Effect of Salinity Stress on Morphological, Physiological and Biochemical Parameters in Mungbean [Vigna radiata (L.) Wilczek]
Автор: Shankhadip Naskar, Supatra Sen
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 4 т.21, 2025 года.
Бесплатный доступ
The study was conducted to determine the effect of different concentrations of NaCl salt stress (0, 25, 50, 75, 100mM) on growth and biochemical parameters in mungbean [Vigna radiata (L.) Wilczek] plants. The plants were uprooted and collected for experimental work on the 20th day after sowing i.e. five days after salinity stress. Salt stress caused reduction in morphological parameters like shoot length, root length, leaf area, fresh weight and dry weight. Relative water content (RWC), photosynthetic pigments like chlorophyll and carotenoid contents also significantly decreased with the increasing NaCl concentrations. However other biochemical parameters protein, amino acid content, osmoprotectant like proline and antioxidant enzymes like catalase (CAT) and peroxidase (POD) activity significantly increased with the increasing NaCl salt concentration. At the 100mM NaCl salinity stress level, chlorophyll, carotenoid contents significantly increased and peroxidase (POD) content significantly decreased compared to the 75mM NaCl salt stress. As mungbean is a salt sensitive crop, it shows significant variation in metabolic activities in response to salt stress. Mungbean plants can tolerate and adapt to salinity stress as well as other environmental stresses through their acclimation process.
Abiotic stress, ROS scavenging enzymes, biochemical constituents, stress physiology
Короткий адрес: https://sciup.org/143185127
IDR: 143185127
Текст научной статьи Effect of Salinity Stress on Morphological, Physiological and Biochemical Parameters in Mungbean [Vigna radiata (L.) Wilczek]
Mungbean [ Vigna radiata (L.) Wilczek] is one of the most economically and ecologically important edible legume crops of India with high content of protein and nutritional elements. Mungbean is primarily grown for its protein-rich dry edible seeds and fresh sprouts. The symbiotic association between mungbean roots and Rhizobia help to fix the atmospheric nitrogen into the soil. Due to short life span, low water requirement, atmospheric nitrogen fixing ability and large amount of biomass production, mungbean plants play key role in various cropping systems and the sustainable agricultural production (Sehrawat et al. 2015).
Abiotic stresses such as salt, drought, heat, cold stress affect plant growth, metabolism and disrupt or inhibit many biological and physiological processes. Among the abiotic stresses, salt stress is one of the major problems that lead to severe crop damage and loss of crop productivity. In the present study, various concentrations of NaCl salt stress effect in mungbean has been investigated by studying the changes in morphological and biochemical parameters.
MATERIALS AND METHODS
25 g of healthy overnight soaked mungbean [ Vigna radiata (L.) Wilczek] seeds were thoroughly washed and rinsed with distilled water 2-3 times. The seeds were sown in 25 pots filled with soil. Maximum 10 seeds were sown in every pot. The pots were divided into five groups with three replicas for each treatment: (i) Control (non-saline) (ii) 25mM NaCl (iii) 50mM NaCl (iv) 75mM NaCl (v) 100mM NaCl. Those salt treatments were prepared by mixing a calculated amount of NaCl with distilled water and applied on mungbean plants on the 15th day after sowing. The treatment was continued for 5 days. The control plants were regularly watered. The plants were uprooted and collected for observations on the 20th day after sowing i.e. five days after salinity stress) for further studies.
After uprooting from the pots, the length of shoot and root was measured by a scale in centimetre unit. Leaf area was determined by sketching the outline of each leaf of a plant on a graph paper and estimating the area in sq. cm. After uprooting from the pots, fresh weight of shoot, root and leaves were measured by the help of a digital balance. Now those plant parts were placed in a Petri dish and oven dried at 60°C for a few hours. Then the dry weight of each plant part was recorded.
The penultimate and 3rd from top leaves were collected from each set. Then they were weighed and the data recorded as fresh weight. Those leaves were individually placed in a Petri dish filled with distilled water covering the lids for 2 hours. After 2 hours, the leaves from each set were blotted with blotting paper and immediately weighed as saturated weight. Now the leaves from individual sets were placed in hot air oven at 60°C for 2 hours. Finally, after the stipulated time the dried leaves were weighed as dry weight. From the data collected, RWC was calculated.
Chlorophyll a, chlorophyll b and total chlorophyll content were calculated by the method of Arnon (1949); amount of Carotenoid (carotene and xanthophyll) was estimated by the method of Kirk and Allen (1965); Protein content was estimated according to Lowry et al. (1951); total amino acid content in leaves was estimated according to the Ninhydrin method of Lee and Takahashi (1966) with some modifications. Estimation of proline was done according to Bates et al. (1973); catalase enzyme activity was assayed by the method of Gasper and Lacoppe (1968) with slight modifications and peroxidase enzyme activity was assayed spectrophotometrically according to Chance and Maehly (1955) with slight modifications.
The results obtained in triplicates were subjected to analysis of variance (AN VA) and S.E. (Standard Error) and C.D. (Critical Difference) at 5% and 1% levels calculated.
RESULTS AND DISCUSSION
Morphological parameters like shoot length, root length and leaf area are significantly decreased with the increasing salinity stress level (Table 1). The control plants show highest shoot length (24.77 cm), root length (5.87 cm) and leaf area (4.97sq.cm) and the 100mM salinity stressed plants show lowest shoot length (21.03 cm), root length (2.17 cm) and leaf area (2.65 sq.cm). Fresh weight and of shoot, root and leaves are decreased with the increasing salinity stress level and the highest activity observed in the control plants and lowest in the 100mM salinity stressed plants (Table 2). Dry weight of shoot, root and leaves are decreased with the increasing salinity stress level and the highest activity observed in the control plants and lowest in the 100mM salinity stressed plants (Table 3).
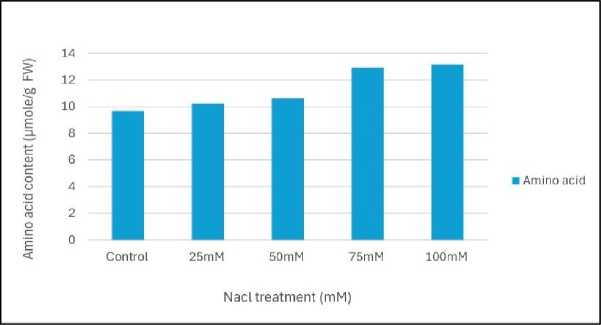
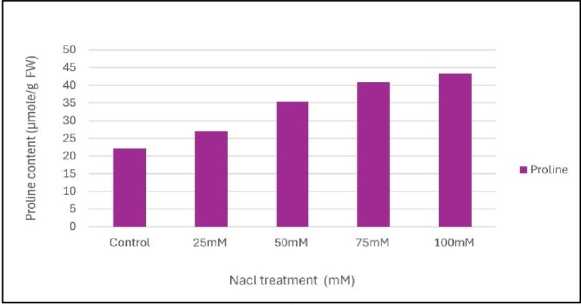
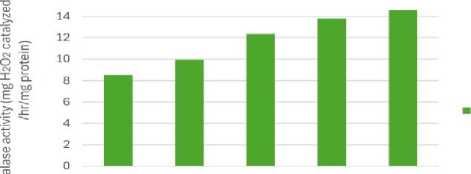
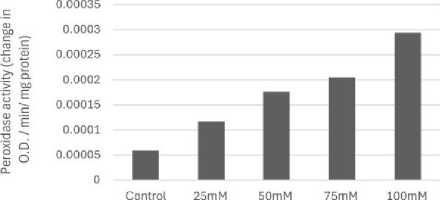
Relative water content (RWC) was the highest in control set and decreased with increasing levels of salinity stress (Fig.1). Chlorophyll content was also found to decrease with increasing salinity, the highest amount being in the control set (Fig. 2). At 100mM NaCl it was found to increase once again. The carotenoid content also followed the same trend (Fig. 3). Protein (Fig. 4), amino acid (Fig. 5) and proline contents (Fig. 6) all followed a similar trend showing increasing content with increasing salinity stress. The two reactive oxygen species (R S) scavenging enzymes catalase (Fig. 7) and peroxidase (Fig. 8) showed an identical trend exhibiting increasing activity with increasing salinity stress.
In the current study, the effect of different concentrations of salinity stress on mungbean ( Vigna radiata L. Wilczek) plants was evaluated. All the morphological parameters like shoot and root length, leaf area, fresh and dry weight (Tables No. 1, 2 and 3) and physiological parameters like Relative water content (RWC) - Fig.1 decreased with increasing salinity concentrations. Due to salinity stress, the suppression of meristematic activity and cell expansion lead to inhibition of root growth. As a result, mungbean plants are unable to collect water from soil which ultimately prevents the plant’s whole growth performance. The excess amount of Na+ ion disrupted the K+ ion uptake which causes loss of cell turgor, prevents cell expansion and disrupts other cellular enzymatic activities which ultimately reduces fresh and dry weight and overall biomass of mungbean plants (Mir et al . 2024).
Photosynthetic activity of mungbean plants are affected by salinity level. Photosynthetic pigments like chlorophyll and carotenoid contents decreased with the increasing salinity level (Figs.2 and 3) could be due to the accumulation of reactive oxygen species (R S) (Sen and Mukherji 1998a, 1999). The activity of chlorophyllase enzyme that degrade chlorophyll, disruption of fine structure of chloroplast and instability of pigment protein complexes by ions could be also the reason for photosynthetic pigments reduction (Saha et al. 2010).
Plants generally accumulate osmolytes in cytoplasm in response to salinity stress to scavenging free radicals, maintaining nitrogen and carbon source for post stress recovery and growth and stabilizing membranes, protein synthesis machinery and protecting cellular components from lipid peroxidation and protein from denaturation (Saha et al . 2010). smolyte accumulation in plant cell results in a decrease of the cell osmotic potential and helps in the maintenance of water absorption and cell turgor pressure, which might contribute to sustaining physiological processes, such as stomatal opening, photosynthesis and expansion growth (Gumi et al . 2013). The increased amount of protein, amino acid (Figs.4 and 5) and proline contents (Fig. 6) were positively correlated to the level of salt tolerance. (Zhang et al. 2020).
Proline is a low molecular-weight osmoprotectant acting as a cytoplasmic osmoticum that helps to preserve structural integrity and cellular osmotic potential within different compartments of the cell. Proline accumulation in plants is known to be a stress effect rather than a cause of stress tolerance. Higher accumulation of proline suggests that the mungbean plants possess a better potential to maintain osmotic balance. In this study, proline content was found to increase with salinity stress. So there is a positive correlation between proline accumulation and abiotic stress (Sen and Mukherji, 1998b). Increasing leaf proline content under salinity stress might be caused by the induction or activation of proline synthesis from glutamate or decrease in its utilization in protein synthesis or enhancement in protein turnover (Sen and Mukherji, 1998c).
The significant increase in antioxidant enzymes viz. catalase and peroxidase is involved in R S detoxification. Under abiotic stress, antioxidant enzymes accumulate in plants to control R S production like H2 2, superoxide radical, singlet oxygen and protect plants from oxidative damage (Sen 2000, 2020, 2023). Increase in antioxidant enzymes viz. catalase and peroxidase activities could be a response to cellular reduced stress severity and thus allowed cell growth to damage induced by NaCl (Figs.7 and 8). This increase occur (Krishnan, 2013).
could not stop the deleterious effects of NaCl, but it
Table 1. Effect of salinity stress on Shoot Length, Root Length and Leaf Area of mungbean plants
|
Treatment |
Mean Shoot Length (cm) |
Mean Root Length (cm) |
Mean Leaf Area (sq.cm) |
|
Control |
24.77 |
5.87 |
4.97 |
|
25mM NaCl |
23.63 |
4.67 |
4.16 |
|
50mM NaCl |
22.37 |
3.37 |
3.59 |
|
75mM NaCl |
21.63 |
2.87 |
3.07 |
|
100mM NaCl |
21.03 |
2.17 |
2.65 |
|
Standard Error (S.E.) |
0.6779 |
0.6614 |
0.4084 |
|
Critical Difference (C.D.) |
0.93 (5%) 1.35 (1%) |
0.91 (5%) 1.33 (1%) |
0.65 (5%) 0.95 (1%) |
Table 2. Effect of salinity stress on fresh weight of mungbean plants
|
Treatment |
Mean Shoot Fresh Weight (g) |
Mean Root Fresh Weight (g) |
Mean Leaves Fresh Weight (g) |
|
Control |
0.385 |
0.056 |
0.167 |
|
25mM NaCl |
0.315 |
0.043 |
0.141 |
|
50mM NaCl |
0.275 |
0.039 |
0.115 |
|
75mM NaCl |
0.204 |
0.031 |
0.103 |
|
100mM NaCl |
0.158 |
0.021 |
0.093 |
|
Standard Error (S.E.) |
0.0401 |
0.0059 |
0.0135 |
|
Critical Difference (C.D.) |
0.29 (5%) 0.43 (1%) |
0.23 (5%) 0.34 (1%) |
0.25 (5%) 0.37 (1%) |
Table 3. Effect of salinity stress on dry weight of mungbean plants
|
Treatment |
Mean Shoot Dry Weight (g) |
Mean Root Dry Weight (g) |
Mean Leaves Dry Weight (g) |
|
Control |
0.045 |
0.020 |
0.021 |
|
25mM NaCl |
0.034 |
0.016 |
0.016 |
|
50mM NaCl |
0.026 |
0.014 |
0.009 |
|
75mM NaCl |
0.023 |
0.009 |
0.008 |
|
100mM NaCl |
0.017 |
0.007 |
0.005 |
|
Standard Error (S.E.) |
0.048 |
0.028 |
0.025 |
|
Critical Difference (C.D.) |
0.30 (5%) 0.44 (1%) |
0.29 (5%) 0.42 (1%) |
0.29 (5%) 0.43 (1%) |
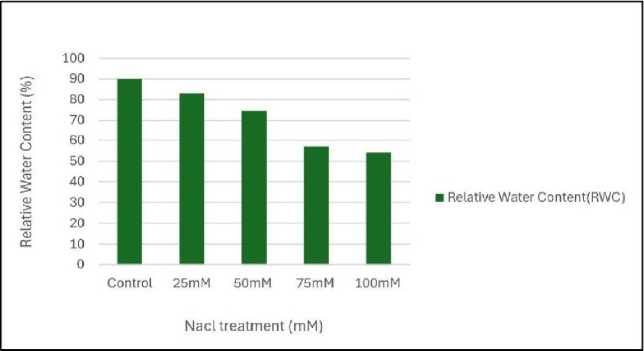
|
Standard Error (S.E.) |
7.0693 |
|
Critical Difference (C.D.) |
7.383 (5%) 10.742 (1%) |
Figure 1: Effect of salinity stress on relative water content (RWC)
0-8
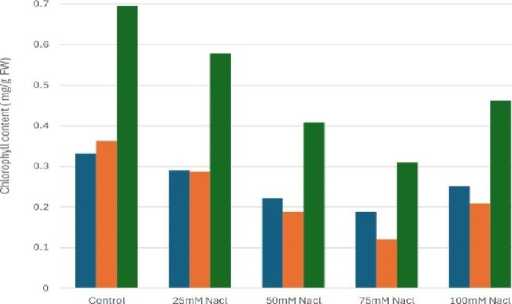
Chlorophyll a
Chlorophyll b
Total Chlorophyll
Nacl treatments (mM)
|
Chlorophyll a |
Chlorophyll b |
Total Chlorophyll |
|
|
Standard Error (S.E.) |
0.025 |
0.041 |
0.067 |
|
Critical Difference (C.D.) |
0.095 (5%) 0.139(1%) |
0.148 (5%) 0.216 (1%) |
0.243 (5%) 0.355 (1%) |
Figure 2: Effect of salinity stress on chlorophyll content
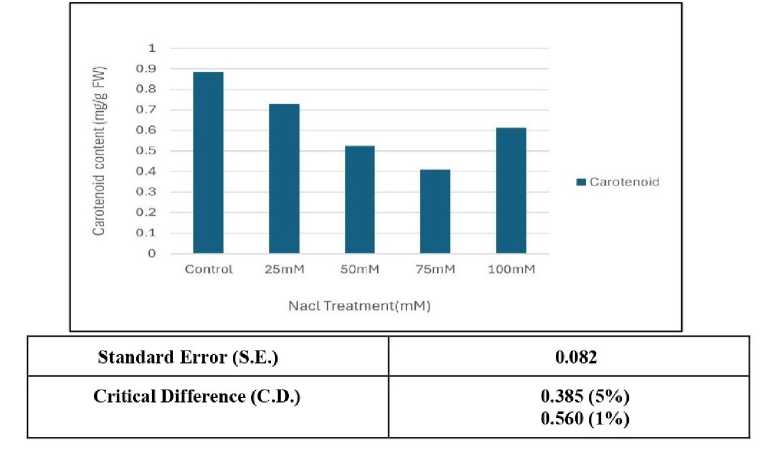
Figure 3: Effect of salinity stress on carotenoid content
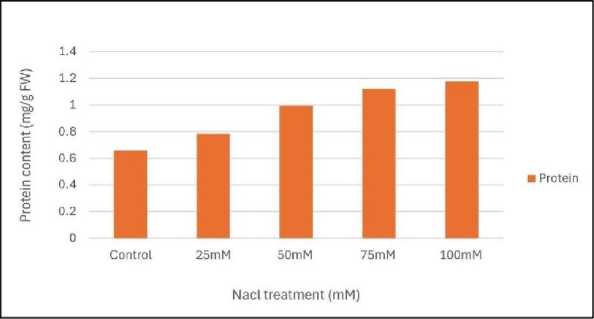
|
Standard Error (S.E.) |
0.098 |
|
Critical Difference (C.D.) |
0.392 (5%) 0.570 (1%) |
Figure 4: Effect of salinity stress on protein content of mungbean leaves
|
Standard Error (S.E.) |
0.7235 |
|
Critical Difference (C.D.) |
1.069 (5%) 1.556 (1%) |
Figure 5: Effect of salinity stress on amino acid content of mungbean leaves
|
Standard Error (S.E.) |
4.0236 |
|
Critical Difference (C.D.) |
4.431 (5%) 6.446 (1%) |
Figure 6: Effect of salinity stress on proline content of mungbean leaves
^ Control 25mM 50mM 75mM lOOmM
Nacl treatment (mM)
|
Standard Error (S.E.) |
0.051 |
|
Critical Difference (C.D.) |
0.203 (5%) 0.296 (1%) |
Figure 7: Effect of salinity stress on catalase enzyme activity
Nacl treatment (mM)
|
Standard Error (S.E.) |
0.025 |
|
Critical Difference (C.D.) |
0.119(5%) 0.173 (1%) |
Figure 8: Effect of salinity stress on peroxidase enzyme activity
CONCLUSIONS
The findings of this study demonstrate that salinity stress significantly impacts the morphological and physiological parameters of mungbean ( Vigna radiata L. Wilczek) plants. As salinity levels increased, there was a marked decrease in shoot length, root length, leaf area, and both fresh and dry weights. This decline can be attributed to the inhibition of meristematic activity and cell expansion, which hinders root growth and water uptake, ultimately affecting the overall growth performance of the plants. Additionally, the reduction in photosynthetic pigments, such as chlorophyll and carotenoids, suggests that salinity stress disrupts photosynthetic activity, likely due to the accumulation of reactive oxygen species (R S) and the degradation of chlorophyll.
verall, the results underscore the importance of understanding the physiological responses of mungbean to salinity stress, which could inform strategies for improving salt tolerance in crops and enhancing agricultural productivity in saline-affected areas.
ACKNOWLEDGEMENT
The authors are thankful to the PG Department of Botany, Asutosh College for providing necessary chemicals and equipment required for the study.
CONFLICTS OF INTEREST
All authors declare that they have no conflicts of interest.

![Effect of Salinity Stress on Morphological, Physiological and Biochemical Parameters in Mungbean [Vigna radiata (L.) Wilczek] Effect of Salinity Stress on Morphological, Physiological and Biochemical Parameters in Mungbean [Vigna radiata (L.) Wilczek]](/file/cover/143185127/effect-of-salinity-stress-on-morphological-physiological-and-biochemical.png)
