Effect of Toxic metals on Seed Germination of Rheum emodi (Wall. Ex Meissn), a Rare Medicinal Plant of Garhwal Himalaya
Автор: Vandana Shukla, Jyoti Thapliyal
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 2 т.17, 2021 года.
Бесплатный доступ
Rheum emodi is a rare medicinal herb in the Himalayan region due to its medicinal cum aromatic properties it is highly over-exploited from nature. Complete Randomized Design was executed and study aimed to understand the effect of different concentration of Lead and Cadmium in growth and biochemical parameters of Rheum emodi (Wall. Ex Meissn) as comparison to control. In plants, heavy metals Quantity were analyzed to show their effects on the animal and human beings who consume them as such or their derived products. In this study, encouraging results were obtained which favoured maximum (53.33%; p<0.05) mean germination in control and minimum in 1.5g concentration in both case of Lead and Cadmium treatment.
Lead, Cadmium, Rheum, Seed germination, Biochemical, Morphological
Короткий адрес: https://sciup.org/143173889
IDR: 143173889
Текст научной статьи Effect of Toxic metals on Seed Germination of Rheum emodi (Wall. Ex Meissn), a Rare Medicinal Plant of Garhwal Himalaya
The great Himalayan region contains thousands of plants which are undoubtedly a boon for human health care. The plant and the plant parts that possess therapeutic values or pharmacological effects on the human body or animal are called medicinal plants. These medicinal plants synthesize and accumulate some secondary metabolites like alkaloids, glycosides, tannins, volatile oils that possess medicinal properties.
Medicinal plants have assumed a fundamental part from the old time frame as they are utilized in customary medication and furthermore as conventional and traditional however egotistical human exercises lead to the destruction of such plants because of this a great deal of plants become extinct from their regular environment and other comes in endangered conditions. Pollution, climate, soil environment, harvesting, and handling are some of the factors, which assume a significant part in the contamination of medicinal plants by metals. Therefore, it is necessary to determine the levels of the metallic elements in the medicinal plants, as these elements when consumed at higher levels become toxic. Toxic metallic stress influences different physiological and biochemical cycles by changing numerous metabolic pathways. Three factors determine the severity of a pollutant i.e. chemical nature, concentration, and persistence. A portion of the basic soil impurities are chlorinated hydrocarbons (CFH), heavy metals, (for example, chromium, cadmium—found in battery-powered batteries, and lead – found in lead paints, avionics fuel, MTBE, zinc, arsenic, and benzene.
Heavy metal affects plants by forming complexes with sulphur, Nitrogen and oxygen ligands (Van A. and Clijsters, 1990) which leads to the alteration of many plant function and plant growth (Woolhause, 1983). They interfere with uptake of minerals (Zhang et al., 2002; Adhikari et al., 2006), function of membrane (Azevedo et al., 2005), relationship with water (Kastori et al., 1992), protein metabolism (Tamas et al., 1997) and germination of seed (Al-Hellal, 1995). Heavy metal’s High concentrations in the soil are toxic to most plant (Macnair, 1993). Today the most commonly anticipate problem is the contamination of soil with toxic chemicals like mercury, chloride, zinc, lead, cadmium, iron etc, which have significant adverse effect on plant productivity.
Cadmium is a significant industrial toxin particularly in zones associated with smelting of zinc and substantial street traffic (Somasekaraiah et al. , 1992; Das et al. , 1997). Cadmium, being a profoundly poisonous metal pollutant of soils, inhibits root and shoot growth and yield production, affects nutrient uptake and homeostasis and a frequently accumulated by agriculturally important crops and then enters the food chain with a significant potential to impair animal and human health (Di Toppi and Gabrielli, 1999).
Lead is a highly noxious and non-disintegrative heavy metal which comprises 0.002% of Earth's crust. Moreover, Lead is the second toxic metal in nature because of its direct toxic effects on living organisms (ATSDR, 2015). Sewage slime containing enormous amount of Pb and different metals which are regularly released on to field and garden soils due to increasing trends in urbanization (Paivoke, 2002).
It has been reported that Lead effects the physiochemical properties and microbial community structure of soil (Akmal and Jianming, 2009) which resulted soil fertility detoriaton which immediately affected the changes of physiological indices and, furthermore, resulted in yield decline (Majer et al. , 2002). Its contamination in soil brings variety of harmful effects on plants i.e. impairment in nutrient uptake, alterations in plant water relations and generation of ROS etc. which results in reduced photosynthesis and cell death leading to substantial decline in crop yield (Uzu et al. , 2009; Hadi, 2015).
Rheum emodi is one of the valuable medicinal plant species. It is the large group of perennial stout herb in the alpine and sub-alpine regions of the world, chiefly in Asia. It is Perennial stout herb, 1.0-3.0m in height, distributed in the temperate and sub tropical region of the world between 2800-3600m altitudes above sea level. Root very stout; leaves radical, long petiole, very large, 30-40cm; flower small, dark purple or pale red in tall axilla panicles (Naithani, 1985). Rheum has five active content namely Emodin, Chrysophanol, Rhein, Rutin and Chrysophenic. Rheum is a mild anthraquinone purgative. It is used as astringent tonic; it stimulating effect combined with aperients properties render it especially useful in a tonic dyspepsia (Chopra et al., 1958). The oil is also sprinkled over ulcer for healing, and powdered roots are used for cleaning teeth. Traditionally, in Garhwal the paste made from the root is mixed with water used to treat boils, wounds and cut. Fresh roots are mixed with Digitalis majalis, boiled over a moderate flame, and applied to cuts and wounds (Maikhuri et al., 2000).
The aim of present study is to survey the impact of various concentrations of lead and cadmium in soil on physiological and biochemical parameters of the Rheum emodi (Wall. ex Meissn).
MATERIALS AND METHODS
Polythene bags filled with amended soil were allowed to stand for 2 days (from the date of 7, Feb. 2019). Therefore, seeds of Rheum emodi were sown. Each treatment had three replicates. Meteorological data also recorded i.e., air temperature, rainfall, relative humidity.
100 seeds of Rheum emodi with triplicate used for moisture content, the percentage moisture content of fresh seeds were determined by oven dry method i.e. 80°C for 24 hours. The percentage moisture content was calculated by the following formula:
Moisture content =
Fresh weight - Dry weight X 100 Fresh weight
Germination percentage, Mean Germination time by Ellis and Roberts (1981), Seedling vigour Index-I (Seedling length X germination) and Index-II (Seedling dry weight X Germination) were calculated (Abdul and Anderson, 1973),
Changes in morphological and biochemical parameters were studied at 30 days interval up to one and half month after initiation of lead and cadmium stress to evaluate the effect of heavy metals treatment in plant growth.
After one month old seedling’s proper growth, under morphology changes Length of root, shoot, leaf & petiole, Width of leaf, Diameter of root, shoot & petiole, Number of root hairs, Total biomass of seedling were observed and recorded at weekly interval. Under Biochemical parameters Chlorophyll, Soluble protein, carbohydrate extraction - estimation followed by Holm method (1954), Bradford method (1976) and Mc. Cready et al. (1950)
Chla ( mg / gfr . wt ) =
( 9.78 xA 662 )-( 0.99 x A 644 ) * volume 1000 * fr . wt
„ . ( 21.4 xA 644 ) - ( 4.65 xA 662 )* volume
Chl b (mg / gfr .w )=1-----------100c fr.w '-------- carotenoids =
( 4.69 x A 440.5 ) - ( Chla + Chlb ) x 0.267 x volume 1000 * fr . wt
Experimental data was analyzed by the software SPSS 25.0 using MANOVA.
RESULTS
The most critical stage of plant life is seed germination (Davies, 2004), effect of biotic and abiotic stresses fully influenced the plant life cycle from beginning to end. These Stresses affect the seed germination and seedling growth either by developing an osmotic pressure that prevents uptake of water or by toxic effects of ions which available in soil (Khan et al. , 2009). The soil of the study site having pH 6.55, available nitrogen, phosphorus, potassium and total nitrogen (Dwivedi et al. , 2015) (Table 1).
Five replicate of seed were used for mean weight of Rheum emodi seed. The mean weight was 17.08±1.99, mean length 4.68±2.68, mean width 3.06±0.35. The moisture content was (4.83±1.97) (Figure 2,c).
In case of Lead condition, morphological parameters data have revealed that all treatments under field condition showed significant variation in measured and observed growth parameters (Table 2). Maximum root length (2.73 cm, p<0.05), shoot length (1.83 cm, p<0.05), leaf length (4.04 cm, p<0.05), total biomass (1.43 gram, p<0.05) measured in control condition soil and minimum root length (0.76 cm, p<0.05), shoot length (1.53, p<0.05), leaf length (1.12 cm, p<0.05), total biomass (0.19 gram, p<0.05) measured in 2 g/kg soil concentration condition (Figure 2, Table 2). With increasing in Pb concentration, there was a gradual decrease observed in plant growth parameters.
In case of Cadmium treatment, maximum root length (1.83; p<0.05), shoot length (2.09; p<0.05), root diameter (1.37; p<0.05), shoot diameter (2.89; p<0.05), leaf width (4.01; p<0.05), leaf length (2.79; p<0.05), petiole length (7.74; p<0.05), petiole diameter (1.52; p<0.05), root numbers (7.84; p<0.05), total biomass (1.24; p<0.05) were found in control condition and minimum found in the highest concentration (2 g/kg soil) of cadmium treatment (Table 3, Figure 3).
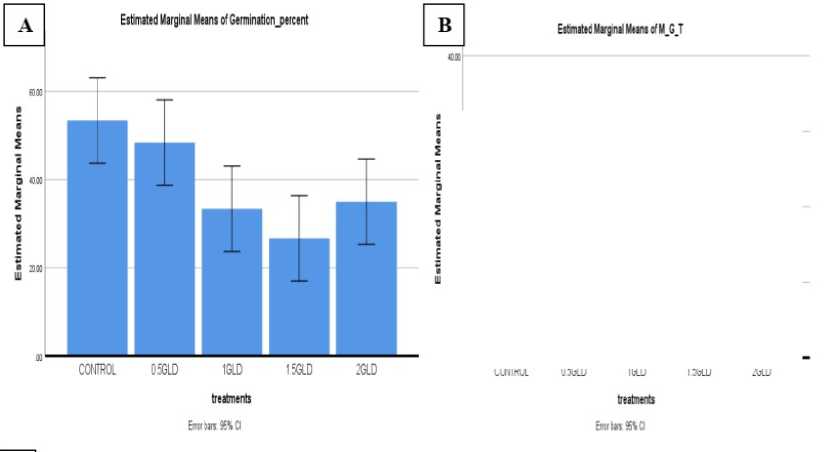
The result of this research showed that the Germination % declined with increasing concentration of Lead metal in a concentration dependent manner, but due to more high concentration of Lead (2 gram) the germination was increased as compare to 0.5gram concentration. Seed % germination highest recorded in control condition (53.33%) followed by at various concentration i.e. 0.5, 1.0, 1.5 gram in soil, it was 38.00%, 33.33%, 26.66% recorded respectively. Seedling vigour index I and II found maximum in control condition (59.21, 43.11) and minimum in 2 g/kg soil Pb (10.25, 9.02) (Table 04).
Table 1: Physio-chemical characteristics of control soil and both treated soil.
|
pH |
Total Nitrogen (kg/ha) |
Available Nitrogen (kg/ha) |
Phosphorous (kg/ha) |
Potassium (kg/ha) |
Organic carbon content |
|
|
Control soil |
6.55 |
100.21 |
25.08 |
26.91 |
163 |
0.22% |
|
0.5gram Pb |
6.35 |
95.14 |
23.21 |
26.12 |
161.01 |
0.20 |
|
1gram Pb |
6.01 |
89.01 |
19.4 |
25.01 |
161.22 |
0.17 |
|
1.5gram Pb |
5.75 |
82.21 |
15.14 |
27.15 |
155.01 |
0.16 |
|
2 gram Pb |
5.12 |
78.14 |
12.01 |
29.15 |
166.01 |
0.14 |
|
0.5gram Cd |
6.12 |
91.21 |
21.12 |
29.14 |
169 |
0.21 |
|
1gram Cd |
5.85 |
71.14 |
18.21 |
29.87 |
174.12 |
0.23 |
|
1.5gram Cd |
5.26 |
65.23 |
14 |
30.1 |
173.01 |
0.19 |
|
2gram Cd |
5.01 |
61.2 |
10.5 |
35.21 |
163.01 |
0.11 |

Figure 1: Preparation of soil for Rheum emodi seed germination.
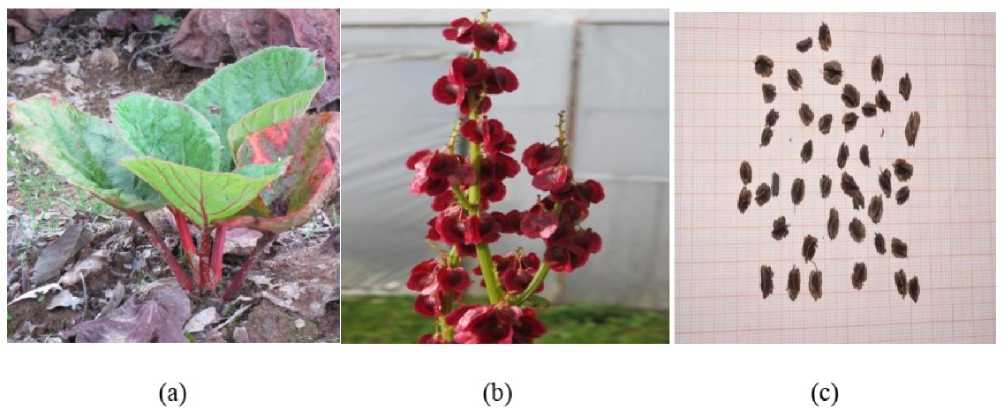
Figure 2: Rheum emodi in its natural site (a) Whole plant (b) Immature seeds (c) Mature seeds
Table 2: Showed effect of Pb (Lead) different concentration in morphological parameters.
|
Treatment (PbNO 3 different concentration) |
Root length (cm) |
Shoot length (cm) |
Root diameter (cm) |
Shoot diameter (cm) |
Leaf width (cm) |
Leaf length (cm) |
Petiole length (cm) |
Petiole diameter (cm) |
Root numbers |
Total biomass (gram) |
|
control |
2.73 |
1.83 |
1.27 |
2.72 |
3.54 |
4.04 |
9.66 |
1.83 |
8.66 |
1.42 |
|
0.5 g/kg soil |
1.66 |
1.72 |
0.67 |
2.43 |
3.11 |
3.12 |
8.76 |
1.19 |
5.51 |
1.12 |
|
1.0 g/kg soil |
1.36 |
1.71 |
0.63 |
2.05 |
1.78 |
2.64 |
7.16 |
1.11 |
4.33 |
0.49 |
|
1.5 g/kg soil |
1.16 |
1.63 |
0.55 |
1.07 |
1.53 |
1.73 |
4.66 |
0.74 |
3.03 |
0.22 |
|
2.0 g/kg soil |
0.76 |
1.53 |
0.34 |
0.89 |
0.73 |
1.12 |
2.24 |
0.71 |
2.12 |
0.19 |
|
Mean |
1.534 |
1.684 |
0.692 |
1.83 |
2.13 |
2.530 |
6.49 |
1.15 |
4.73 |
0.688 |
|
S.D |
0.702 |
0.111 |
0.112 |
0.81 |
0.16 |
1.147 |
3.04 |
0.43 |
1.58 |
0.554 |
|
F |
87* |
54.75* |
47.41* |
55.42* |
56.48* |
47.15* |
45.6* |
75.45* |
85.45* |
60.21* |
|
CL(P=0.05) |
P<0.05 |
P<0.05 |
P<0.05 |
P<0.05 |
P<0.05 |
P<0.05 |
P<0.05 |
P<0.05 |
P<0.05 |
P<0.05 |
*= signigicant at p value 0.05
Table 3: Showed effect of CdNO 3 different concentration in morphological parameters.

A

Figure 3 : A. Morphological comparison of R. emodi seedlings after the effect of using four different Pb concentrations. B. Morphological comparison of R. emodi seedlings grown under the effect of Cd concentration.
B
|
Treatment (CdNO 3 different concentration |
Root length (cm) |
Shoot length (cm) |
Root diameter (cm) |
Shoot diameter (cm) |
Leaf width (cm) |
Leaf length (cm) |
Petiole length (cm) |
Petiole diameter (cm) |
Root numbers |
Total biomass (gram) |
|
control |
1.83 |
2.09 |
1.37 |
2.89 |
4.01 |
2.79 |
7.74 |
1.52 |
7.84 |
1.24 |
|
0.5 g/kg soil |
1.47 |
1.72 |
1.29 |
2.73 |
2.79 |
2.54 |
7.67 |
1.46 |
3.89 |
1.12 |
|
1.0 g/kg soil |
1.36 |
1.64 |
1.19 |
2.32 |
2.43 |
2.21 |
5.12 |
1.26 |
2.21 |
0.88 |
|
1.5 g/kg soil |
1.11 |
1.55 |
0.13 |
1.63 |
1.21 |
1.15 |
3.21 |
0.98 |
1.21 |
0.62 |
|
2.0 g/kg soil |
0.98 |
1.31 |
0.09 |
1.24 |
1.09 |
0.99 |
2.01 |
0.21 |
0.55 |
0.39 |
|
Mean |
1.35 |
1.66 |
0.814 |
2.16 |
2.36 |
1.93 |
5.15 |
1.08 |
3.14 |
0.85 |
|
S.D |
0.33 |
0.28 |
0.64 |
0.70 |
1.20 |
0.81 |
2.58 |
0.53 |
2.91 |
0.12 |
|
F value |
84.64* |
37.11* |
14.55* |
77.77* |
50.43* |
38.92* |
56.82* |
16.37* |
21.48* |
226.87* |
|
p=0.05 |
<0.05 |
<0.05 |
<0.05 |
<0.05 |
<0.05 |
<0.05 |
<0.05 |
<0.05 |
<0.05 |
<0.05 |
(*= significant at p value 0.05)
Table 4: Effect of different concentration of Pb (Lead) treatments in soil showed the various characteristics of Rheum emodi .
|
Lead (Pb) Treatments |
Germination percentage |
Mean Germination Time |
Mean Daily Germination |
Speed of Germination |
Seedling vigour index I |
Seedling vigour index II |
|
Control |
53.33 |
24.00 |
0.18 |
0.38 |
59.21 |
43.11 |
|
0.5 g/kg soil |
38.00 |
24.33 |
0.20 |
0.45 |
38.15 |
32.00 |
|
1 g/kg soil |
33.33 |
23.66 |
0.11 |
0.24 |
26.04 |
15.21 |
|
1.5 g/kg soil |
26.66 |
16.00 |
0.15 |
0.32 |
19.45 |
10.56 |
|
2 g/kg soil |
35.00 |
12.33 |
0.80 |
0.15 |
10.25 |
9.02 |
|
Mean |
37.26 |
20.06 |
0.14 |
0.30 |
30.62 |
21.98 |
|
S.Em(±) |
2.40 |
1.40 |
0.01 |
0.02 |
3.21 |
2.14 |
|
F value |
3.40 |
3.13 |
3.47 |
4.02 |
2.41 |
1.25 |
|
P=0.05 |
NS |
NS |
P<0.05 |
P<0.05 |
P<0.05 |
NS |
ЕпмЬи 95% Cl

EfiOfiOT 95% О
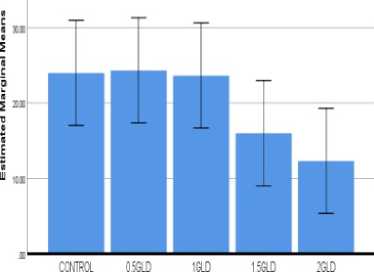
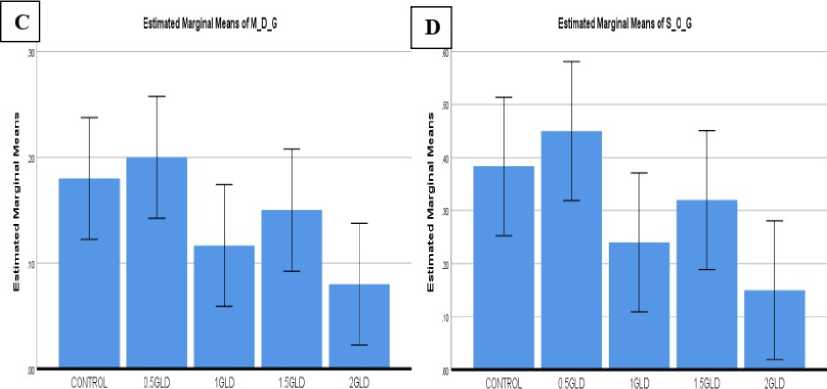
Figure 4: Graph 01: Lead concentration showed the effect of germination percentage (A) Seed germination (B) Mean Germination time (C) Mean Daily germination (D) Speed of germination
Table 5: Effect of different concentration of Cd treatments in soil showed the germination parameters of Rheum emodi .
|
Cadmium (Cd) Treatments |
Germination percentage |
Mean Germination Time |
Mean Daily Germination |
Speed of Germination |
Seedling vigour index I |
Seedling vigour index II |
|
Control |
65.00 |
19.00 |
0.19 |
0.41 |
58 |
42.12 |
|
0.5 g/kg soil |
51.66 |
21.00 |
0.17 |
0.38 |
54.25 |
39.14 |
|
1 g/kg soil |
50.00 |
23.00 |
0.22 |
0.52 |
46.21 |
32.14 |
|
1.5 g/kg soil |
35.00 |
15.66 |
0.15 |
0.28 |
35.21 |
25.21 |
|
2 g/kg soil |
43.33 |
21.66 |
0.19 |
0.43 |
22.12 |
15.21 |
|
Mean |
49.00 |
20.06 |
0.18 |
0.40 |
43.15 |
30.76 |
|
S.Em(±) |
2.80 |
1.90 |
0.01 |
0.04 |
2.01 |
1.25 |
|
F value |
3.12 |
0.42 |
0.61 |
0.69 |
1.02 |
0.15 |
|
P=0.05 |
NS |
NS |
NS |
NS |
P<0.05 |
NS |
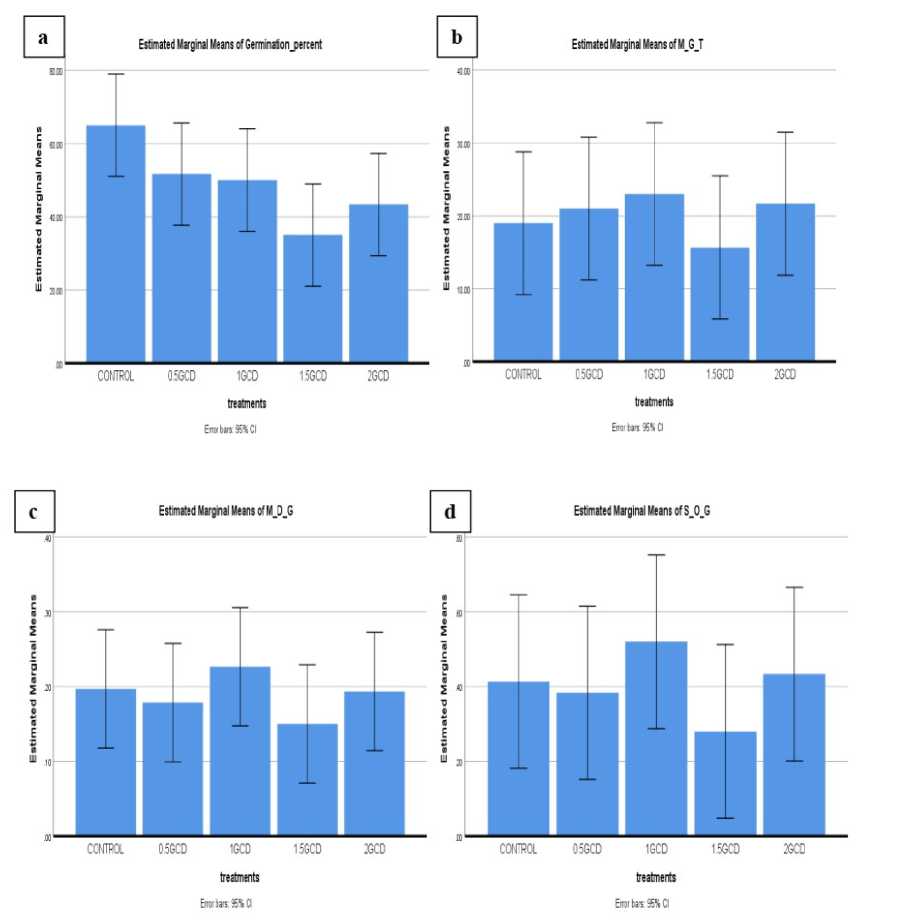
Figure 5: Graph 02: Cadmium concentration showed the effect of germination percentage (A) Seed germination (B) Mean Germination time (C) Mean Daily germination (D) Speed of germination
Table 6: Variation in the photosynthetic content, protein and total amino acid in Rheum emodi seedlings under the different concentration of Pb and Cd
|
Treatments |
Chl a (mg/g) |
Chl b (mg/g) |
Carotenoid |
Protein (mg/g) |
Amino acid |
|
Control |
20.12 |
17.25 |
0.46 |
25.41 |
46.85 |
|
0.5 g lead |
17.12 |
15.24 |
0.68 |
28.50 |
42.67 |
|
1.0 g lead |
14.25 |
12.49 |
1.30 |
31.21 |
38.92 |
|
1.5 g lead |
10.42 |
9.41 |
1.41 |
29.15 |
37.23 |
|
2.0 g lead |
8.12 |
9.15 |
1.54 |
24.61 |
35.38 |
|
Control |
26.44 |
19.45 |
0.89 |
21.34 |
38.40 |
|
0.5 g cadmium |
24.31 |
14.20 |
0.95 |
21.45 |
36.55 |
|
1.0 g cadmium |
20.15 |
11.11 |
1.11 |
22.35 |
31.32 |
|
1.5 g cadmium |
15.45 |
10.21 |
1.15 |
17.78 |
28.90 |
|
2.0 g cadmium |
13.42 |
8.06 |
1.24 |
11.18 |
26.16 |
In this study, five germinated seedlings were randomly selected for estimation of pigment, protein and total amino content. Total three leaves were randomly taken from each selected seedlings and wash two times with tap water and two times with distilled water before the extraction and estimation procedure start. Chl a , Chl b and carotenoid content found maximum in control condition as compare to all different concentration treatments. Protein 25.41mg/g highest found in control condition and lowest found in 24.61mg/g) in 2.0 g/kg soil Pb and (11.18 mg/g) in 2.0 g/kg soil Cd. Highest total amino acid 46.85 mg/g in control condition and lowest total amino content 26.16 mg/g in 2.0 g/kg soil Cd. The seed characteristics parameters data in both treatments graphically represented in Figures 4 and 5.
DISCUSSION
The present study was undertaken to find out the relative response of Rheum emodi to increasing concentration of cadmium and lead in soil. The result clearly show that due to treatment with Cd and lead, there were change in almost all the soil parameters, morphological characteristics and biochemical compared to control .
With increasing concentration of lead and cadmium all the soil parameters were effected compared to control soil. With increasing concentration of both the lead and cadmium, pH of soil decreased.
In treated soil level of total nitrogen and available nitrogen decreased as the concentration of lead and cadmium increased. In case of lead there is constant declined in the total and available nitrogen but in case of cadmium a sharp decline has been seen in the level of available nitrogen and total nitrogen. This is the same as the data collected and calculated by Zhou et al. (2016).
In case of lead treated soil availability of phosphorus first slightly declined with the increasing concentration (0.5 g and 1 g/kg soil) but after that level of phosphorus started to increase (1.5 g and 2 g/kg soil). In case of cadmium, with increasing concentration availability of phosphorus were also increase. Level of potassium in soil also get affected by the increasing amount of lead and cadmium. In case of lead decrease of level of potassium has been observed but in case of cadmium increase in level of phosphorus observed which slightly declined at highest concentration (2 g/kg soil).
Organic carbon content also get effected by the increase concentration of lead and cadmium. In case of lead and cadmium decline of organic carbon content have been observed which is same as observed by Zhou et al. (2016).
Seed germination was found to be reduced after cadmium and lead treatment at all concentrations as compared with control. Decrease in seed germination percentage has been observed as the concentration of Cd increased. In the case of lead total seed germination percent declined with the increasing of concentration of
Lead but compare to the low concentration (0.5 g/kg soil) high germination percentage is seen in the high concentration (2.0 g/kg soil) of Lead.
In Rheum emodi seedlings study, the total chlorophyll content in the test plant showed a decrease in Chl a , Chl b content with the treatment of Cadmium and Lead. Carotenoid content were increased by the increasing amount of Cadmium and Lead 0.5g, 1.0g, 1.5g, 2.0g with compared to control. this is the same as observed by Bhardwaj et al (2009) in the plant species Phaseolus vulgaris L.
Cadmium and Lead exhibited significantly reduction in chlorophyll, protein and amino acid content in leaves. These biochemical parameters showed a negative correlation with leaf area, root length, root diameter, shoot length, shoot diameter, petiole length, petiole diameter and total biomass of the plant at all treatment levels. Most of the changes were observed by dependent toxic metals concentrations. Hence, the morphological and biochemical traits may serve to determine suitable bio indicators of heavy metal pollution and also for the classification of the species as tolerant or sensitive to heavy metals.
Germination % and speed of germination of treated Rheum emodi decreased step by due to increasing conc. of Cadmium and Lead compared to untreated seedlings. In biochemical experiments, due to the increasing concentration of Cadmium and Lead the biochemical contents are all decreased compared to control condition.
CONCLUSION
Our study has tried to hypothesise specific physiological and pathophysiological mechanisms which might be involved in changes of individual blood pressure components. It is fascinating to notice that none of the observed factors (physiological and pathological) has any correlation with Heart Rate. Our subjects were at rest and hence, heart rate was normal, showing no variations. This article is useful to evaluate/predict outcome of specific factor modification influencing specific blood pressure components. It also helps to understand probable underlying mechanisms for the same.
ACKNOWLEDGMENTS
The authors are very thankful to acknowledge the Director HAPPRC. HNB Garhwal University, for providing all the necessary facilities.
CONFLICTS OF INTEREST
Список литературы Effect of Toxic metals on Seed Germination of Rheum emodi (Wall. Ex Meissn), a Rare Medicinal Plant of Garhwal Himalaya
- Abdul B., Andreson A. (1973) Biochemical aspects of seed vigour. Hortic Sci 15, 765–771
- Adhikari T., E. Tel-Or, Y. Libal and M. Shenker (2006). Effect of cadmium and iron on rice (Oryza sativa L.) plant in chelator-buffered nutrient solution. J. Plant Nutr., 29, 1919-1940 (2006).
- Akmal M., Jianming X. (2009) Microbial biomass and bacterial community changes by Pb contamination in acidic soil. J. Agric. Biol. Sci. 1, 30–37.
- Al-Helal, A. A. (1995) Effect of cadmium and mercury on seed germination and early seeding growth of rice and alfalfa. J. Univ. Kuwait (Sciences) 22, 76-83 (1995).
- ATSDR (2015) Priority List of Hazardous Substances. Agency for Toxic Substances andDisease Registry. Public Health Service, United States Department of Health and Human Services, Atlanta, Georgia.
- Azevedo H., Pinto C. G. and Santos C. (2005) Cadmium effect in sunflower: Membrane permeability and changes in catalase and peroxidase activity in leaves and calluses. J. Plant Nutr., 28, 2233-2241.
- Bhardwaj P., Chaturvedi A., & Pratti P. (2009). Effect of Enhanced Lead and Cadmium in soil on Physiological and Biochemical attributes of Phaseolus vulgaris L. Nature and Science. 7. 63-75.
- Chopra R. N., Chopra, I. C., Hnda K. L. and Kapuo L. D. (1958) Indigenous drugs of India. Dhar & Sons Pvt., Ltd., Calcutta.
- Davies P.J. (2004) Plant hormones: biosynthesis, signal transduction, action. Kluwer Academic Press, Dordrecht, pp 89–102.
- Das P., Somantary S. and Rout G.R. (1997) Studies on cadmium toxicity in plant: A review. Environ. Pollut., 98, 29-36.
- Dwivedi B.S. and Sharma G.D. 2015. Management of soil health: Challenges and Oppurtunities. Labortory manual. ICAR. pp. no : 19-31.
- Khan H.A., Ayub C.M., Pervez M.A., Bilal R.M., Shahid M.A., Ziaf K. (2009) Effect of seed priming with NaCl on salinity tolerance of hot pepper (Capsicum annuum L.) at seedling stage. Soil Environment 28(1), 81–87
- Kastori R., M. Petrovic and N. Petrovic (1992) Effect of excess lead, cadmium, copper and zinc on water relations in sunflower. J. Plant Nutr., 15, 2427-2439.
- Maikhuri R. K., Nautiyal S., Rao K. S. and Sexena K. G. (2000). Conservation Policy-people conflicts: An aces study from Nanda Devi Biosphere Reserve (a world heritage site), India. Forest Policy & Economics Report.
- Majer B. J., Tscherko D., Paschke A., (2002) Effect of heavy metal contamination of soils on micronucleus induction in Tradescantia and on microbial enzyme activities: a comparative investigation. Mut. Res.515, 111-124.
- Naithani B. D. (1984),(1985) Flora of Chamoli. Vol.I & II. Botanical Survey of India, Howarh.
- Macnair M.R. (1993) The genetics of metal tolerance in vascular plants. New Phytol., 124, 541-559.
- Pourrut B., Shahid M., Dumat C., Winterton P., Pinelli E. (2011) Lead uptake, toxicity, and detoxification in plants. Rev Environ Contam Toxicol. 213:113-36. doi: 10.1007/978-1-4419-9860-6_4. PMID: 21541849.
- Somashekaraiah B.V., Padmaja K. and Prasad A. P. K. (1992) Phytotoxicity of cadmium ion in germinating seedling of Mung bean (Phaseolus vulgaris): Involvement of lipid peroxides in chlorophyll degradation. Physiol. Plant, 85, 85-89.
- Tamas L., Huttova J. and Zigova Z. (1997) Accumulation of stress protein in intracellular spaces of barley leaves induced by biotic and abiotic factors. Biol. Plant, 39, 387-394.
- Uzu G., Sobanska S., Aliouane Y., Pradere P., Dumat C. (2009) Study of lead phytoavailability for atmospheric industrial micronic and sub-micronic particles in relation with lead speciation. Environ Pollut 157(4):1178–1185
- Van Asshe F. and Clijsters H. (1990) Effects of metals on enzyme activity in plant. Plant Cell Environ., 13, 195-206.
- Woolhouse H.W. (1983) Toxicity and tolerance in the responses of plants of metals. Encyclopedia of plant physiology. (Eds.: O.L. Lange, P.S. Nobel, C.B. Osmond and H. Ziiegler). New Series, Vol. 12C, Springer-Verlag, Berlin. Pp, 245-300
- Zhang, G., M. Fukami and H. Sekimoto. (2002) Influence of cadmium on mineral concentrations and yield components in wheat genotypes differing in Cd tolerance at seedling stage. Field Crop Res., 77, 93-98.
- Zhou, T. & Li, Lijiuqi & Zhang, X. & Zheng, Jennie & Joseph, S. & Pan, Gen-Xing. (2016). Changes in organic carbon and nitrogen in soil with metal pollution by Cd, Cu, Pb and Zn: A meta-analysis. European Journal of Soil Science. 67. 237-246.


