Effects of catechol containing fraction and other fractions of Nauclea latifolia aqueous root-bark extract on blood glucose, lipid profile and serum liver enzymes in streptozotocin - induced diabetic Wistar albino rats
Автор: Ochalefu D.O., Adoga G.I., Luka C.D., Abu A.H.
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 1 т.20, 2024 года.
Бесплатный доступ
Background. Diabetes mellitus has been a menace to healthy human condition from antiquity. There has been continuous search for plant medicinal substances for drug development with an aim to managing this ailment with minimal drug side effects. In this research work, effects of fractions of aqueous root-bark extract of Nauclea latifolia on blood glucose, lipid profile and serum liver enzymes in diabetic rats were investigated. Thirty -five Wistar albino rats weighing 164.1 - 171.6 grammes were used for this study involving aqueous root-bark extract fractions A, B, C and D. The rats were divided into 7 groups of 5 rats each. Group 1 was normal non-diabetic control, group 2 was diabetic control and groups 3, 4, 5 and 6 were rats treated with 250 mg/kg body weight of varying root-bark fractions while group 7 was diabetic rats treated with 5mg/kg. body weight of glibenclamide, the standard anti-diabetic drug. Fasting blood glucose levels were determined using digital glucometer (Acuu-chek, Mannheim, Germany). Lipid profile was determined using standard procedures. Serum liver enzymes were determined using assay kits. Fraction A was analyzed using gas chromatography- mass spectroscopy and nuclear magnetic resonance spectroscopy.
Diabetes mellitus, nauclea latifolia, aqueous extract, catechol
Короткий адрес: https://sciup.org/143182400
IDR: 143182400
Текст научной статьи Effects of catechol containing fraction and other fractions of Nauclea latifolia aqueous root-bark extract on blood glucose, lipid profile and serum liver enzymes in streptozotocin - induced diabetic Wistar albino rats
Diabetes mellitus has been a threat to humanity from ancient times (Jonathan et al. , 2018). This disease has continued to inflict havoc globally (Tabish, 2007). It is a non-communicable disease characterized by metabolic disorder of various aetiologies described by sustained hyperglycaemia with disorders of carbohydrate, protein and fat metabolism due to abnormality in insulin secretion, its action or both (Alberti and Zimmet, 1998; Lowell et al. , 2021). Diabetes mellitus has been recognized as one of the significant killer diseases and a major cause of death in low- and middle income countries, WHO (2014). The disease, with time, affects adversely vital organs like the kidney, eye, heart, liver and the brain (Deshpande et al 2008; Al-Lawati, 2017). It is described as a global fatal trouble (Al-Lawati, 2017).
These complications increase the suffering of these patients as well as causing financial burden on them (Scully, 2012). The complications have been linked with short life expectancy in the diabetic patients (Sean et al., 2017). Whereas majority of diabetic patients need oral hypoglycaemic drugs and or insulin, some of these patients can be managed on diet alone (Aguora et al. , 2015; Forouhi et al. , 2018). Presently, there are many natural or synthetic anti-diabetic drugs in pharmaceutical market. However, these drugs are not readily affordable in addition to their adverse side effects (Akomas et al. , 2014; Ayinla et al., 2014; Aguora et al., 2015). These disadvantages have led to the increased interests in researching towards the discovery of affordable and safer products for the management of diabetes mellitus in recent times ( Remigio et al., 2022; Dasofunjo et al., 2013; Thomson et al., 2007). The World Health Organization has also encouraged research into plant hypoglycaemic agents (Aguora et al., 2015).
The use of plant agents for the management of diseases like diabetes mellitus is not a recent phenomenon. Since ancient times, humanity has looked for solutions for their disease conditions from the plant kingdom (Stojanoski, 1999; Biljana, 2012). The first recorded proof of medicinal plant utilization dates back to as far as 5000 years ago (Kelly, 2009). For instance, the Chinese book on roots and grasses referred to as ‘Pen T’Sao” which was written by Emperor Shen Nung
Circa 2500 BC described 365 drugs (dried parts of medicinal plants) many of which are still in use today and include Rhei rhisoma , Camphor , Theae folium , Podophyllum , the great yellow Gentian, Ginseng , Jimson weed, Cinnamon bark and Ephedra (Biljana, 2012). In this study, fractions of aqueous root-bark extract of Nauclea latifolia plant were investigated for their effects on blood glucose level, lipid profile and serum liver enzymes in streptozotocin - induced diabetic Wistar albino rats as well as the identification of the bioactive principle in the Nauclea latifolia root-bark fraction A.
Nauclea latifolia (African peach) is a herbal medicinal plant commonly used by the Idoma natives of North Central Nigeria for the management of diabetes mellitus (Ochalefu et al, 2018). It is an evergreen multi-stemmed tree and grows to a height of between 10 – 30 metres. The plant is mainly found in the humid tropical rain forest zone and the savannah wood lands of West and Central Africa (Okwori et al. , 2008).
MATERIALS AND METHODS
Sample collection and preparation
The root bark of Nauclea latifolia was harvested from the wild around the Federal University of Agriculture Makurdi, Benue State, Nigeria. The plant material was identified at the Federal School of Forestry, Jos, Plateau State, Nigeria where it was assigned voucher number FHJ 279 and deposited at the school’s herbarium. The root bark was air-dried to a constant weight under shade and then pulverized into powder and stored in air-tight container until its usage.
Extraction
One hundred grammes of the pulverized root-bark was macerated in 1000 ml distilled water at a ratio of 1:10 (powder / solvent) (Das, Tiwari and Shrivastava, 2010). This was stirred intermittently at room temperature for 48 hours, followed by its filtration using muscilin cloth and Whatman No 1 filter paper size 110 mm. The filtrate was concentrated to dryness using water bath at 45oC. This extract was stored in the refrigerator at 4oC.
Fractionation of the Aqueous Root-bark Extract using Column Chromatography
Twenty grammes of the extract was completely mixed with forty grammes of silica gel (silica gel 60-120 mesh) and then dissolved in 100 ml of methanol to form slurry. This was allowed to dry forming a powder. The column was blocked at the stop cock lower down the column. The powder was then carefully poured into the column and mixtures of solvents (methanol, n-hexane and ethyl acetate) were used to run the column. After the column has been loaded, the stop cock was opened to enable the solvent level to drop to the top of the bed (packing). Necessary precaution was taken to ensure that the solvent layer did not go below this point as allowing the solvent level going below the stationary phase will result in air bubbles and channel formation leading to poor separation. The solvent was allowed to elute through the column one drop at a time. Eighteen fractions were collected. The fractions were pooled together to form four major fractions (fractions A, B, C and D) based on their polarity using thin layer chromatography. The fractions were put in water bath at 45oC to enable evaporation of their solvent.
Administration of Nauclea latifolia aqueous rootbark extract fractions to streptozotocin-induced diabetic rats
Thirty- five male Wistar albino rats weighing 164.1171.6 grammes were used for the study involving aqueous root-bark extract fractions A, B, C and D. The rats were divided into 7 groups of 5 rats each. Group 1 was normal, non-diabetic control, group 2 was diabetic control and groups 3, 4, 5 and 6 were diabetic rats treated with 250 mg/kg. body weight of Nauclea latifolia root-bark fractions A, B, C and D respectively while group 7 was diabetic rats treated with 5 mg/kg. body weight of glibenclamide, the standard anti-diabetic drug.
Evaluation of effects of fractions of aqueous root-bark extract of Nauclea latifolia on blood glucose level.
The fractions of aqueous root-bark of Nauclea latifolia were administered orally via intra-pharyngeal feeding canula to the diabetic rats at a dose of 250mg/kg. body weight following the determination of their initial blood glucose levels. The Nauclea latifolia fractions and the standard anti-diabetic drug, glibenclamide (5mg/ kg. body weight) were administered daily for 10 days.
The blood of the experimental rats was collected for fasting blood glucose determination on days 3, 7 and 10 by tail tipping method. The sample of blood was taken through a tiny incision on the tail tip. The blood sample was dropped on the dextrostix reagent pad that was inserted into the digital glucometer (Accu-chek, Mannheim, Germany) and the readings were recorded.
Determination of lipid profile and serum liver enzymes
Total cholesterol (TC), high density lipoprotein cholesterol (HDL-C) and triacylglycerol were determined using assay kits from Agape Diagnostic Switzerland. Very low density lipoprotein cholesterol (VLDL-C) was calculated according to the method of Burstein and Samaile (1960) by dividing the concentration of triacylglycerol by a factor of 5. The estimation of low density lipoprotein cholesterol (LDL-C) was done using the method of Friedwald, Levy and Fredrickson (1972) that entails the differential subtraction of the sum of the cholesterol fractions from total cholesterol
LDL = TC - HDL – VLDL
Assays of serum alanine aminotransferase and aspartate aminotransferase (Reitman and Frankel, 1957) were done according to procedures outlined in the manuals of Randox Laboratories, Antrim, United Kingdom. Serum alkaline phosphatase was analyzed using kits from Teco Diagnostic Anaheim, United States of America.
Further purification of root-bark fraction A using column chromatography
Fraction A having been found to have more positive effects on the biochemical parameters was subjected to further column chromatographic analysis to enhance its purity (This procedure was carried out after the Gas Chromatography – Mass Spectroscopy analysis of the fraction). Similar thin layer chromatographic procedure was performed for all the sub- fractions collected from the column chromatography of fraction A. Sub-fractions with similar polarity were also combined. Evaporation of the solvent left behind dried whitish crystal substance which was subjected to Nuclear Magnetic Resonance Spectroscopy (NMR- Spectroscopy) analysis for characterization and structural elucidation (Figure 1).
Gas Chromatography-Mass Spectroscopy (GC-MS) analysis of Nauclea latifolia Root- bark Fraction A
The root-bark fraction A was analyzed using Gas Chromatography-Mass Spectroscopy at the Multi-user Signs Research Laboratory, Ahmadu Bello University, Zaria, Nigeria.
The conditions were set thus:
i In-port temperature 230oC ii Initial temperature 105oC iii Ramp rate 5o per 5 minutes till 300oC iv Eluates were detected using a Mass Spectrum Detector (MSD).
Peaks were identified using comparison of their Kovat’s indices, literature reports and National Institute for Standards (NIST) – 14 Libraries of compounds.
Determination of the purified sample using Nuclear Magnetic Resonance (NMR) Spectroscopy
The proton NMR spectrum of the purified sample was acquired on a Bruker AV III 400MHZ spectrophotometer at the Strathclyde Institute of Pharmacy and Biological Sciences, University of Strathclyde, Glasgow, Scotland. Deuterated Chloroform, CDCl 3 , was used as solvent.
Statistical Analysis
The statistical analysis was done using statistical package for social sciences (SPSS version 24) software package programme. The results were expressed as Mean ± SEM (standard error of mean), where n = 5, analyzed by one – way Analysis of Variance (ANOVA) and the level of significance determined by least significant difference (LSD). The p values of 0.05 and less were taken to imply statistical significance between the means.
RESULTS
Effects of fractions of aqueous root- bark extract of Nauclea latifolia on blood glucose levels in diabetic Wistar albino rats.
Table 1 shows the effects of fractions of root-bark extract of Nauclea latifolia and glibenclamide on blood glucose levels of the diabetic rats. Root-bark fraction A reduced the blood glucose level significantly (p < 0.05) on days 3, 7 and 10 when compared with the diabetic control rats. Blood glucose level was decreased significantly (p<0.05) by fraction B on day 10 while both fractions C and D led to significant reduction (p< 0.05) of the blood glucose level on day 7 only.
Effects of fractions of aqueous root-bark extract of Nauclea latifolia on serum lipid profile of diabetic Wistar albino rats
Table 2 shows a significant increase (p < 0.05) in the levels of total cholesterol, triacylglycerol, very low density lipoprotein and low density lipoprotein cholesterol in the diabetic control rats compared with the normal control rats whereas the high density lipoprotein cholesterol level was significantly lowered (p < 0.05) in the diabetic control rats compared with the normal control rats. Nauclea latifolia root-bark fractions A, B, C and glibenclamide significantly reduced (p < 0.05) the total cholesterol in the diabetic treated rats compared with the diabetic control rats. The decrease in total cholesterol in the diabetic rats treated with root-bark fraction D was non-statistically significant (p > 0.05) compared with the diabetic control. Fractions A, B and D increased non-statistically significant (p > 0.05) high density lipoprotein cholesterol level in the diabetic treated rats compared with the diabetic control. Treatment of the diabetic rats with fraction C led to nonsignificant decrease in high density lipoprotein cholesterol when compared with the diabetic control.
Fractions A and B brought about a significant decrease (p <0.05) in triacylglycerol in the diabetic treated rats compared with the diabetic control. Fractions C and D reduced triacylglycerol insignificantly (p > 0.05). Very low density lipoprotein cholesterol levels were reduced significantly (p > 0.05) in the diabetic treated rats with fractions A, B, D and glibenclamide when compared with the diabetic control. There was non-significant reduction (p > 0.05) in very low density lipoprotein cholesterol by fraction C. Fractions A, C and glibenclamide decrease significantly low density lipoprotein cholesterol in the diabetic treated rats compared with the diabetic control. There was nonsignificant reduction (p > 0.05) of low density lipoprotein cholesterol by fractions B and D in the diabetic treated rats compared with the diabetic control rats (Table 2).
Effects of fractions of aqueous root-bark extract of Nauclea latifolia on serum liver enzymes in streptozotocin –induced diabetic Wistar albino rats.
Serum aspartate aminotransferase, alanine aminotransferase and alkaline phosphatase levels were significantly increased (p < 0.05) in the diabetic control rats when compared with the normal control. Fraction A and glibenclamide reduced significantly (p <0.05) the level of aspartate aminotransferase in the treated rats compared with the diabetic control rats. Fractions B, C and D did not impact significantly (P> 0.05) on aspartate aminotransferase level.
Alanine aminotransferase level was significantly reduced (p < 0.05) by fractions A, B, C and glibenclamide. Fraction D did not affect its level significantly. Fraction A and glibenclamide caused significant reduction (p < 0.05) in alkaline phosphatase level in the diabetic treated rats whereas fractions B, C and D have no significant effects on them (p > 0.05) (Table 3).
Gas Chromatography-Mass Spectroscopy (GC-MS) result of aqueous root-bark extract fraction A
The result of the GC-MS analysis showed catechol to be the most abundant bioactive principle in fraction A. Other compounds were found in trace amounts only. Catechol has a high match value of 97 (Table 4).
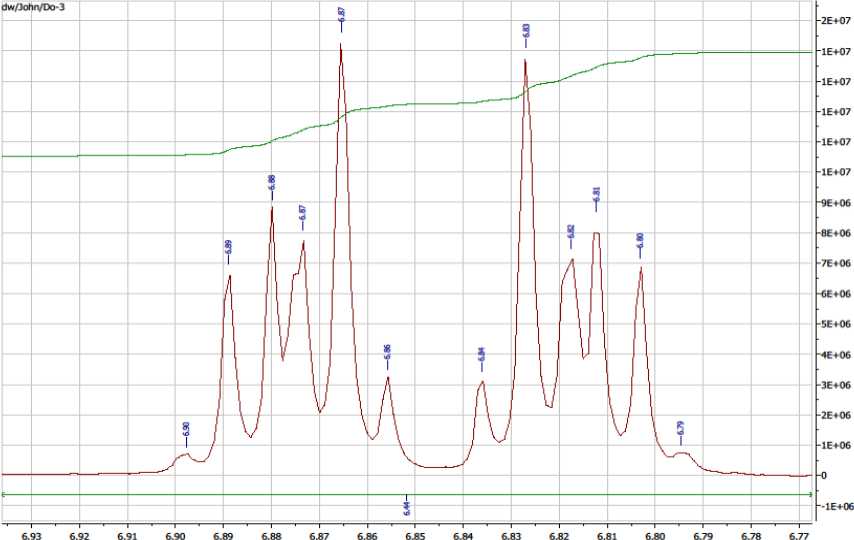
Result of Nuclear Magnetic Resonance (NMR) Spectroscopy confirming that the bioactive principle in Nauclea latifolia aqueous root-bark extract fraction A to be catechol
The following signals at δ 6.90, 6.89, 6.88, 6.87, 6.87, 6.86, 6.84, 6.83, 6.82, 6.81, 6.80 and 6.79 ppm were typical for a disubstituted aromatic system and were consistent with literature reports for catechol (Guadalupe et al , 2010; Lambert et al., 1975; Urara et al. , 2015). Hence the purified substance in fraction A, DO3, (NMR sample number) was identified as catechol (Figure 2 and 3).
|
Treatment/Group |
Day 0 |
Day3 |
Day7 |
Day 10 |
|
Normal control (Group A) |
99.40±2.98 |
101.40±4.05 |
99.60±3.61 |
99.20±2.18 |
|
Diabetic control (Group B) |
328.40±10.50 |
357.20±9.68** |
362.60±11.50** |
365.00±7.65** |
|
NL -root fraction A (Group C) |
299.80±26.40 |
252.20±34.35* |
235.20±28.86* |
198.00±27.59* |
|
NL -root fraction B (Group D) |
302.80±5.72 |
342.60±6.61 |
354.00±6.15 |
265.60±3.37* |
|
NL -root fraction C (Group E) |
352.00±33.38 |
341.20±34.40 |
293.80±27.99* |
334.00±32.36 |
|
NL -root fraction D (Group F) |
368.80±26.21 |
350.60±26.54 |
299.80±24.12* |
347.00±23.14 |
|
Glibenclamide (Group G) |
330.20±12.54 |
270.60±14.18 * |
250.00±17.96* |
169.40±10.70* |
Values are Mean ± SEM of 5 determinations
*= Statistically significant when compared to diabetic control at (p < 0.05)
**= Statistically significant when compared to normal control at (p < 0.05)
|
Treatment /Group |
Total cholesterol (Mmol/L) |
HDL-C (Mmol/L) |
Triacylglycerol (Mmol/L) |
VLDL-C (Mmol/L) |
LDL-C (Mmol/L) |
|
Normal control (Group 1) |
1.88±0.05 |
0.61±0.03 |
0.80±0.09 |
0.16±0.02 |
1.11±0.05 |
|
Diabetic control (Group 2) |
2.32±0.09** |
0.43±0.03** |
1.39±1.27** |
0.28±0.03** |
1.61±0.08** |
|
NL -root fraction A (Group 3) |
1.69±0.05* |
0.53±0.05 |
1.10±0.06* |
0.22±0.01* |
0.94±0.07* |
|
NL- root fraction B (Group 4) |
2.06±0.13* |
0.45±0.05 |
1.13±0.04* |
0.23±0.01* |
1.38±0.13 |
|
NL -root fraction C (Group 5) |
1.98±0.07* |
0.41±0.03 |
1.25±0.04 |
0.25±0.01 |
1.32±0.09* |
|
NL -root fraction D (Group 6) |
2.14±0.09 |
0.50±0.22 |
1.16±0.10 |
0.23±0.02* |
1.41±0.08 |
|
Glibenclamide (Group 7) |
1.72±0.90* |
0.59±0.08* |
0.84±0.09* |
0.17±0.02* |
0.97±0.09* |
HDL-C = High density lipoprotein cholesterol
VLDL-C = Very low density lipoprotein cholesterol
LDL-C = Low density lipoprotein cholesterol
Values are Mean±SEM of 5 determinants
-
* = Statistically significant when compared to diabetic control at (p < 0.05)
-
* * = Statistically significant when compared to normal control at (p < 0.05)
|
Treatment |
AST (IU/L) |
ALT (IU/L) |
ALP (IU/L) |
|
Normal control (Group 1) |
48.40± 4.86 |
18.40± 0.81 |
21.64± 1.48 |
|
Diabetic control (Group 2) |
74.40± 3.96** |
39.00±3.16** |
33.16± 2.52** |
|
NL - root fraction A (Group 3 ) |
65.80± 8.29* |
23.60± 1.44* |
26.32± 2.40* |
|
NL -root fraction B (Group 4) |
73.00± 2.78 |
31.40± 1.60* |
33.64± 4.26 |
|
NL- root fraction C (Group 5) |
74.80± 4.50 |
25.80± 2.50* |
29.40± 1.24 |
|
NL- root fraction D (Group 6) |
68.00± 8.31 |
37.80± 2.52 |
32.74± 2.97 |
|
Glibenclamide (Group 7) |
63.00±5.00* |
20.40± 1.44* |
26.22± 1.78* |
AST= Aspartate aminotransferase
ALT= Alanine aminotransferase
ALP= Alkaline aminotransferase
Values are Mean± SEM of 5 determinations.
-
* = Statistically significant when compared to diabetic control at (p < 0.05)
-
**= Statistically significant when compared to normal control at (p < 0.05).
Table 4: Gas chromatography-mass spectroscopy (GC-MS) result of aqueous root-bark fraction A
Peak number
Retention time Library ID
Reference number
CAS number
Quality of Match
5
15.879 catechol
5860
000120-80-9
97

Figure 1: The purified whitish crystal substance subjected to Nuclear Magnetic Resonance (NMR) Spectroscopy analysis for characterization and structural elucidation (Picture snapped by the researcher).
H (ppm)
Figure 2. Nuclear Magnetic Resonance (NMR) Spectroscopy Spectrum of catechol, the active principle in Nauclea latifolia aqueous extract of root-bark fraction A.
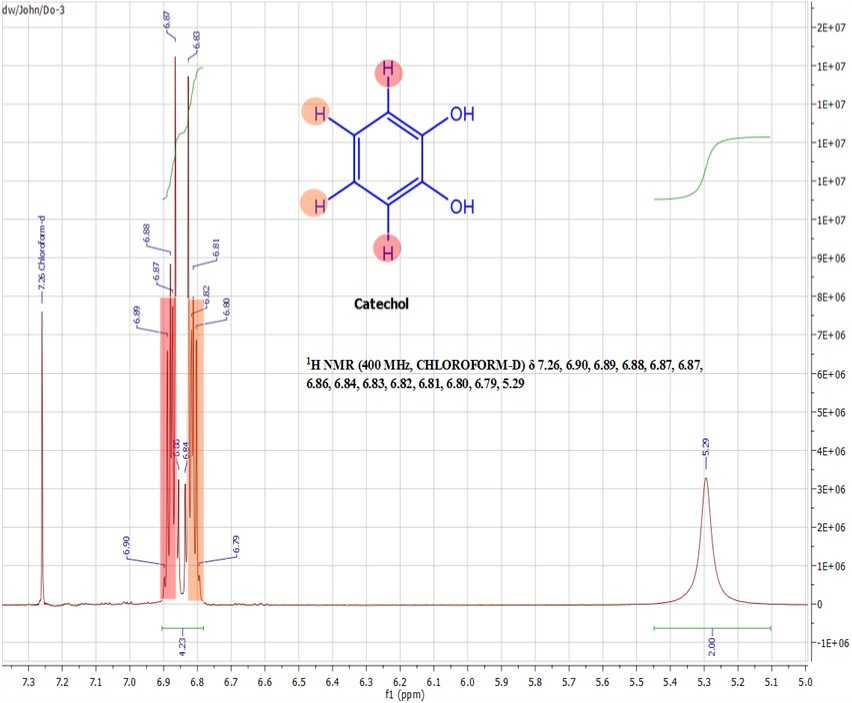
Figure 3. The structure of catechol the bioactive principle in Nauclea latifolia aqueous extract of root-bark fraction A
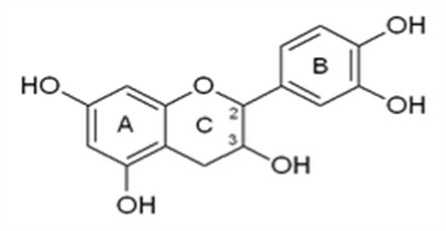
Figure 4. Chemical structure of catechin (a flavonoid) containing catechol (Courtesy of Royal Society of Chemistry, 2015).
DISCUSSION
In this study fraction A of the aqueous root-bark extract of Nauclea latifolia was found to demonstrate the most effective anti-diabetic property in terms of lowering blood glucose level and causing favourable effects on both blood lipid and serum enzyme levels when compared with fractions B, C and D. This fraction was found to contain the bioactive compound, catechol. Catechol which is also known as Pyrocatechol or 1, 2 – dihydrobenzene is an organic compound with molecular formular, C6H4 (OH) 2. The compound occurs as a feathery white crystal that is very rapidly soluble in water. It exists either as a free molecule or a substituent of flavonoids like catechin, quercetin, fisetin and eriodictyol where it represents a 1, 2 dihydrobenzene group (Fitzgerald, 2011; Kapiszewska et al., 2003). Flavonoids are made up of two aromatic rings (A and B rings) connected by a 3 – carbon chain that forms an oxygenated heterocyclic ring (C ring). The B ring is the orthohydroxyl (catechol) structure of flavonoids. (Figure 4).
This B ring hydroxyl configuration in flavonoids is said to be the most important determinant of reactive oxygen species scavenging activity of flavonoids. This is because it donates hydrogen and an electron to hydroxyl, hydroperoxyl and peroxynitrite radicals (Spencer et al., 2012). The catechol structure of the B ring confers anti-oxidative potential to flavonoids (Krych and Gebieka, 2013). This enhances their ability to improve the release of insulin through their anti-oxidative effects on pancreatic β- cells which protect them against further hyperglycemia – induced destruction particularly in type 1 diabetes mellitus (Shi et al., 2019). Many studies support blood glucose lowering activity of flavonoids with catechol moiety (Yeon et al., 2015; Zhang et al, 2015).
Catechin, a flavonoid containing catechol moiety have been found to have hypoglycaemic activity (Cremonini et al. , 2019; Roghani and
Baluchnejadmojarad, 2010; Zhang et al., 2011). Earlier researches have reported the inhibiting effects of catechol containing flavonoids on alpha – glucosidase (Zhenhua et al. , 2014). This membrane bond enzyme is located in the brush border of the small intestine. It catalyses the final step in the digestive process of carbohydrates to release monosaccharides that are absorbable thereby causing increased blood glucose level (Bras et al., 2014). Alpha-glucosidase inhibitors are used to control blood sugar levels in diabetes mellitus (Zhenhua et al. , 2014). The presence of catechol system in the B ring of flavonoids contributes to the distribution of electron cloud that then becomes accessible to give hydrogen atoms to form hydrogen bonds with active site residues of alpha- glucosidase. This plays a critical role in inhibiting its action (Vaya et al., 2003; Carina et al., 2017).
The reduction in blood glucose levels with the administration of catechol moiety containing flavonoids is also attributed to the stimulation of insulin secretion from the remaining portion of beta cells of the pancreas (Chung et al., 2012). Other mechanisms of action of these flavonoids in lowering blood glucose is that they might carry out insulin like effect on peripheral tissues by either enhancing glucose uptake or inhibiting hepatic gluconeogenesis (Chen et al., 2020). The flavonoid, quercetin which contains catechol moiety has been investigated to raise the activity of glycogen synthase, the rate limiting enzyme in glycogen synthesis. This enhances the conversion of excess glucose to its storage form, glycogen. It increases glycolysis while decreasing gluconeogenesis (Yeon et al., 2015).
Catechol containing flavonoids ameliorate lipid profile by inhibiting the major enzymes involved in lipid synthesis in addition to reducing intestinal lipid absorption. They lead to significant decrease in serum levels of triacylglycerol, very low density lipoprotein cholesterol, low density lipoprotein cholesterol (Babu and Liu, 2008; Zheng et al., 2011; Courtney et al., 2017). These results observed in the lipid profile might be due to a reduction in the activity of 3 – hydroxyl - 3-methyl glutaryl coenzyme A (HMG- CoA reductase) and an increase in the activities of plasma lipoprotein lipase and lecithin cholesterol acyltransferase (LCAT) (Zheng et al., 2011; You et al. , 2008; Prince and Kannan, 2006). LCAT plays a key role in the maturation of high density lipoprotein composition, structure, intravascular metabolism and plasma concentration (Chen et al., 2008; Zheng et al., 2011).
The mechanism of the hypolipidaemic effects of flavonoids containing catechol also includes improvement of insulin / glucagon ratio which entails low fatty acid biosynthesis in the liver via the reduction of the expression of sterol regulation element binding protein (SREBP – 1). SREBP – 1 is a transcription factor which controls de novo lipogenesis through induction of lipogenic enzymes (Hwang et al., 2011; Liu et al., 2011; Sharma et al., 2011). The ameliorating effect of catechol containing flavonoids on lipid profile confers on them health benefits in the management of cardiovascular diseases (Babu and Liu, 2008; Ciumărnean et al., 2020). They slow the progression of atherosclerotic cardiovascular disease. Their consumption has been associated with a decrease in endothelium -1 (a molecule involved in blood pressure regulation) and a reduction in myocardial ischaemic perfusion injury (Jun-Ying et al. , 2021; Peregrin, 2005).
There are previous reports on the hepatoprotective effects of catechol containing flavonoids (Tapas et al., 2008; Wu et al., 2006). The administration of catechin which contains catechol to alloxan- induced diabetic mice led to a significant reduction in the serum liver enzymes, aspartate aminotransferase, alanine aminotransferase and alkaline phosphatase (Kadhim, 2013).
CONCLUSION
Fraction A of the aqueous root-bark extract showed the most effective anti-diabetic potential compared to fractions B, C and D of the extract. This fraction was found to contain the bioactive compound, catechol using both Gas chromatography- Mass spectroscopy and Nuclear magnetic resonance spectroscopy. Previous reports on catechol show that it either exists as a free molecule or a substituent of flavonoids and that it confers on them their anti-diabetic property. From the findings it can be deduced that catechol containing fraction of Nauclea latifolia plant has the potential for use in the management of diabetes mellitus and its complications in clinical medicine.
ETHICAL APPROVAL
The ethical guidelines for the care and use of research animals were closely followed.
ACKNOWLEDGEMENTS
The authors express their gratitude to Dr. John Vershima Anyam for allowing the use of his laboratory for part of the initial work. We also appreciate every other persons who assisted us in one way or the other at various stages of this research work.
CONFLICTS OF INTEREST
The authors declare that they have no potential conflicts of interest.
Список литературы Effects of catechol containing fraction and other fractions of Nauclea latifolia aqueous root-bark extract on blood glucose, lipid profile and serum liver enzymes in streptozotocin - induced diabetic Wistar albino rats
- Aguora S O, Unekwe P C, Okechukwu E C (2015). Evaluation of anti-diabetic activity of Nauclea latifolia chloroform root extract in normal and alloxan-induced diabetic rats. IOSR Journal of Pharmacology and biological Sciences, 10 (3):53-57.
- Akomas S C, Okafor A I, Nnah I S (2014) Glucose level, haematological parameters and lipid profile in ficus sur treated diabetic rats. Comprehensive Journal of Agriculture and Biological sciences, 2 (1): 5-11
- Al-Lawati J. A. (2017). Diabetes Mellitus: A Local and Global Public Health Emergency!. Oman medical journal, 32(3), 177-179.
- Albert K G M M, Zimmet P Z (1998). WHO consultation, definition, diagnosis and classification of diabetes mellitus and its complication. Diabetes Medicine, 5 (7): 539-553.
- Aynila M T, Owoyele B V, Yakubu M T (2014). Antidiabetic activity of aqueous extract of Senna fistula leaves in streptozotocin - induced diabetic rats. Nigerian Journal of Biochemistry and Molecular Biology, 29(2): 93-106.
- Babu P V, Liu D (2008). Green tea catechins and cardiovascular health. An update. Current Medicinal Chemistry, 15(18), 1840-1850.
- Biljana B P (2012) Historical review of medicinal plants' usage. Pharmacogen, 6(11):1-5.
- Bras N F, Cerqueira N M F, Ramos M J, Fernandes P A (2014). Glycosidase inhibitors: a patent review (2008-2013). Expert Opinion on Therapeutic Patients, 24(8), 857-874
- Burstein M, Samaile J (1960). Sur un dosage rapide du cholesterol lié aux a-et aux p-lipoprotéines du sérum. Clinical Chem. Acta 5(4): 609 https://doi.org/10.1016/0009-8981(60)90075-9
- Carina P, Marisa F, Daniela R, Eduardo F T O, Joana L C S, Sara M T, Maria J R, Artur M S S, Pedro A F, Eduarda F (1917). Alpha - Glucosidase inhibition by flavonoids: an in vitro and in silico structure activity relationship study. J. Enzyme Inhib. Med Chem., 32(1); 1216-1228.
- Chen L T Shen, Zhang C P, Xu B L, Qiu Y Y, Xie X Y, Wang Q, Lei T (2020) quercetin and isoquercitrin inhibiting hepatic gluconeogenesis through LKB1-AMPK and pathway. Acta Endocrinologica (Buchar), 16 (1): 9-14 doi: 10.4183/aeb.2020.9
- Chen Z Y, Jiao R, Ma K Y (2008). Cholesterol lowering nutraceuticals and functional foods. Journal of Agricultural Food Chemistry, 56, 8761-8773.
- Chen, S. C. C., Tsai, S. P., Jhao, J. Y., Jiang, W. K., Tsao, C. K., & Chang, L. Y. (2017). Liver fat, hepatic enzymes, alkaline phosphatase and the risk of incident type 2 diabetes: a prospective study of 132,377 adults. Scientific reports, 7(1), 4649.
- Chung H C, Geg C N, Rozita Y (2012). A brief review of antidiabetic plants; global distribution, active ingredients, extraction techniques and acting mechanisms. Pharmagn. Rev., 6(11), 22-28.
- Courtney L M, Quinn D, Christopher N B (2017). Effects of dietary flavonoids on reverse cholesterol transport, HDL metabolism, and HDL function. Advances in Nutrition, 8 (2): 226-239.
- Cremonini E, Fraga C G, Oteiza P I (2019). Epicatechin in the control of glucose homeostasis: Involvment of redox-regulated mechanisms. Free Radic. Biol. Med., 130, 478- 488.
- Das K, Tiwari R K S, Shrivastava D K (2010). Techniques for evaluation of medicinal plant products as anti-microbial agent: current methods and future trends. Journal of Medicinal Plants Research, 4 (2): 104-111.
- Dasofunjo K, Nwodo O F C, Johnson J T, Ukpanukpong R U, Ugwu M N and Ayo U I (2013). Phytochemical screening and effect of ethanolic leaf extract of Piliostigma thonningii on serum lipid profile of male albino rats. J. Nat. Prod. Plant Resour. , 3 (2): 5-9.
- Despande A D, Harris- Hayes M, (2008). Epidemiology of diabetes and diabetes related complications. Physical Therapy, 88(11): 1254-1264.
- Fitgerald P A (2011). Adrenal medulla and paraganglia. In: Gardner, shobakk D, (Eds) Greenpans Basic and Clinical Endocrinology (9th ed.) New York, M C Grant-Hill.
- Forouhi N. G., Misra, A., Mohan, V., Taylor, R., & Yancy, W. (2018). Dietary and nutritional approaches for prevention and management of type 2 diabetes. BMJ, 361.
- Friedwald W T, Levy R I, Fredrickson D S (1970). Estimation of the concentration of low density lipoprotein cholesterol in plasma without use of the preparative ultracentrifuge. Clinical Chemistry, 18: 499-502.
- Guadalupe A, Williams B, Vitaly A R, Robert H S (2010). Absortption spectrum, mass spectrometric properties and electronic structure of 1, 2 benzoquinine. Journal of Physical Chemistry A, 114 (28):7470-7478.
- Hwang Y P, Choi J H, Yun H J, Han, E H K, J Y, Park B H, Khanal T, Choi J M, Chung Y C, Jeong H G (2011). Purple sweet potato Anthocyanins attenuate hepatic lipid accumulation through activating adenosine monophosphate-activated protein kinase in human HepG2 cells and obese mice. Nutrition Research, 31(12), 896-906.
- Jonathan E E, Esther O A, Ibrahim A O, Osede I I (2018) Antidiabetic effects of ethanolic root extract of Uvaria chamae P Beauv (Annonaceae) in alloxan -induced diabetic rats: A potential alternative treatment for diabetes mellitus. Advances in pharmacological and Pharmaceutical Sciences volume 2018/ Article ID 1314941
- Jun-Ying J, Er-huan Z, Li-Juan L V, Qiu-yu L, Chun-Hua Z, Min-hui L (2020). Flavonoids in myocardial ischaemic- reperfusion: Therapeutic effects and mechanisms. Chinese Herbal Medicines, 13(1): 4963 doi; 10.1016/j.chmed.2020.09.002.
- Kadhim K. G. (2013). Effect of alcoholic catechin extract in hyperglycaemia, hyperlipidaemia and liver functions in alloxan diabetic mice. Baghdad Science Journal, 11 (3), 1192-1200
- Kapiszenska M, Malgorzata K, Urszula W, Agnieszka C (2003). The COMT-mediated metabolism of flavonoids and oestrogen and its relevance to cancer risk. Polish Journal of Food and Nutrition Sciences, 12(1), 141-146.
- Kelly K (2009) History of medicine. New York: Facts on file, pp. 29-50.
- Krych J, G L (2013). Catalase is inhibited by flavonoids. International of Biological Macromolecules, 58, 148-153.
- Lambert F, Ellenberger M, Merlin L, Chohen Y (1975) NMR study of catechol and some catecholamines. Organic Magnetic Resonance, 7, 266-273.
- Liu J F, Ma Y, Wang Y, Du Z Y, Shen J K, Peng H L (2011). Reduction of lipid accumulation in HepG2 cells by luteolin is associated with activation of AMPK and initiation of oxidative stress. Phytotherapy Research, 25(4), 588-596.
- Ciumarnean, L., Milaciu, M. V., Runcan, O., Vesa, S. C., Rachisan, A. L., Negrean, V., Perne, M. G., Donca, V. I., Alexescu, T. G., Para, I., & Dogaru, G. (2020). The Effects of Flavonoids in Cardiovascular Diseases. Molecules (Basel, Switzerland), 25(18), 4320.
- Lowell D, Aldeam F and Felix O (2021). Diabetes mellitus and its complications: The role of adipose tissues. Int. J. Mol. Sci. 22 (14):7644
- Ochalefu D O, Adoga G I, Luka CD, Abu H A, Amali O O E, Agada S A and Alonyenu E I (2018). Phytochemical composition and effect of Nauclea latifolia aqueous extracts on blood glucose levels of streptozotocin-induced diabetic Wistar albino rats. Journal of Biomedical Research and Clinical Practice, 1(2): 157-163.
- Okwori A E J, Okeke C I, Uzoechina A, Etukudoh N S, Amali M N, Adetunji J A (2008) The anti-diabetic potential of Nauclea latifolia. African Journal of Biotechnology, 7 (10): 1394-1399.
- Peregrin T (2005). Wine a drink to your health. Journal of American Diet Association, 105(7), 1053-1054.
- Prince P, Kannan N K (2006). Protective effect of rutin on lipids, lipoprotein, lipid metabolizing enzymes and glycoproteins in streptozotocin-induced diabetic rats. Journal of Pharmacy and Pharmacology, 58, 1373-1383.
- Reitman S, Frankel (1957). A colorimetric method for determination of SGOT and SGPT. American Journal of clinical Pathology, 28: 56-63.
- Remigio U, Stephen M, Freeborn R (2022). Natural products for treatment and management of diabetes mellitus in Zimbabwe- a review. Front Pharmacol. 13: 980819 Doi;10:3389/fphar.2022.980819.
- Roghani M, Baluchnejadmojarad T (2010). Hyperglycaemic and hyperlipidaemic effect and antioxidant activity of chronic epigallo catechin-gallate in streptozotocin-induced diabetic rats. Pathophysiology, 17, 55-59.
- Scully T. 'Diabetes in numbers' Nature vol. 485 no 7398, pp.S2-S3.
- Sharma A.K, Barti S, Goyal S, Arora S, Nepal S, Kishore K, Joshi S, Kumari S, Arya D.S (2011). Up-regulation of PPAR gamma, heat, shock protein-27 and -72 by naringin attenuates insulin resistance, beta cell dysfunction, hepatic steastosis and kidney damage in a rat model type 2 diabetes mellitus. British Journal of Nutrition, 106(11), 1713-1723.
- Shi G J, Li Y, Cao Q H, Wu H X, Tang X Y, Gao X H, Yu J Q, Chen Z, Yang Y (2019). In -vitro and vivo evidence that quercetin protects against diabetes and its complications: A systematic review of the literature. Biomed. Pharmacother. 2019, 109, 1085 -1099.
- Spencer J P, Vafeiadou K, Williams, R. J. (2012). Neuroinflammation: modulation by flavonoids and mechanisms of actions. Molecular Aspects Medicine, 33(1), 83-97.
- Stojanoki N (1999) Development of health culture in Veles and its region from the past to the end of the 20th Century. Veles: Society of Science and Arts, 13-34
- Tabish S A (2007) Is diabetes becoming the biggest epidemic of the twenty- first century? International Journal of Health Sciences 1 (2): 5-8
- Tapas A R, Sakarkar, D M, Kakole R B (2008). Flavonoids as nutraceuticals: a review. Tropical Journal of Pharmaceutical Research, 7, 1089-1099.
- Thomson M, Al-Arim Z, Al-Quttan K K, Shaban, H L, Ali M (2007). Antidiabetic and hypolipidaemic properties of garlic (Allium sativum) in STZ-induced diabetic rats. Int. J. Diabetes and Metabolism, 15: 108-115.
- Urana H, Masaki M, Hiroshi U, Andre J V (2015). Catechol - bearing block copolymer micelles: structural characterization and anti-oxidant activity. Polymer, 66, 1-7.
- Vaya J, Mahmood S, Goldblum A (2003). Inhibition of LDL oxidation by flavonoids in relation to their structure and calculated enthalpy. Phytochemistry, 62(1), 89-99.
- World Health Organisation (2014). Global health estimates 2013: Deaths by cause, age, sex and country 2000 - 2002, WHO, Geneva, Switzerland.
- Wu Y, Wang F, Zheng Q (2006). Hepatoprotective effect of total flavonoids from Laggera alata against carbon tetrachloride-induced injury in primary cultured neonatal rat hepatocytes and in rats with hepatic damage. Journal of Biomedical Science, 13(4), 569-578.
- Yeon J Y, Bae Y J, Kim E Y (2015). Association between flavonoid intake and diabetes risk among the Koreans. Clinica Chima Acta, 439, 225-230.
- You C L, Su C L, Zhon C L (2008). Study on effect of mechanisms of Scutellaria baicalensis stem, leaf total flavonoid in regulating lipid metabolism. Chinese Journal of Clinical Mater. Medicine, 33, 1064-1066
- Zhang B, Kang M, Xie Q, Xu B, Sun C, Chen K, Wu Y (2011). Anthocyanins from Chinese bayberry extract protect ß-cells from oxidative stress mediated injury via HO-1 up -regulation. Journal of Agricultural Food Chemistry, 59, 537-545.
- Zhang, X, Huang H, Zhao X, Ding R, Lu W, Wang, Y (2015). Effects of flavonoids - rich Chinese bayberry pulp extracts on glucose consumption in human HepG2 cells. Journal of Functional Foods, 14, 144-153.
- Zhenhua Y, Wei Z, Fajin F, Yong Z, Wenyi K. (2014). Alpha-glucosidase inhibitors isolated from medicinal plants. Journal of Food Science and Human Wellness, 3(3), 136-174.


