Effect of metal ions, chelating agent and SH-reagents on radish (Raphanus sativus L.) root -amylase
Автор: Sarowar Jahan M.G., Shaela Pervin M., Shariar Shovon M., Dev Sharma S.C., Roy Narayan, Habibur Rahman M.
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 3 т.8, 2012 года.
Бесплатный доступ
Metal ions play vital roles in enzymes. They may also show sensitivity to various sulfhydryl reagents and chelating reagents. Effect of some metal ions, EDTA and sulfhydryl reagents on the activity of partially purified β-amylase of radish root were studied. Amylolytic activity of purified enzyme was increased substantially in the presence of Ca2+, Mg2+, and Zn2+. Some other divalent cations Cu2+, Pb2+, Sn2+, and Hg2+ almost completely ceased the enzyme activity. Cobalt (II), Manganese (II), and Iron (III) exhibited moderate activating effects on the activity. Of the monovalent cations, Na+ and Ag+ reduced the β-amylase activity, while K+ increased. The chelating agent EDTA was found to be effective in the enzyme. Sulfhydryl reagents, Iodoacetic acid and N-Ethylmaleimide showed marginal inhibitory effect, but p-hydroxymercuribenzoic acid (PCMB) almost completely stopped the enzyme activity. The addition of thiol compounds such as cysteine could reverse the inhibitory effect of heavy metals and PCMB. The results indicate that sulfhydryl groups of radish root β-amylase were essential for the activity although it is not clear whether the sulfhydryl groups were directly involved in catalysis.
Β-amylase, chelating agent, metal ions, radish, sulfhydryl reagent
Короткий адрес: https://sciup.org/14323661
IDR: 14323661
Текст научной статьи Effect of metal ions, chelating agent and SH-reagents on radish (Raphanus sativus L.) root -amylase
β-amylase is an important enzyme used in the food processing, brewing and distil industries (Priest, 1984). Its main form of introduction is in the preparation of syrups from starch via saccharification processes (Boivin, 1997). The practical interest of β-amylase centres on its capacity to produce maltose syrup from starch (Boivin, 1997; Dicko et al., 2000). This maltose containing syrups can be used as moisture conditioners, crystallisation inhibitor, stabilizer, carriers, and bulking agents (Badal et al., 1989). The extensive application of amylases in the food, starch liquefaction and saccharification, detergent, textile, paper, brewing and distilling industries has paved a way for their large-scale commercial production (Gupta et al., 2003). With the advent of new frontiers in biotechnology, the spectrum of amylase application has expanded into many other fields, such as clinical, medical and analytical chemistries.
Many substances alter the activity of an enzyme by combining it in a way that influences the binding of the substrate (Dogan et al ., 2007). These substances can be called as effectors either being activators or inhibitors. Metal ions can be considered as good examples, different metals exhibiting different behaviors in their ability to act as effectors. Li et al ., (2005) also stated that metal binding to enzymes plays an important role in their activation and stabilization. Most amylases are known to be metal ion dependent enzymes (Pandey et al ., 2000; Gupta et al ., 2003; Ramachandran et al ., 2004). According to Leveque et al ., (2000), a metal ion present at high concentrations might compete with another metal present at a lower concentration and replace it at a metal-binding site, even if its affinity for the binding site is lower. An accepted property of amylases is their content of Ca2+ as an integral part of the enzyme molecule and their consequent activation by Ca2+ and inhibition by chelating reagents (Mar et al ., 2003; Kiran and Chandra, 2008). Besides, amylases have been reported to be inhibited by heavy metal ions such as Ag+, Hg2+, Cu2+, Fe2+, etc., suggesting the involvement of cysteine residue in enzyme activity. The importance of cysteine residues for catalysis has been described for amylases independent of their origin (Pandey et al ., 2000; Lo et al ., 2001; Diaz et al ., 2003). The thiol of a cysteine residue occasionally acts as a ligand of metal ions (Tatara et al ., 2005). Amylases are also found sensitive to various sulfhydryl modifying reagents as free thiol or sulfhydryl groups are considered to be useful in stability, redox behaviour, metal binding, acidity,
182 nucleophilicity and catalytic activity (Bardwell, 2005; Giles et al ., 2003).
In order to obtain an insight into these, an attempt has been made to determine the effect of various metal ions and the chelating reagent EDTA on the radish root β-amylase activity. Besides, the possible effects of sulfhydryl reagents (p-hydroxymercuribenzoic acid, N-Ethylmaleimide and Iodoacetic acid) on amylase activity have also been investigated.
MATERIALS AND METHODS
Materials
Mature, healthy Raphanus sativus (radish) roots were collected directly from the cultivation field during the winter season and these roots were used. Sephadex G-150 was from Pharmacia Fine Chemicals Uppsala, Sweden. Soluble potato starch and Bovin serum albumin (BSA) were obtained from Sigma Co. p-hydroxymercuribenzoic acid (PCMB), N-Ethylmaleimide and Iodoacetic acids were purchased from Fluka. All others chemicals were of analytical grade and also purchased from Sigma Inc., USA.
Preparation of the crude extract
Fresh, mature and healthy radish roots were washed thoroughly with distilled H 2 O, cut into small pieces and ground with distilled H 2 O and sand in a morter at 4oC. The suspension was then filtered through few layers of cheesecloth in a cold room. The filtrate was clarified further by centrifugation at 10,000 g for 15 min at 4oC. The supernatant was then dialyzed against distilled H 2 O for 48h at 4oC and centrifuged at 10,000 g for 15 min at 4oC. The clear supernatant obtained was used as crude enzyme extract.
Purification of β-amylase
All steps of purification were carried out at 40C. The enzyme was purified to homogeneity by the sequential steps of acetone precipitation, followed by DEAE- cellulose chromatography and gel filtration on Sephadex G-150.
Enzyme precipitation
The crude extract was initially fractioned by 50% (v/v) chilled (-20oC) acetone saturation. After centrifugation at 10,000 g for 15 min, the precipitated pellets were collected and resuspended in two-pellet volume of cold buffer. The solution was dialyzed against 10 mM phosphate buffer of pH 7.0 for overnight. Undissolved particles were removed by centrifugation and the clear solution was used for further purification.
DEAE- cellulose chromatography
The enzyme preparation obtained from the previous step was passed over a DEAE-cellulose column (20.0 X 1.0 cm) previously equilibrated with 10 mM phosphate buffer of pH 7.0 at 4oC. The buffer was first passed through the column and then the enzyme was eluted with the same buffer containing a linear gradient of NaCl (0-500 mM) at a flow rate of 30 ml/h. Fraction with β-amylase activity was pooled and dialyzed against 10 mM phosphate buffer of pH 7.0 at 4oC and concentrated by Millipore.
Gel filtration chromatography
The dialyzed, concentrated enzyme preparation resulting from the anion exchange chromatography was applied to a column of Sephadex G-150 (150 X 3.0 cm) preequilibrated with 10 mM phosphate buffer of pH 7.0 at 4oC. The protein was eluted with the same buffer at a flow rate of 15 ml/h. Enzymatically active fraction from gel filtration step was pooled separately, concentrated by commercial sucrose and applied for molecular weight determination by gel filtration and electrophoresis.
Enzyme assay
One milliliter of enzyme was added to 1 ml of 1% soluble starch containing 0.1 M phosphate buffer pH of 6.7 and the mixture was incubated at 37oC for 15 min. The amount of reducing sugar produced was determined by Somogyi (1952) and Nelson (1944) methods. One unit of enzyme activity was defined as the amount; which catalyzed the formation of 1 μmol of maltose under the assay conditions.
Protein concentration was determined by Lowry’s phenol method (1951), using crystalline BSA as the standard.
SDS-polyacrylamide gel electrophoresis (SDS-PAGE)
SDS-polyacrylamide gel electrophoresis was carried out according to the method of Laemmli (1970). The PAGE was performed with 7% gels at 110 V and 30 mA. The protein bands on the gel was dyed by using of 0.25% Coomassie brilliant blue R25 (CBB) solution containing 50 % methanol and 10 % acetic acid.
Effect of metal ions, EDTA and sulfhydryl reagents on β-amylase activity
The effect of various metal ions, chelating agent and inhibitors on the activity on β-amylase was examined by incubating the enzyme with substrate solution (comprising of 1% starch in 0.1 M phosphate buffer pH 6.7) at 37oC in the presence of the respective ions or chemicals for 5 min and at the end of incubation period, aliquots were withdrawn and assayed. In all of the above cases, the β-amylase activity is expressed as a percentage of the control enzyme activity (100%).
RESULTS AND DISCUSSION
Purification of radish root β-amylase
The crude enzyme solution was precipitated by the addition of water-miscible organic solvent, acetone. After centrifugation, the concentrated dialyzed enzyme solution was passed over a column of DEAE-cellulose for separation on the basis of ionic property of β-amylase. The active fraction obtained from the ion exchange chromatography was applied to a column of Sephadex G-150 (50 X1.0 cm) for further separation. The molecular weight of radish root β-amylase, determined from the experiment with Sephadex G-150 gel filtration column was 62 kda (Data not shown). This was in good agreement with the molecular weight (61 kDa) determined by SDS-PAGE (Data not shown). Hence, radish root β-amylase was a monomer. The present value (61 kDa) was very close to the molecular weights of β-amylase isolated from yam tuber (60 kDa) and ginseng (63 kDa) reported by Arai et al ., (1991) and Yamasaki et al .,(1989), respectively. Larger multimeric β-amylases have been reported from leaves of potato (111 kDa) (Vikso-Nelson et al ., 1997) and potato tubers (122 kDa) (Yamasaki et al ., 1989).
The effect of metal ions on radish root β-amylase
In the enzyme action, metallic cofactors are important because their presence or absence regulates enzyme activity. The presence of specific metallic ions along with food content can inhibit or enhance amylase activity, and therefore the rate of digestion. Various metal ions were tested for activation/inhibition effects that were shown in Table 2. Among monovalent cations K+ increased the enzyme activity substantially, but incubation the enzyme with Na+ and Ag+ reduced the activity. Divalent cations like Ca2+, Mg2+, and Zn2+ increased the activity of the purified β-amylase. Usually, the role of Ca2+ in maintaining the stability and structure of the amylase is well documented (Parkin, 1993). Similar to other plant β-amylases, the radish root β-amylase was severely inhibited by Cu2+, Pb2+, Sn2+, and Hg2+. Notably Fe2+ moderately reduced the amylolytic activity.
Effect of EDTA on radish root β-amylase activity
It is well known that amylases contain Ca2+ as an essential component of the enzyme molecule and are often inhibited by the chelating reagent EDTA (Mar et al ., 2008; Kiran and Chandra, 2008) presumably because of its chelating properties. Interestingly, radish root β-amylase increased activity with the addition of EDTA (Table 3).
Effect of sulfhydryl reagents on radish root β-amylase activity
The effect of the sulfhydryl reagents (p-hydroxymercuribenzoic acid, N-Ethylmaleimide and lodoacetic acid) on β-amylase activity are presented in Table 3. The minimum amount of these reagents inducing a detectable inhibition of activity in radish root β-amylase amylase was found to be 0.1 mM.
From the above results, it was found that Iodoacetic acid and N-Ethylmaleimide reduced the activity of β-amylase moderately. The enzyme was almost completely inhibited by the presence of heavy metals such as Cu2+, Pb2+, Sn2+ Hg2+ and by SH-inhibitor such as PCMB, the activity being restored by thiol compounds such as cysteine. The result indicates that an SH group exits in the molecular structure, as in other plant β-amylase. The inactivation caused by PCMB was similar to that found in other plant β-amylases (Marshall, 1975). Plant β-amylase has been reported to require free sulfhydrylgroups for activity and is inhibited by heavy metals as well as other binding reagents
(Thoma et al ., 1971; Trachuk and Tipples, 1966).
Table 1: Summary of radish root β-amylase purification
|
Purification Step |
Total Protein (mg) |
Total Activity (unit) |
Specific Activity (unit/mg) |
Yield (%) |
Purification (fold) |
|
Crude Extract |
1625 |
4468.75 |
2.75 |
100 |
1 |
|
Acetone Precipitation & Dialysis |
485.5 |
3997.5 |
8.2 |
89.18 |
2.9 |
|
DEAE-cellulose |
59.4 |
2108.7 |
35 |
47.18 |
12.7 |
|
Sephadex G-150 |
3.6 |
468 |
130 |
10.47 |
47.2 |
Table 2: Effects of various metal ions on radish root β-amylase
|
Metal Ions |
Concentration (mM) |
Residual Activity (%) |
Induction (%) |
Inhibition (%) |
|
Control |
- |
100 |
- |
- |
|
NaCl |
1 |
96 |
4 |
|
|
KCl |
1 |
141 |
141 |
|
|
AgNO 3 |
1 |
13 |
87 |
|
|
CaCl 2 |
0.1 |
175 |
175 |
|
|
MgCl 2 |
0.1 |
160 |
160 |
|
|
ZnCl 2 |
0.1 |
152 |
152 |
|
|
MnCl 2 |
1 |
113 |
113 |
|
|
CoCl 2 |
0.1 |
117 |
117 |
|
|
FeCl 2 |
1 |
33 |
67 |
|
|
CuCl 2 |
1 |
18 |
82 |
|
|
PbCl 2 |
1 |
17 |
83 |
|
|
SnCl 2 |
1 |
33 |
67 |
|
|
HgCl 2 |
1 |
13 |
87 |
|
|
FeCl 3 |
1 |
103 |
103 |
Table 3: Effects of chelating reagent EDTA and sulfhydryl reagents on radish β-amylase
|
Chemical Reagent |
Concentration (mM) |
Residual Activity (%) |
Induction (%) |
Inhibition (%) |
|
Control |
- |
100 |
- |
- |
|
EDTA |
0.1 |
120 |
120 |
|
|
*PCMB |
0.1 |
5 |
95 |
|
|
Iodoacetic acid |
0.1 |
87 |
13 |
|
|
N-Ethylmaleimide |
0.1 |
97 |
3 |
* p-hydroxymercuribenzoic acid
-е Protein -А - Enzyme
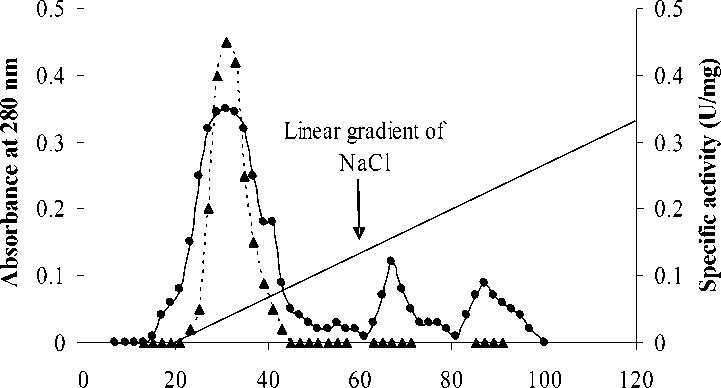
Fraction No. (3ml/tube
Figure 1: Elution profile of radish root β-amylase on DEAE-cellulose column. The enzyme solution (485.5mg protein) obtained after acetone precipitation and dialysis was applied to the column previously equilibrated with 10 mM phosphate buffer pH 7.0 at 40C eluted with the same buffer containing a linear gradient of NaCl (0-500 mM).
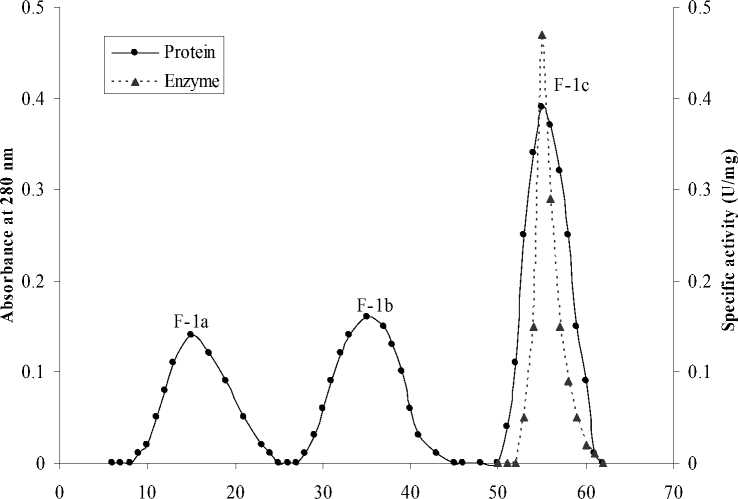
Fraction No. (3 ml/tube)
Figure 2: Elution profile of F-1 fraction on Sephadex G-150 column. Protein (59.4 mg) was applied to column pre-equilibrated with 10 mM phosphate buffer pH 7.0 at 40C. The protein was eluted with the same buffer with same pH.
ACKNOWLEDGEMENT
The authors gratefully acknowledge the research facilities provided by the Department of Biochemistry and Molecular Biology, University of Rajshahi, Rajshahi-6205, Bangladesh.
Список литературы Effect of metal ions, chelating agent and SH-reagents on radish (Raphanus sativus L.) root -amylase
- Arai, T., Kawabta, A. and Taniguchi, H. (1991). Purification and some properties of Ichoimo β-Amylase. Agric. Biol. Chem., 55(2), 399-405.
- Badal C., Saha T. and Zeikus J. (1989). Improved method for preparing high maltose conversion syrups. Biotechnology and Bioengineering, 34, 299-303
- Bardwell, J. (2005). Thiol modifications in a snapshot. Nature Biotechnol., 23, 42-43.
- Boivin P. (1997). Les enzmyes en brasserie. In Multon, J.L. (ed.), Enzymes en Agroalimentarie. Colletion Science et Techniques Agroalimenaires, Londres, Paris, New York, pp. 138-168.
- Diaz, A., Sieiro, C. and Villa, T.G. (2003). Production and partial characterization of a β-amylase by Xanthophyllomyces dendrorhous. Lett. Applied Microbiol., 36, 203-207.
- Dicko M.H., Searle-Van L., Hilhorst R. and Traore A.S. (2000). Extration, Partial purification and Characterisation of β-amylase from the bulb of G. Klattianus. Biosource Technology, 73, 183-185
- Dogan, S., Turan, P., Dogan, M., Alkan, M. and Arslan, O. (2007). Inhibition kinetics of polyphenol oxidase by glutamic acid. Eur. Food Res. Technol., 225, 67-73.
- Giles, N.M., Giles, G.I. and Jacob, C. (2003). Multiple roles of cysteine residue in biocatalysis. Biochem. Biophys. Res. Commun., 300, 1-4.
- Gupta, R., Gigars, P., Mohapatra, H., Goswami, V.K. and Chauhan, B. (2003). Microbial a-amylase: A biotechnological perspective. Process. Biochem., 38, 1599-1616.
- Kiran, K.K. and Chandra T.S. (2008). Production of surfactant and detergent-stable, halophilic and alkalitolerant alpha-amylase by a moderately halophilic Bacillus sp. Strain TSCVKK. Applied Microbiol. Biotechnol., 77, 1023-1031.
- Laemmli, U.K. (1970). Cleavage of structural proteins during the assembly of head of bacteriophage T4. Nature, 227, 680-685.
- Leveque, E., Haye, B. and Belarbi, A. (2000). Cloning and expression of an a-amylase encoding gene from the hyperthermophilic archaebacterium Thermococcus hyclrothermalis and biochemical characterization of the recombinant enzyme. FEMS Microbiol. Lett., 186, 67-71.
- Li, W.F., Zhou, XX. and Lu, P. (2005). Structural features of thermozymes. Biotechnol. Adv., 23, 271-281.
- Lo, H.F., Lin, L.L., Chen, H.L., Hsu, H.H. and Chang, C.T. (2001). Enzymatic properties of a SDS-resistant Bacillus sp. TS-23 a-amylase produced by recombinant Escherichia coli. Process Biochem., 36, 743-750.
- Lowry, O.H., Roserbrough, N.J., Farr A.L. and Randall, R.J. (1951). Protein measurement with the follin phenol reagent. J. Biol. Chem., 193, 265-275.
- Mar, S.S., Mori, H., Lee, H.J., Fukuda, K. and Kimura, A. (2003). Purification, characterization and sequence analysis of two alpha-amylase isoforms from azuki bean, Vigna angularis, showing different affinity towards beta-cyclodextrin sepharose. Biosci. Biotechnol. Biochem., 67, 1080-1093.
- Marshal, J.J. (1975). Inhibition of plant and bacterial β-amylases. Mol. Cell. Biochem., 7, 127-129.
- Nelson, N. (1944). A photometric adaptation of the Somogyi method for the determination of glucose. J. Biol. Chem., 153, 375-380.
- Pandey, A., Nigam, P., Soccol, C.R., Soccol, V.T., Singh, D. and Mohan, R. (2000). Advances in microbial amylases. Biotechnol. Applied Biochem., 31,135-152.
- Parkin, K.L. (1993). Environmental Effects on Enzyme Activity. In Nagodawithana, T. and Reed, G. (ed.), Enzymes in Food Processing, 3rd Edn., Academic Press Inc., San Diego, pp. 480.
- Priest F.G. (1984). Commercialisation in Extracellular Enzymes. In Cole, S. (ed.), Applied Microbiology, Vol.19, American Society of Microbiology, Washington DC, pp. 32-50
- RamaChandran, S. Patel, A.K., Nampoothiri, K.M., Chandran, S., Szakacs G., Soccol, C.R. and Pandey, A. (2004). Alpha amylase from a fungal culture grown on oil cakes and its properties. Braz. Arch. Biol. Technol., 47, 309-317.
- Somogyi, M. (1952). Note on sugar determination. J. Biol. Chem., 195, 19-23.
- Tatara, Y., Yoshida, T. and Ichishima, E. (2005). A single free cysteine residue and disulphide bond contribute to the thermostability of Aspergillus saitoi 1, 2-a-omannosidase. Biosci. Biotechnol. Biochem., 69, 2101-2108.
- Thoma, J.A., Sprandlin J.E. and Dygert, S. (1971). Plant and animal amylases. In Boyer, P.S., (ed.), The Enzymes, Academic Press, New York, pp.115-189.
- Trachuk, R. and Tipples K.H. (1966). Wheat β-amylase. II. Characterization. Cereals Chem., 43, 62-79.
- Vikso-Nelson, A., Christensen, T.M.I.E., Bojko, M. and Marcussen, J. (1997). Purification and Characterization of β-amylase from leaves of potato (Salanum tuberosum). Physiol. Plant., 99, 190-196.
- Yamasaki, K., Yokoyama, H., Miyano, K., Nunoura, Y., Higashihara, M., Kitahata, S., Yoneda, K. and Umezawa, C. (1989). Purification and characterization of β-amylase from ginseng. Chem. Pharm. Bull., 37, 973-978.


