Effect of weed extracts on enzymatic analysis of Vigna unguiculata (L.) Walp. and Lablab purpureus (L.) Sweet
Автор: Shah B., Prajapati M., Patel R.
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 2 т.21, 2025 года.
Бесплатный доступ
Important field crops grow less successfully contaminated by weeds. The environment and human health have suffered severely from improper weed control methods and the unnecessary and inappropriate application of chemical fertilizers, and caused an increase in weed variety resistance. The phytochemicals found in allelopathic plants have gained importance as potential biological equivalents for synthetic herbicides. The enzyme activity of Lablab purpureus (L.) sweet and Vigna unguiculata (L.) Walp can be impacted allopathically by weed extracts. In Vigna unguiculata (L.) Walp and Lablab purpureus (L.) sweet, the enzyme activities of Phyllanthus niruri (L.) and Chloris barbata Sw. weed extracts treated as foliar sprays were assessed throughout the pre-flowering, flowering, and post-flowering stages. This work shows that an aqueous weed extract of Lablab purpureus (L.) sweet and Phyllanthus niruri (L.) and Chloris barbata Sw. can enhance enzyme activity in Vigna unguiculata (L.) Walp, hence providing the possibilities for the development of plant growth.
Chloris barbata sw, enzyme activity, lablab purpureus sweet, phyllanthus niruri, vigna unguiculata
Короткий адрес: https://sciup.org/143184703
IDR: 143184703
Текст научной статьи Effect of weed extracts on enzymatic analysis of Vigna unguiculata (L.) Walp. and Lablab purpureus (L.) Sweet
The current study was conducted at M. N. College, Visnagar, Mehsana, Gujarat (India). The coordinates of the city are 23°41'54.78" N 72°33'7.56" E.
Preparation of extracts
Vigna unguiculata and Lablab purpureus fresh leaves collected after 1 gm leaves pest was prepared using phosphate buffer with the help of mortal-pastel. Centrifuged at 8000 rpm for 7 minutes, followed by recentrifugation at 17,000 rpm for 11 minutes and finally at 27,000 rpm for 5 min. All the centrifugations were carried out at 4°C of the tube temperature. The clear supernatant was collected and used this extract for enzyme activity.
Protease
The activity of the protease enzyme was determined by the Basha and Beevers method (1975).
Take the substrate solution, prepare a 1% casein solution in 0.1 M phosphate buffer (pH 6.0). 0.5 ml of the above-mentioned casein solution was mixed with 0.5 ml of enzyme extract. Incubation the reaction mixture at 37°C for 1 hour then 1 ml of 15% Trichloroacetic Acid (TCA) was added to precipitate the protein. The mixture was then centrifuged to get the extracted amino acids in the supernatant. The enzyme activity was estimated by Lowry et al. (1951) method with 0.5 ml of supernatant. The specific activity of the protease enzyme was determined given the known concentration of tyrosine
(standard) and expressed as mg/g protein.
Polyphenol Oxidase
The activity of polyphenol oxidase enzyme was measured by Van Leyveld and Pretorius method (1973).
Substrate solution has 1% catechol in 0.1 M phosphate buffer at pH 6.0. to 0.1 ml of enzyme extract, 3 ml of substrate solution was added. The absorbance value of the colored solution, developed after 60 seconds, was measured at 485 nm using a spectrophotometer. The specified activity was expressed as a ΔA/min protein.
Peroxidase
Peroxidase activity was calculated with guaiacol as the substrate, as by George,1953 illustrated. The assay mixture contained 1 ml of 0.1 M phosphate buffer with a pH of 6.4, 1 ml of 20 mM guaiacol, H2O2, and 0.1 mL crude enzyme extract. Enzyme activity was measured by recording the optical density (OD) at 420 nm using a spectrophotometer, and the results were expressed as moles H2O2 destroyed per minute per gram of protein.
RESULTS AND DISCUSSION
Proteases are essential enzymes in both prokaryotic and eukaryotic organisms, regulating nutrition and function. In both plants and animals, proteolytic enzymes have a role in metabolic and developmental processes such as germination, senescence, apoptosis, and inflammation. Proteases have been isolated from plants, animals, bacteria, and fungi and employed in medical, food, brewing, molecular biology, leather, detergent, and textile industries. Interestingly, plant-derived proteases have been especially used for a variety of industrial applications because they have beneficial industrial properties such as broad substrate specificity, stability at various pH and temperature levels, and performance in the presence of organic compounds and additives (Aehle, 2004).
In the present study, The effect of Phyllanthus niruri L. and Chloris barbata L. weed aqueous extracts in different concentration protease activity from the leaves of Cowpea (Vigna unguiculata (L.) Walp.) and Indian bean (Lablab purpureus L.) for the estimation of protease which is determined through spectrophotometer as shown in Fig. 1. The results showed that different leaf proteins of Cowpea (Vigna unguiculata (L.) Walp.) and Indian bean (Lablab purpureus L. Sweet) attained higher levels of protease enzyme activity followed by 100 % of Phyllanthus niruri L. in Indian bean (Lablab purpureus L.) as compared to control. Tyrosine was used as a standard for these studies.
Improving crop yields during abiotic and biotic stress is a significant agricultural target. Polyphenol oxidase (PPO) enzymes occur in most plant species, and their foliar production may play a role in tolerance or stress response, as evidenced by enzyme location and sensitivity to environmental factors. PPO has been found in all plants on earth evaluated so far, including Arabidopsis (Tran et al., 2012).
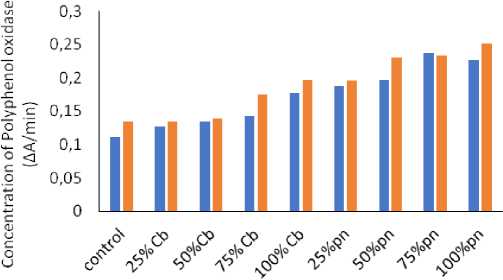
The effect of Phyllanthus niruri L. and Chloris barbata L. weed aqueous extracts in different concentrations of polyphenol oxidase (PPO) activity from the leaves of Cowpea ( Vigna unguiculata (L.) Walp.) and Indian bean ( Lablab purpureus L.) for the estimation of PPO activity was determined through spectrophotometer as shown in Fig. 2, 3 and 4 . The results showed that different leaves proteins of Cowpea ( Vigna unguiculata (L.) Walp.) and Indian bean ( Lablab purpureus L.) observed maximum polyphenol oxidase enzyme activity followed by 100 % of Phyllanthus niruri L. in Cowpea ( Vigna unguiculata (L.) Walp.) and lowest activity shown in 25% Chloris barbata L. extract on Indian bean ( Lablab purpureus L.) compared to control in 40days, 60days and 80days plants. Catechol was used as a substrate.
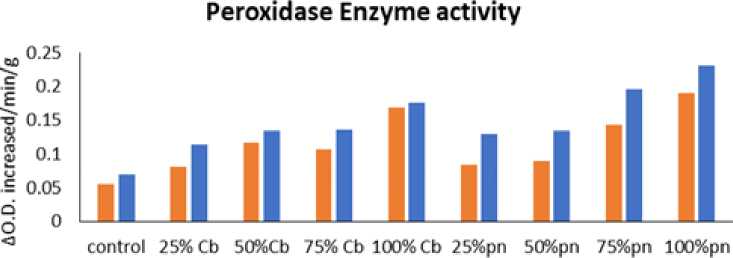
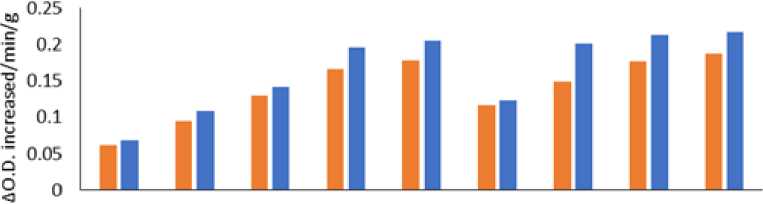
The results showed that Cowpea ( Vigna unguiculata (L.) Walp.) and Indian bean ( Lablab purpureus L.) observed higher levels of peroxidase activity followed by 100 % of Phyllanthus niruri L. in Cowpea ( Vigna unguiculata L.) Walp and low activity showed in 25% Chloris barbata L. extract in Indian bean ( Lablab purpureus L.) compared to control in 40days, 60days and 80days plants as shown in Fig. 5,6 and 7 . Guaiacol was used as a substrate.
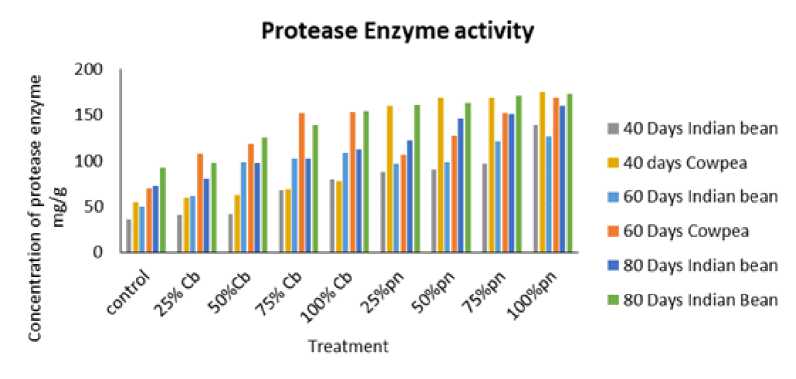
Figure 1: Effect of Phyllanthus niruri L. and Chloris barbata L. extracts on Cowpea ( Vigna unguiculata (L.) Walp.) and Indian bean ( Lablab purpureus L.) Pn- Phyllanthus niruri L. extracts and Cb- Chloris barbata L. extracts
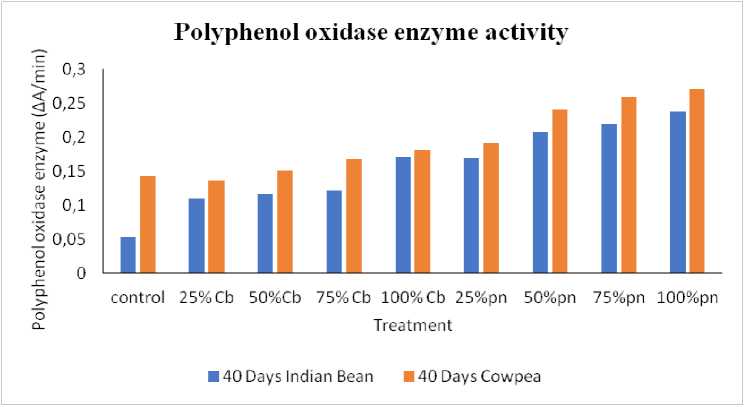
Figure 2: Polyphenol oxidase on Effect of Phyllanthus niruri L. and Chloris barbata L. extracts on Cowpea ( Vigna unguiculata (L.) Walp.) and Indian bean ( Lablab purpureus L.). Pn- Phyllanthus niruri L. extracts and Cb-Chloris barbata L. extracts
Polyphenol oxidase enzyme activity
■ bO Days Indian Bean
60 Days Cowpea
Treatment
Figure 3: Effect of Phyllanthus niruri L. and Chloris barbata L. extracts on Cowpea ( Vigna unguiculata (L.) Walp.) and Indian bean ( Lablab purpureus L.). Pn- Phyllanthus niruri L. extracts and Cb- Chloris barbata L. extracts
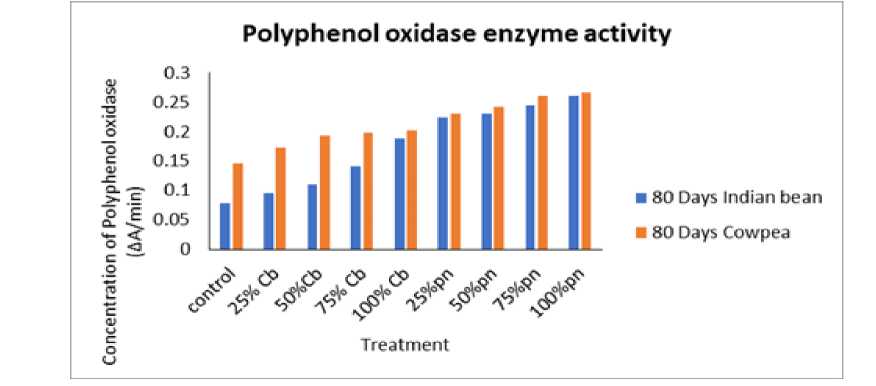
Figure 4: Effect of Phyllanthus niruri L. and Chloris barbata L. extracts on Cowpea ( Vigna unguiculata (L.) Walp.) and Indian bean ( Lablab purpureus L.). Pn- Phyllanthus niruri L. extracts and Cb- Chloris barbata L. extracts
Treatment
-
■ 40 Days Indian bean ■ 40 Days Cowpea
Figure 5: Figure: 5 Effect of Phyllanthus niruri L. and Chloris barbata L. extracts on Cowpea ( Vigna unguiculata (L.) Walp.) and Indian bean ( Lablab purpureus L.) Pn- Phyllanthus niruri L. extracts and Cb- Chloris barbata L. extracts
Peroxidase Enzyme activity
control 25% Cb 50%Cb 7 5% Cb 100% Cb 25%pn 5 0%pn 7 5%pn 100%pn Treatment
-
■ 60 Days Indian bean ■ 60 Days Cowpea
Figure 6: Figure: 6 Effect of Phyllanthus niruri L. and Chloris barbata L. extracts on Cowpea ( Vigna unguiculata (L.) Walp.) and Indian bean ( Lablab purpureus L.). Pn- Phyllanthus niruri L. extracts and Cb- Chloris barbata L. extracts
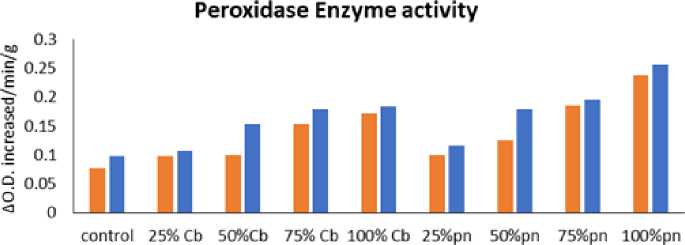
Treatment
■ 80 Days Indian bean ■ 80 Days Cowpea
Figure 7: Effect of Phyllanthus niruri L. and Chloris barbata L. extracts on Cowpea ( Vigna unguiculata (L.) Walp.) and Indian bean ( Lablab purpureus L.). Pn- Phyllanthus niruri L. extracts and Cb- Chloris barbata L. extracts
CONCLUSION
This study was conducted to observe allelopathic effects of Phyllanthus niruri L. and Chloris barbata L. aqueous extracts on Vigna unguiculata (L.) Walp and Lablab purpureus (L.) sweet. Higher enzyme activities were observed in Phyllanthus niruri L. extract effect on Vigna unguiculata (L.) Walp compared to control. Plant necessary enzymes for growth and production of energy. The difference in enzyme activity impacts the effectiveness of enhancing plant development and might be utilized as a rapid method to determine incompatibility.
CONFLICTS OF INTEREST
The authors declare that they have no potential conflicts of interest.