductive traits is well known in Solanaseous crops [1-3,6,26,30], and there is enough information about the effect of the cold damages in juvenile phase on the floral and fruit traits at growth stages of pepper. In fact, recently it was found that the selected pepper accessions under LT condition were primarily involved in the positive trends with the reproductive index including NFR, FL, FD, and TY traits and recommended to utilize for pepper breeding programs to develop LT tolerant cultivars [24].
The results of the analysis of LCD influence on the flowering period indicate that in cold damaged plants flowering started relatively later than plants in NT and varietal differences were observed. No significant effect of LCD on the development of the N FL was observed, whereas LCD effected the development of NFR but among accessions differences persist in the varietal aspect, where susceptible accession “Neokgwang” distinguished with high NFR and relatively FS per plant than in NT plants.
In the agreement with our previous studies of pepper genotypes under LT condition [24], our current finding also showed that the N FL among pepper accessions showed no remarkable difference between the LCD and NT.
The effect of LCD on the agronomical traits with NT plants was more distinctive in the correlations between LMA , FD , TY, PFW and NFL, and a small correlation between FD and FL was observed. Same correlations were also found between TY in NT and that in LCD, which indicates that accessions with high yield in NT can also perform well in terms of harvest in LCD plants, which was also reported in previous research results [24,31].
Based on N FR or FS various genotypes from Solanaceae family were selected and used as predictor for abiotic stress tolerance [9 ,27,32,33]. However, in our study chilling susceptible accession “Neokgwang” in seedling stage showed good physiological traits performance such as high rates of NFR, FS and TY in comparison with screened one as tolerant in seedling stage “PE -J-2”. It means that cold tolerance rate in seedling stage is not always associated with reproductive traits.
In addition, recently, a similar results revealed in evaluation of the physiological and biochemical parameters related to heat tolerance and to determine critical leaf heat damage levels for selecting heat-tolerant in hot pepper genotypes [34]. Leaf heat damage levels over 25% in seedling stage was critical for selecting heat-tolerant genotypes in pepper, and remarked that constant photosynthetic rate via increased transpiration rate as well as high proline content in heat stress condition confers faster recovery from heat damage of heat-tolerant cultivars in seedlings stages. In accordance with these results, further studies need to focus on elucidating the mechanism and role of the impact of physiological parameters on vegetative and reproductive traits in pepper plants with different low temperature tolerance.
Conclusion
O verall, our results imply that, the genotypes screened in juvenile stages as cold tolerant cannot always manifest good agronomical traits performance at growth stages and it may range depending on the genotype specific features. LCD within and over 25% may contribute to increase the index of PFW but might decrease the LMA and FL delaying the days to flowering regardless of the tolerance of the pepper accessions to low temperature. Moreover, agronomic traits associated in LCD plants differed by genotype specific. And, future researches on screening and selection for pepper abiotic stress tolerance should be evaluated at the vegetative and reproductive stages with consideration of reproductive parameters and molecular functions, combined with DNA- and RNA-seq.
Об авторах:
Чо Мёнг Чеол – PhD (сельское хозяйство),
зав. лабораторией, Отдел овощных культур, ,
Жеонг Хёо Бонг – старший научный сотрудник, Отдел овощных культур, ,
Aboutthe authors:
Myeong-Cheoul Cho – PhD (Agriculture),
Head of Laboratory, ,
Hyo-Bong Jeong – Senior Researcher, ,
Список литературы Effect of leaf cold damage after chilling temperature treatment on growth and reproductive parameters of Chilli pepper plants
- Bakker J.C. and Van Uffelen J.A.M. The effects of diurnal temperature regimes on growth and yield of sweet pepper. Netherlands J. Agril. Sci. 1998;36(20):1-208. https://doi.org/10.1018174/njas.v36i3.16670
- Venema J.H., Posthumus F., de Vries M., van Hasselt P.R. Differential response of domestic and wild Lycopersicon species to chilling under low light: growth, carbohydrate content, photosynthesis and the xanthophyll cycle. J. Physiologia Plantarum. 1999;105:81-88. https://doi.org/10.1034/J.1399-3054.1999.105113.X
- Fang X., Zaiqiang Y., Liyun Z. Low temperature and weak light affect greenhouse tomato growth and fruit quality. J. Plant Sci. 2018;6:16-24. https://doi.org/10.11648/j.jps.20180601.14
- Thomashow M.F. Plant cold acclimation: freezing tolerance genes and regulatory mechanisms. Annu. Rev. Plant Biol. 1999;50:571-599. https://doi.org/10.1146/annurev.arplant.50.1.571
- Chinnusamy V., Zhu J. K. & Sunkar, R. Gene regulation during cold stress acclimation in plants. Methods Mol. Biol. 2010;639:39-55. https://doi.org/10.1007/978-1-60761-702-0_3
- Erickson A.N., Markhart A.H., Flower developmental stage and organ sensitivity of bell pepper (Capsicum annuum L.) to elevated temperature. Plant Cell Environ. 2002; 25:123-130. https://doi.org/10.1046/j.0016-8025.2001.00807.x.
- Adams S.R., Cockshull K.E., Cave C.R. Effect of temperature on the growth and development of tomato fruits. Ann. Bot. 2001;88:869-877. https://doi.org/10.1006/anbo.2001.1524
- Der Van Ploeg A., Heuvelink E. Influence of sub-optimal temperature on tomato growth and yield: a review. J. Hortic. Sci. Biotech. 2005;80:6652-659. https://doi.org/10.1080/14620316.2005.11511994
- Yang E.-Y., Rajametov S.N., Cho M.-C., Jeong H.-B., Chae W.-B. Factors Affecting Tolerance to Low Night Temperature Differ by Fruit Types in Tomato. Agriculture. 2021;11:681. https://doi.org/10.3390/agriculture11070681
- Munir Sh., Liu H., Xing Y., Hussain S., Ouyang B., Zhang Y., Li H., Ye Z. Overexpression of calmodulin-like (ShCML44) stress-responsive gene from Solanum habrochaites enhances tolerance to multiple abiotic stresses. Sci. Rep. 2016;6;31772. https://doi.org/10.1038/srep31772
- Ding F., Liu B., Zhang Sh. Exogenous melatonin ameliorates coldinduced damage in tomato plants. Sci. Hortic. 2017;219:264-271. https://doi.org/10.1016/j.scienta.2017.03.029
- Rihan H.Z., Al-Issawi M., Fuller M.P. Advances in physiological and molecular aspects of plant cold tolerance. J. Plant Interact. 2017;12:143-157. https://doi.org/10.1080/17429145.2017.1308568
- Liu H., Ouyang B., Zhang J., Wang T., Li H., et al. Differential Modulation of Photosynthesis, Signaling, and Transcriptional Regulation between Tolerant and Sensitive Tomato Genotypes under Cold Stress. PLoS ONE. 2012;7(11):e50785. https://doi.org/10.1371/journal.pone.0050785
- Chen H., Chen X., Chen D, Li J., Zhang Y., Wang A. A comparison of the low temperature transcriptomes of two tomato genotypes that differ in freezing tolerance: Solanum lycopersicum and Solanum habrochaites. J. BMC Plant Biology, 2015;15:132. https://doi.org/10.1186/s12870-015-0521-6
- Hu T., Wang Y., Wang Q., Dang N., Wang L., Liu Ch, et al. The tomato 2-oxoglutarate-dependent dioxygenase gene SlF3HL is critical for chilling stress tolerance. J. Horticulture Research. 2019;6:45. https://doi.org/10.1038/s41438-019-0127-5
- Goodstal F.J., Kohler G.R., Randall L.B., Bloom A.J., St Clair D.A. A major QTL introgressed from wild Lycopersicon hirsutum confers chilling tolerance to cultivated tomato (Lycopersicon esculentum). J. Theor. Appl. Genet. 2005;111:898-905. https://doi.org/10.1007/s00122-005-0015-2
- Somerville C. Direct tests of the role of membrane lipid composition in low temperature-induce photoinhibition and chilling sensitivity in plants and cyanobacteria. Proc. Natl. Acad. Sci. (USA). 1995;92:6215-6218. https://doi.org/10.1073/pnas.92.146215
- Foolad M., Lin G. Relationship between cold tolerance during seed germination and vegetative growth in tomato: Germplasm evaluation. J. Am. Soc. Hortic. Sci. 2000;125:679-683. https://doi.org/10.21273/JASHS.125.6.679
- Park E., Hong S.J., Lee A.Y., Park J., Cho B.K., Kim G. Phenotyping of Low-Temperature Stressed Pepper Seedlings Using Infrared Thermography. J. of Biosystems Eng. 2017;42(3):163-169. https://doi.org/10.5307/JBE.2017.42.3.163
- Kato K. Flowering and fertility of forced green peppers at lower temperatures. J. Jpn. Soc. Hortic. Sci. 1989;58:113-121.
- Shaked R., Rosenfeld K., Pressman E. The effect of low night temperatures on carbohydrates metabolism in developing pollen grains of pepper in relation to their number and functioning. Sci. Hortic. 2004;102:29-36. https://doi.org/10.1016/j.scienta.2003.12.007
- Seo J.-U.; Hwang J.-M.; Oh S.-M. Effects of night temperature treatment of raising seedlings before transplanting on growth and development of pepper. J. Bio-Env. Con. 2006;15:149-155.
- Rajametov S.N., Lee K., Jeong H.B., Cho, M.C., Nam C.W., Yang E.Y. Physiological Traits of Thirty-Five Tomato Accessions in Response to Low Temperature. J. Agriculture. 2021;11:792. https://doi.org/10.3390/agriculture11080792
- Rajametov S.N., Lee K., Jeong H.B., Cho M.C., Nam C.W., Yang E.Y. The Effect of Night Low Temperature on the Agronomical Traits of ThirtyNine Pepper Accessions (Capsicum annuum L.). J. Agronomy. 2021;11:1986. https://doi.org/10.3390/agronomy11101986
- Zhang C., Liu J., Zhang Y., Cai X., Gong P., Zhang J., Ye Z. Overexpression of SlGMEs leads to ascorbate accumulation with enhanced oxidative stress, cold, and salt tolerance in tomato. J. Plant Cell Rep. 2011;30:389-398. https://doi.org/10.1007/s00299-010-0939-0
- Hu Y., Wu Q., Sprague S., Park S., Oh M., et al. Tomato expressing Arabidopsis glutaredoxin gene AtGRXS17 confers tolerance to chilling stress via modulating cold responsive components. J. Hortic. Res. 2015;2:15051. https://doi.org/10.1038/hortres.2015.51
- Rajametov S.N., Yang E.Y., Jeong H.B., Cho M.C., Chae S.Y., Paudel N. Heat Treatment in Two Tomato Cultivars: A Study of the Effect on Physiological and Growth Recovery. J. Horticulturae. 2021;7:119. https://doi.org/10.3390/horticulturae7050119
- Rajametov Sh., Yang E.Y., Cho M.Ch., Chae S.Y., Bong J.H. Screening of pepper (Capsicum L.) seedlings tolerance to low temperature. The Agrarian Sci J. 2020;11:78-82. https://doi.org/10.28983/asj./2020i11pp78-82
- Hoek H.I.S., Hanisch Ten Cate C.H., Keijzer C.J., Schel J.H., Dons H.J.M. Development of the fifth leaf is indicative for whole plant performance at low temperature in tomato. J. Ann. Bot. 1993;72:367-374.
- Xu J., Wolters-Arts M., Mariani C., Huber H., Rieu I. Heat stress affects vegetative and reproductive performance and trait correlations in tomato (Solanum lycopersicum). J. Euphytica. 2017;213:156. https://doi.org/s://doi:10.1007/s10681-017-1949-9
- Rajametov Sh., Yang E.Y., Cho M.Ch., Chae S.Y., Won B.Ch. Physiological traits associated with high temperature tolerance differ by fruit types and sizes in tomato (Solanum lycopersicum L.). J. Hortic. Environ. Biotechnol. 2020;61:837-847. https://doi.org/10.1007/s13580-020-00280-4
- Abdul-Baki A.A., Stommel J.R. Pollen viability and fruit set of tomato genotypes under optimum and high-temperature regimes. J. Hort. Sci. 1995;30:115-117. https://doi.org/10.21273./HORTSCI.30.1.115
- Sato S., Peet M.M., Thomas J.F. Physiological factors limit fruit set of Tomato (Lycopersicon esculentum Mill.) under chronic high temperature. Plant Cell Environ. 2000;23:719-726. https://doi.org/s://doi:10.1046/j.1365-3040.2000.00589.x
- Rajametov S., Yang E.Y., Cho M.C., Bong J.H., Chae S.Y., Chae W.B. Heat-tolerant hot pepper exhibits constant photosynthesis via increased transpiration rate, high proline content and fast recovery in heat stress condition. Sci. Rep. 2021;11:14328. https://doi.org/10.1038/s41598-021-93697-5


