Effect of temperature on fatty acid profile of Nostoc spongiaeforme (freshwater) and marine water Nostoc calcicola (marine water): A comparative study
Автор: Prabha Tiwari, Prabhat Kumar Sharma
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 4 т.16, 2021 года.
Бесплатный доступ
The Fatty acid profiling in Nostoc spongiaeforme and Nostoc calcicola was undertaken at varying growth temperature. The extraction of fatty acid was done by chloroform: methanol method and identification of various SFA, MUFA and PUFA was performed using GC-MS. The result of our study showed that amount of fatty acid content varied with varying temperature. At low temperature, the content of unsaturated fatty acid was maximum in both the Nostoc species but the amount of saturated fatty acid was reduced under the same condition. Whereas, at extreme temperature unsaturated fatty acid was least in both Nostoc species and saturated fatty acid was maximum. Both species showed higher amount of fatty acid content at 20°C and 30°C. Our study concluded that the best temperature for mesophilic cyanobacteria to produce higher amount of fatty acid content is in the range of 20-30°C.
Cyanobacteria, Fatty acids, GC-MS, Nostoc, Temperature
Короткий адрес: https://sciup.org/143178320
IDR: 143178320
Текст научной статьи Effect of temperature on fatty acid profile of Nostoc spongiaeforme (freshwater) and marine water Nostoc calcicola (marine water): A comparative study
Abbreviations: SFA, Saturated fatty acids; MUFA, Monounsaturated fatty acids; PUFA, Polyunsaturated fatty acids; GC-MS, Gas chromatography- Mass spectroscopy
Rapid industrialization and population growth have put pressure on finite energy sources (Petroleum-derived fuel) (Shafiee and Topal, 2009). Due to this, the emission of greenhouse gases has also intensified and hence causing climate change and hampering the biosphere (Chisti, 2007). Due to the above mention reasons, there is a need to call for alternative sustainable and renewable energy sources (Demirbas and Demirbas, 2011). Production of biofuel from different biomass could be an important alternative and sustainable step to fossil-derived fuels (Li et al., 2011). Biofuels are characterized into three generations based on the type of feedstock used. The first-generation biofuels are derived from different crops like corn, soybean, sunflower, palm oil, and rapeseed. atropha Miscanthus, Switchgrass and other organic wastes are used to derive second-generation biofuel. As microalgae and cyanobacteria does not require agricultural or arable land for production and has high photosynthetic efficiencies, biomass productivities and high lipid content. They are considered as the third and most reliable material for biofuel production (Hariskos and Posten, 2014; Tredici, 2010). Moreover, in microalgae storage lipids are in the form of neutral lipids or triacylglycerols, which can be esterified to fatty acid methyl esters (FEMSs), most suitable for biofuel production (Ge et al., 2017). Moreover, cyanobacteria have a great adaptation ability to various environmental conditions and easy to cultivate.
Among the different abiotic factors, temperature is one of the vital parameters which influence the overall growth of microalgae and cyanobacteria. Temperature is also responsible for directly influencing the lipid content and fatty acid composition of algae and cyanobacteria but the nature and magnitude of temperature effect may vary species to species (Guschina and Harwood, 2006). In order to thrive the change in the ambient temperature, organisms alter their fatty acid composition which is considered as a mechanism to maintain normal cellular function (Thompson, 1996). The decrease in the growth temperature increases the ratio of unsaturated to saturated fatty acids (Renaud et al., 2002). In order to sustain low temperature and to maintain greater membrane fluidity at low temperature, organisms often increase the ratio of unsaturated fatty acid over saturated fatty acid whereas, at high temperature, the organisms cell membrane becomes more saturated to attain higher rigidity (Hilditch and Williams, 1964). For instance, the tobacco plant was made tolerance to cold by introducing the omega-3 fatty acid desaturase gene (fad7), which increased the trienoic fatty acid (16:3 and 18:3) content. At the same time, suppression of fad7 has resulted in a decrease in the trienoic fatty acid which helped the tobacco plant to get acclimatize to high temperatures (Murakami et al., 2000). However, it is vital to note that the response of growth temperature on the organism is species-specific, with no overall consistent relationship between fatty acid unsaturation and temperature (Guschina and Harwood, 2006).
Mesophilic cyanobacteria cultures grow within a high diurnal temperature range of 25-30°C throughout the year. We are curious to study the effect of different growth temperature on the fatty acid composition of mesophilic nitrogen-fixing cyanobacteria from two different habitats. The purpose of our work was to explore the effect of different growth temperatures, within the range of 10°C-40°C, on the fatty acid composition of two Nostoc species i.e. Nostoc spongiaeforme (Freshwater)and Nostoc calcicola (Marine water) nitrogen-fixing cyanobacteria.
MATERIALS AND METHODS
Culture of N. spongiaeforme and N. calcicola
Temperature
Once the exponential growth phase was set, both Nostoc species were grown at different experimental temperatures (10, 20, 30, and 40 °C) on the 13th and 25th day in sterilized BG-11 and ASN III medium.
Fatty acid extraction
50 mg of lyophilized tissue was transferred to 50ml graduated centrifuge tube with 5ml of pre-cooled (4°C) 1:2 (v/v) chloroform: methanol and spin-vortex for 3 min and sonicated for 15min in a sonicator. Samples were centrifuged at 4°C, 10,000 x g for 10 min and supernatant was pooled in a fresh 50 ml tube. To the pellet, 2.5 ml pre-cooled 1:1 (v/v) chloroform: methanol was added, vortexed and centrifuged for 10min at 10,000 x g at 4°C and supernatant were pooled out and this step was repeated for 3-4 times. Pooled supernatant was filtered using Whatman filter paper and wash by mixing with an equal volume of potassium phosphate buffer (50 nm; pH-7.5). The filtrate was centrifuged for 10 mints at 10,000 x g at 4°C and the lower organic phase was collected and total lipids were dried under N 2 using a sample concentrate and total dried lipid was stored at -20°C for further analysis.
Conversion of total lipid to fatty acid methyl esters (FAME) by transmethylation
The fatty acid profile of N. spongiaeforme and N. calcicola were performed by Gas Chromatography coupled with Mass spectrophotometer. Total lipid dissolved in 1 ml of methanolic NaOH and kept for drying in the oven at 80°C for 5 hours. After drying, 2ml of 5% methanolic HCL was added and vortex for 20 sec. Tubes were kept for drying at 80°C for 5 hours. To this 1 ml of water (Milli Q) and 2 ml of 100% Hexane (HPLC grade) was added, spine and allow to settle down. Top layer was transferred into a new vial and this step was repeated 3 times. At the last stage, the upper layer was collected and 1 ml saturated sodium bicarbonate was added, spine and allow to settle down and the upper layer was collected into the new vial. To this, 1 ml sterile water was added with few crystals of CaCl2 and allow for settle down and again upper layer was transferred into the new vail and dry with N2 and before injecting into the GC-MS injector, the sample was dissolved in 500 µl of 100% hexane.
GC-MS analysis
Chromatography was performed using a Shimadzu Mass spectrometer-2010 plus (CIF, SPPU, Pune) equipped with GC-MS-TQ8030, equipped with an autosampler (AOC-20s). An electron ionization system coupled with an ionization energy of 70eV was used for GC-MS detection. The carrier gas used was He at a flow rate of 1ml per minute. Column oven and injector temperatures were set 50°C and 270°C. The compound was identified by matching the mass spectra with the inbuilt library National Institute of Standard and Technology (NIST).
RESULTS
Fatty acid methyl ester (FAME) composition of two Nostoc species at different temperatures is shown in (Fig.1, 2 and Table 1, 2). From our study, it was shown that the saturated and mono-unsaturated fatty acid increased by increasing the temperature in N. spongiaeforme . N. spongiaeforme , at 40°C showed the maximum percentage of saturated and monounsaturated fatty acid (38.28 and 36.24% respectively) as compared to control (30°C). Whereas, under the same conditions, the poly-unsaturated fatty acid was reduced to 3.14% compared to control. Through our study, it was also observed that N. spongiaeforme when grown at a relatively lower temperature (20°C), resulting in an increase in the poly-unsaturated fatty acid 43.71% as compared to control. Whereas, at 10°C only 19.33% poly-unsaturated fatty acid was observed which was far less than control (39.44%).
N. calcicola also showed variation in the fatty acid composition when grown at different growth temperatures. The percentage of saturated fatty at 40°C (30.77%) was more or less similar to control (31.52%), whereas mono-unsaturated fatty acid was increased to 30.73% compared to control (14.48%). Where, at same temperature, the poly-unsaturated fatty acid was reduced 17.91% compared to control (38.36%). Cultures grown at 10°C, resulted into reduce saturated and monounsaturated fatty acid compared to higher temperature treatment, whereas poly-unsaturated fatty acid was increased to 41.45% as compared to control. On the other hand, 20°C showed highest amount of saturated fatty acid and the least amount of monounsaturated fatty acid among all the growth temperatures whereas, 33.46% of poly-unsaturated fatty acid was detected.
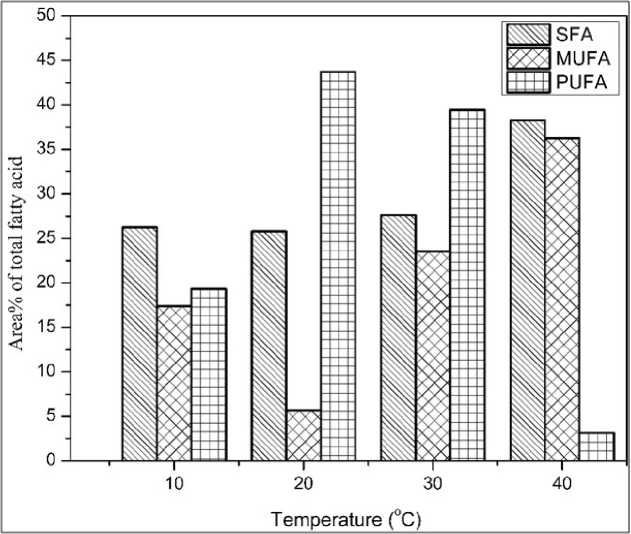
Figure 1: Effect of different growth temperatures on the total methyl esters of fatty acids of N. spongiaeforme . Abbreviations - SFA: saturated fatty acid; MUFA: monounsaturated fatty acid; PUFA: polyunsaturated fatty acid. N. spongiaeforme : Nostoc spongiaeforme.
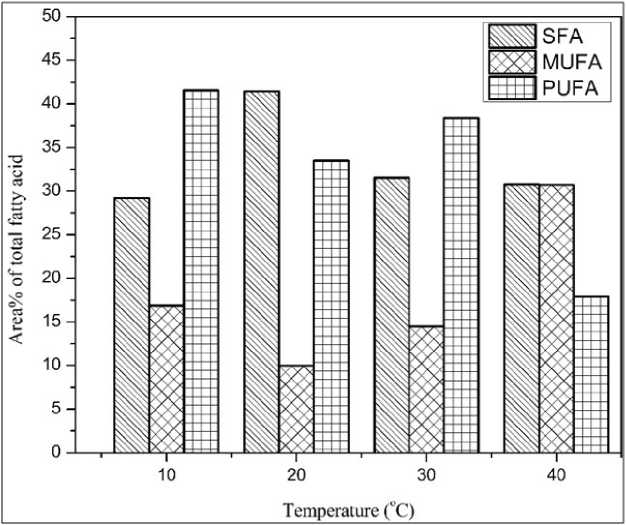
Figure 2: Effect of different growth temperatures on the total methyl esters of fatty acids of N. calcicola . Abbreviations - SFA: saturated fatty acid; MUFA: monounsaturated fatty acid; PUFA: polyunsaturated fatty acid; N. calcicola : Nostoc calcicola
|
Table 1: Analysis of Fatty acid profile based on the methyl ester of fatty acids (Area% of fatty acids) of Nostoc |
||||
|
Fatty acid |
Percent of fatty acids (% of total fatty acids) |
|||
|
10°C |
20°C |
30°C |
40°C |
|
|
9-Hexadecenoic acid, methyl ester (C16:1) |
3.02 |
ND |
15.18 |
3.97 |
|
Hexadecenoic acid, methyl ester (C16:0) |
13.92 |
21.55 |
23.94 |
17.38 |
|
9,12-Octadecadienoic acid, methyl ester (C18:2) |
10.28 |
2.32 |
18.98 |
2.86 |
|
9,12,15-Octadecatrienoic acid, methyl ester |
8.34 |
27.16 |
18.93 |
ND |
|
9-Octadecenoic acid methyl ester (C18:1) |
14.36 |
5.01 |
7.62 |
32.27 |
|
Octadecanoic acid (C18:0) |
10.81 |
3.99 |
3.16 |
14.69 |
|
Glycidyl palmitate |
1.52 |
ND |
0.29 |
0.62 |
|
Ethyl 9,12-hexadecadienoate |
0.71 |
1.95 |
ND |
ND |
|
Methyl tetradecanoate (C14:0) |
ND |
0.20 |
0.23 |
ND |
|
Tridecanoic acid, 4,8,12-trimethyl, methyl ester |
ND |
0.22 |
0.18 |
ND |
|
Cis-10-Heptadecenoic acid |
ND |
0.67 |
ND |
|
|
Linoleic acid ethyl ester |
ND |
12.28 |
ND |
ND |
|
Methyl 5,11,14-eicosatrienoate |
ND |
ND |
1.35 |
ND |
|
Pentadecanoic, methyl ester (C15:0) |
ND |
ND |
ND |
1.35 |
|
7,10-Hexadecadienoic acid, methyl ester |
ND |
ND |
ND |
0.28 |
|
Heneicosanoic acid, methyl ester |
ND |
ND |
ND |
1.6 |
|
SFA MUFA PUFA |
26.25 17.38 19.33 |
25.80 5.68 43.71 |
27.62 23.74 39.44 |
38.28 36.24 3.14 |
Analysis of Fatty acid profile based on the methyl ester of fatty acids (Area% of fatty acids) of Nostoc spongiaeforme grown under different temperature.
ND: Not detected; SFA: saturated fatty acid; MUFA: mono-unsaturated fatty acid; PUFA: poly-unsaturated fatty acid
Table 2: Analysis of Fatty acid profile based on the methyl ester of fatty acids (Area% of fatty acids) of Nostoc calcicola (Marine cyanobacteria) grown under different temperature.
|
Fatty acids |
Percent of fatty acids (% of total fatty acids) |
|||
|
10°C |
20°C |
30°C |
40°C |
|
|
9-Hexadecenoic acid, methyl ester (C16:1) |
6.19 |
7.05 |
5.95 |
2.44 |
|
Hexadecenoic acid, methyl ester (C16:0) |
23.66 |
28.92 |
26.28 |
21.77 |
|
9,12-Octadecadienoic acid, methyl ester (C18:2) |
13.62 |
10.62 |
17.63 |
17.91 |
|
9,12,15-Octadecatrienoic acid, methyl ester |
27.51 |
22.4 |
20.73 |
ND |
|
9-Octadecenoic acid methyl ester (C18:1) |
10.68 |
2.53 |
7.38 |
28.29 |
|
Octadecanoic acid (C18:0) |
5.21 |
11.57 |
4.7 |
7.84 |
|
Glycidyl palmitate |
0.33 |
0.3 |
0.27 |
0.65 |
|
8,11,14-Docosatrienoic acid, methyl ester |
0.41 |
0.36 |
ND |
ND |
|
Methyl tetradecanoate (C14:0) |
ND |
0.34 |
0.27 |
ND |
|
Cis-10-Heptadecenoic acid |
ND |
0.4 |
0.46 |
ND |
|
Octadecanoic acid, 3-hydroxy-methyl ester |
ND |
0.31 |
ND |
ND |
|
Cis-10-Nonadecenoic acid, methyl ester (C19:1) |
ND |
ND |
0.69 |
ND |
|
2,6,10-Trimethyltridecane |
ND |
ND |
ND |
0.57 |
|
Cyclopropaneoctanoic acid, 2-octyl-methyl ester |
ND |
ND |
ND |
0.51 |
|
SFA |
29.20 |
41.44 |
31.52 |
30.77 |
|
MUFA |
16.87 |
9.98 |
14.48 |
30.73 |
|
PUFA |
41.54 |
33.46 |
38.36 |
17.91 |
DISCUSSION
temperature, whereas in the same condition unsaturated fatty acid (MUFA and PUFA) increased. Due to this, the degree of unsaturated fatty acid in both the Nostoc species increased. However, N. calcicola showed higher poly-unsaturated fatty acids as compared to control. It could be due to this reason N. calcicola showed a better growth rate compared to N. spongiaeforme (data not shown). As high degree of unsaturated may help the cells to maintain the membrane fluidity under cold conditions. Seto et al., (1984) reported an increase in the polyunsaturated fatty acid at low temperature in Chlorella which could be due to the sensitivity of the fatty acid biosynthetic enzymes. A similar pattern was observed by Chen et al. (2008) who reported an increase in the polyunsaturated fatty acid in Nitzschia laevis at low temperature. Our study was also on par with iang and Chen, 2000, their study also showed an increase in the polyunsaturated fatty acid at low temperature in Crypthecodinium cohnii a marine microalga. On the other hand, when the temperature was increased to 40°C there was a drastic reduction in the degree of unsaturated fatty acid. The decrease in the unsaturated fatty acid was escorted by the enhancement of saturated fatty acid. This may suggest that both Nostoc species was able to survive at higher growth temperature by adapting themselves to producing more saturated fatty acid compared to low temperature ( iang and Chen, 2000). Through our result, it was also seen that, both the Nostoc species produced a maximal degree of polyunsaturated fatty acid when grown at 20°C and 30°C (control).
CONCLUSION
Our study concludes that the varying growth temperature showed effects on the fatty acid composition of N. spongiaeforme and N. calcicola. The degree of fatty acid saturation increased at higher growth temperature which may have helped the Nostoc species to withstand the extreme temperature stress. Whereas, in order to maintain the fluidity of the membrane, on the other hand, low temperature resulted in the enhancement of unsaturated fatty acid. The temperature between 20°C to 30°C showed maximum fatty acid production in both the Nostoc species. Hence,
20°C-30°C is recommended temperature for producing maximum amount of fatty acid content in mesophilic nitrogen fixing cyanobacteria for biofuel production.
ACKNOWLEDGEMENT
The authors are very grateful to CIF, SPPU, Pune for analyzing the fatty acid samples. This research was supported by a grant from University Grant CommissionSpecial Assistant Program (UGC-SAP).
CONFLICTS OF INTEREST
The authors declare that they have no potential conflicts of interest.
Список литературы Effect of temperature on fatty acid profile of Nostoc spongiaeforme (freshwater) and marine water Nostoc calcicola (marine water): A comparative study
- Chen, G. Q., Jiang, Y., & Chen, F. (2008). Variation of lipid class composition in Nitzschia laevis as a response to growth temperature change. Food Chemistry, 109(1), 88-94. doi:10.1016/j.foodchem.2007.12.022
- Chisti, Y. (2007). Biodiesel from microalgae. Biotechnology Advances, 25(3), 294306. doi:10.1016/j.biotechadv.2007.02.001
- Demirbas, A., & Fatih Demirbas, M. F. (2011). Importance of algae oil as a source of biodiesel. Energy Conversion and Management, 52(1), 163-170. doi:10.1016/j.enconman.2010.06.055
- Ge, S., Champagne, P., Plaxton, W. C., Leite, G. B., & Marazzi, F. (2017). Microalgal cultivation with waste streams and metabolic constraints to triacylglycerides accumulation for biofuel production. Biofuels, Bioproducts and Biorefining, 11(2), 325-343. doi:10.1002/bbb.1726
- Guschina, I. A., & Harwood, J. L. (2006). Lipids and lipid metabolism in eukaryotic algae. Progress in Lipid Research, 45(2), 160-186. doi:10.1016/j.plipres.2006.01.001
- Hariskos, I., & Posten, C. (2014). Biorefinery of microalgae-opportunities and constraints for different production scenarios. Biotechnology Journal, 9(6), 739-752. doi:10.1002/biot.201300142
- Hilditch, T. P., & Williams, P. N. (1964). The chemical constitution of natural fats. The chemical constitution of natural fats (4th ed)
- Jiang, Y., & Chen, F. (2000). Effects of temperature and temperature shift on docosahexaenoic acid production by the marine microalge Crypthecodinium cohnii. Journal of the American Oil Chemists' Society, 77(6), 613-617. doi:10.1007/s11746-000-0099-0
- Li, Y., Lian, S., Tong, D., Song, R., Yang, W., Fan, Y., ... and Hu, C. (2011). One-step production of biodiesel from Nannochloropsis sp. on solid base Mg-Zr catalyst. Applied Energy, 88(10), 33133317. doi:10.1016/j.apenergy.2010.12.057
- Murakami, Y., Tsuyama, M., Kobayashi, Y., Kodama, H., & Iba, K. (2000). Trienoic fatty acids and plant tolerance of high temperature. Science, 287(5452), 476-479. doi:10.1126/science.287.5452.476
- Renaud, S. M., Thinh, L. V., Lambrinidis, G., & Parry, D. L. (2002). Effect of temperature on growth, chemical composition and fatty acid composition of tropical Australian microalgae grown in batch cultures. Aquaculture, 211(1-4), 195-214.
- doi:10.1016/S0044-8486(01)00875-4 Stanier, R. Y., Deruelles, J., Rippka, R., Herdman, M., & Waterbury, J. B. (1979). Generic assignments, strain histories and properties of pure cultures of cyanobacteria. Microbiology, 111(1), 1-61. doi:10.1099/00221287-111-1-1
- Seto, A., Wang, H. L., & Hesseltine, C. W. (1984). Culture conditions affect eicosapentaenoic acid content ofChlorella minutissima. Journal of the American Oil Chemists' Society, 61(5), 892-894. doi:10.1007/BF02542159
- Shafiee, S., & Topal, E. (2009). When will fossil fuel reserves be diminished? Energy Policy, 37(1), 181189. doi:10.1016/j.enpol.2008.08.016
- Thompson, Jr., G. A. (1996). Lipids and membrane function in green algae. Biochimica et Biophysica Acta, 1302(1), 17-45. doi:10.1016/0005-2760(96)00045-8
- Tredici, M. R. (2010). Photobiology of microalgae mass cultures: Understanding the tools for the next green revolution. Biofuels, 1(1), 143-162. doi:10.4155/bfs.09.10
- Wang, S., Zhang, D., & Pan, X. (2012). Effects of arsenic on growth and photosystem II (PSII) activity of Microcystis aeruginosa. Ecotoxicology and Environmental Safety, 84, 104-111. doi:10.1016/j.ecoenv.2012.06.028


