Effects of exogenous silicic (Si) and salicylic acid (SA) applied individually or in combination on barley growth and nitrogen uptake under various watering regimes
Автор: Kurdali F.
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 4 т.19, 2023 года.
Бесплатный доступ
This pot experiment investigated the effect of treatment with exogenous silicic (Si) and/or salicylic acids (SA) on dry matter (DM), nitrogen yield (NY) and soil N uptake from soil (Ndfs) and from fertilizer (Ndff) nitrogen use efficiency (%NUE) of barley plants ( Hordeum vulgare L.) grown under varying levels of watering regimes (high stress I1, mild stress I2 and well-watered I3). Results showed that, foliar application of Si and/or SA markedly improved the overall studied growth parameters of barley plants and decreased the water-deficit influences. The synergistic effects of Si + SA application were more effective as compared with Si or SA applied separately indicating that Si and SA supports each other’s in improving plant performance which was more pronounced under high water-deficit than other watering regimes. A proper application of Si and SA might represent a suitable agricultural approach and result in increased production of barley, particularly, in the semi-arid areas under rain-fed conditions.
Water stress, salicylic acid, silicon, barley, 15n
Короткий адрес: https://sciup.org/143180992
IDR: 143180992
Текст научной статьи Effects of exogenous silicic (Si) and salicylic acid (SA) applied individually or in combination on barley growth and nitrogen uptake under various watering regimes
Barley ( Hordeum vulgare ) is one of the most important widely grown cereal grain crops worldwide with multipurpose use as animal feed, human food, and brewing material (Badea and Wijekoon 2021). In semiarid areas of the Mediterranean basin, barley is cultivated on a large scale under rain-fed conditions, where rain is scarce, irregularly distributed, and variable from one year to another. Thus, water deficit occurring during the growing periods considered the major environmental constraint affecting the agronomic production of barley ( amarah, 2005).
The improvement of barley’s yield could be achieved by several approaches including plant breeding (Cai et al., 2020) and best agricultural practice managements. Among the agricultural practices, the use of beneficial elements such as silicon (Etesami and Jeong 2023, Maghsoudi et al., 2019) or exogenous application of plant growth regulators ( GR) such as salicylic acid which can effectively alleviate the adverse effects of abiotic stresses and consequently enhance plant growth, development and yield ( irasteh-Anosheh et al., 2015; Al-Chammaa et al., 2019; Ahmad et al., 2021; Mir et al., 2021).
Both i and A have been reported to be effective in mitigating the harmful effects of environmental stresses by promoting the endurance of plants against a variety of stresses due to induced changes in many physiological and biochemical processes (Etesami and Jeong 2023, Maruri-López et al. 2019; Gorni et al. 2021; Torun et al., 2020). ilicon ( i) has been reported to be effective in mitigating the harmful effects of environmental constraint including salinity (El-Moukhtari et al., 2022), drought (Kurdali et al., 2019), heavy metal toxicity and nutritional imbalance stresses (Etesami and Jeong 2023) in plants. Moreover, salicylic acid ( A), when sprayed, results in increased photosynthetic activity in plants due to A-induced changes in physiological and biochemical processes (Bandurska and troinski 2005; Maruri-López et al. 2019; Gorni et al. 2021). Moreover, many recent studies intensively focused on the indispensable role of A as an internal signal arbitrating plant defense behavior against both biotic and abiotic stresses (Liu et al. 2022; Mir et al. 2021; Aldoude et al., 2019). Individual application of i or A has been reported to be effective in mitigating the harmful effects of drought on barley plants. Bandurska and troinski (2005) reported that ABA and proline can contribute to the development of anti-stress reactions induced by A in barley. Also, irasteh-Anosheh et al., (2015) reported that growth and yield of barley were improved due to A applied as a foliar spray in both stressed and non-stressed plants. Habibi (2012) suggests that the improvement of A on drought tolerance of barley plants was associated with the increase of antioxidant defense abilities and maintenance of photosynthesis under drought, which may elucidate the physiological mechanism of A in improvement of drought tolerance of barley plants. In regards to i influences, Kurdali and Al-Chammaa (2013) reported that i might be involved in saving water loss through reducing transpiration rate and facilitating water uptake, consequently, increasing water use efficiency in barley under water deficit. A number of recent studies showed that application of i in combination with A was more effective in enhancing plant growth in many plant species including wheat (Maghsoudi et al. 2019), cotton (Barros et al., 2019) and tomato (Khan et al., 2019). ince i and A have been noted for their particular role in enhancing drought tolerance of crops, there are still few studies that address their beneficial effects on growth and N-uptake of barley plants in response to drought tolerance particularly, when they are applied in combination. Hence, a better understanding of the interaction between i and A applications and plant responses will contribute to more efficient agricultural practices, particularly under water stress conditions. Therefore, the objective of this study was to assess the effect of exogenous i and A applied individually or in combination on dry matter yield, nitrogen uptake and N fertilizer use efficiency in barley plants grown under waterdeficit and non-deficit conditions using 15N.
MATERIALS AND METHODS
Site Description, Soil Properties and Plant Materials
A pot experiment was conducted at the research field of Deir AL-Hajar station” located south east of Damascus, yria (36° 28'E, 33° 21' N; altitude 617 m) in the growing seasons of 2020. The site is located within a dry
Mediterranean semiarid area with hot-dry summer and cold winter. The total annual rainfall is about 120 mm, and most precipitations occur between November and early April. For the last ten years, the average minimum temperature in winter was 1.3°C in January, while it increases to the average maximum temperature of 38.3 °C in July.
eeds of local genotypes of barley (cv. Arabi Aswad) were sown in plastic pots containing of thoroughly mixed soil (7 kg) collected from the field at the depth of 0 to 20 cm. oil was air dried before being ground and sieved (2 mm) to remove small rocks and plant litter. The main physical and chemical soil properties were: pH 7.85, EC e 0.52 d m-1, organic matter 0.54%, cations (Ca++4.08, Mg++ 13.21, K+0.01 and Na+0.08 mmol L-1), anions ( O 4 -- 1.27, HCO 3 - 4.0 and Cl- 1.02 mmol L-1), available (Olsen) 5.6 μgg-1, total N 0.02%, NO 3 - 8.9 μgg-1, NH 4 +19.2 μgg-1. Exchangeable cations (Ca++ 46.08 and Mg++ 6.33 meq 100 g-1 soil). The soil is classified as a clay loam, with average 54.2% clay, 32.5% silt, and 13.3% sand.
After germination, plants were thinned to two plants per pot. The pots were placed outdoor under natural climatic conditions, and protected from rainfall by manually operated shelter equipped with movable sheet of transparent flexible plastic. The pots were kept weed-free and any drainage was prevented.
Experimental Procedures and Treatments and 15N-Application
A split-plot based on randomized complete block design (RCBD) with three replicates was carried out in a pot experiment. The main factor was soil moisture level (low, 45–50%; medium, 55–60% and high, 75–80% of field capacity) which abbreviated as I1, I2, and I3, respectively. The sub-factor was different spraying compounds consisted of: silicic acid ( i+), salicylic acid ( A+), i+ plus A+. For non-treated plants, leaves were sprayed with distilled water and set as control (W). oil water content in all pots was maintained at around 75% of field capacity from planting up to the tillering stage (i.e., main shoot plus three tillers). Thereafter, plants were subjected to the three above-mentioned soil moisture regimes (i.e., I1, I2 and I3).
Foliar application with silicon ( i) was applied in the form of silicic acid i(OH)4 at a rate of 150 mg L-1. One hundred mL per plant of i solution were applied as foliar spray. First spray was given two weeks after appearance of the first leave, and further 3 sprays were given regularly at 15 days interval. alicylic acid ( A), C6H4 (OH) COOH, was applied at a rate of 10-5mol L-1 as foliar spray.
praying of the plants with A was started at the same time of applying soil moisture regimes, and performed six times at seven days intervals. For untreated i and A plants, leaves were sprayed with distilled water and set as control (W). praying with i and/or A was made using a manual sprayer early in morning, even full wet. On the other hand, equivalent amounts of 75 kg 2 O 5 ha-1 in the form of phosphoric acid and of 75 kg K 2 O ha-1 in the form of potassium sulfate were applied to each pot prior planting to all treatments. Moreover, an equivalent rate of 20 kg N ha-1 of 15N labeled urea (5% 15N atom excess) was applied at planting to estimate the fractional contribution of nitrogen derived from soil (Ndfs) and from fertilizer (Ndff), using 15N isotopic dilution method.
Plant Sampling, Isotopic Composition and Statistical Analysis lants were harvested at the spike formation stage. hoots and roots were dried at 70 °C for 72 h, weighed for dry matter determinations. amples were then ground to a fine powder using a mill having a 0.5 mm sieve. Total nitrogen was determined by Kjeldahl procedure, and 15N/14N isotope ratio was measured with an emission spectrometer (Jasco-150, Japan). The N fractions derived from the available sources (i.e., soil Ndfs and fertilizer Ndff) were calculated using the isotopic dilution method by applying equations previously described (Zapata, 1990). Nitrogen fertilizer use efficiency (%NUE) was calculated as fertilizer N recovery in the whole plant.
Statistical Analysis
Collected data were subjected analysis of variance (ANOVA) using the statistical program tatview, 4.57® Abacus Concepts, Berkley, Canada. Means were compared using the least significant difference (Fisher’s L D) test at the 0.05 probability level.
RESULTS AND DISCUSSION
Effect of Si and/or SA on Dry Matter Yield and Nitrogen uptake everal studies indicate that exogenous application of silicon ( i) or salicylic acid ( A) may be a sustainable technique to help mitigate the various environmental stresses in various crop plants (Etesami and Jeong 2023; Mir et al., 2021). This study investigated the effect of treatment with exogenous silicic and/or salicylic acids ( A) in barley plants (Hordeum vulgare L.) grown under waterdeficit and non-deficit conditions. Exogenous application of i and A could positively affect dry matter yield of barley plants by improving the uptake of nitrogen and decreasing the water-deficit influences.
In i, A and i+ A treatments, total dry matter yield (DM) was 13%, 11% and 19% higher, respectively, than with no foliar application under high water stress (I1). However, the effect was less pronounced under mild water stress (I2) where DM was 8%, 8%, and 16% higher, respectively than the control. Under well watering regime (I3), the significant increment was only obtained in the combined treatment of i+ A (14% over the control), (Fig. 1). These results were in harmony with those of Maghsoudi et al., (2019) who reported that in i, A and i+ A treatments, grain yield of two wheat cultivars was 10-16%, 16-17% and 19-24 % higher, respectively than with no foliar application under water stress in two wheat cultivars. imilarly, data of total N yield were consistent with those of DM. In i, A and i+ A treatments, NY was 27, 23 and 33% higher, respectively than with no foliar application under high water stress. Under mild water stress (I2), although separate application of i and A enhanced NY, the dual application of i+ A resulted in a significant increase of NY by 15% over the control. In the well irrigated plants, NY yield increased by 16%, 20% and 40%, over the control in i, A and i+ A treatments, respectively (Fig.1). hoot DM data were in consistence with those of total DM. i and /or A treated plants resulted in significant greater shoot DM than the control in stressed and non-stressed plants (Table 1), whereas, root DM was significantly higher in i and/or A treatments than with no foliar application under high water stress (I1). However, under mild water stress (I2) and in well-watered plants (I3), no apparent and significant increases were found in root DM. Also, it can be noticed that the dual application of both compounds was more effective than those of sole applications. Our results are similar to findings reported by other workers demonstrating that foliar application of i, A and especially their combination, markedly improved yield components in a variety of plant species under environmental stresses including drought (Maghsoudi et al., 2019; Li et al. 2009; Zhang et al. 2017). Moreover, the present investigation demonstrated that synergistic effects of i+ A application on plant performance were greater than of i or A applied separately. imilarly, de ouza Junior et al. (2021) reported that, applying A, especially when associated with foliar i, favored dry weight production in peanut plants. Moreover, Barros et al. (2019) reported that i foliar application associated with A favors the physiological variables, increasing the photosynthesis, stomatal conductance and water use efficiency reflecting on the increase of cotton yield. Likewise, Maghsoudi et al. (2019) reported that synergistic effects of i + A application on physiological parameters and yield of water stressed wheat plants were apparent compared with i or
A applied separately. Khan et al. (2019) reported that exogenously applied i and A enhance the activation of the antioxidant defense system, modulate key hormones, increase the potassium concentration and stimulate the expression of the critical genes involved, which confers successful tolerance of tomato plants to alkaline conditions. Therefore, it can be concluded that the dual application of i and A to barley plants is more effective than those of sole applications, indicating that i and A underpins each other’s in improving plant growth performance which was more pronounced under high water-deficit than the other watering regimes.
Effect of Si and/or SA on nitrogen derived from soil, fertilizer and its use efficiency
Decreasing water availability under drought generally results in reduced nutrient uptake and frequently reduced concentrations of elements in crop plants (Marschner, 1995). In this study, both Ndfs and Ndff increased with increasing soil moisture content under various spraying treatments (Fig. 2). ositive and significant impacts on soil nitrogen uptake (Ndfs) and nitrogen derived from fertilizer (Ndff) in response to i and/or A were generally observed in plants grown under various watering regimes (Fig. 2).On percentage basis, amount of Ndfs in water stressed plants (I1) increased by 28%, 22 and 32% in response to i, A and i+ A treatments, respectively. Under mild water stress (I2), although separate application of i enhanced Ndfs, solely applied A or in combination with i significantly increased by 14% as compared with no foliar application. Under well irrigated conditions, Ndfs increased by 18%, 22% and 39%, over the control in i,
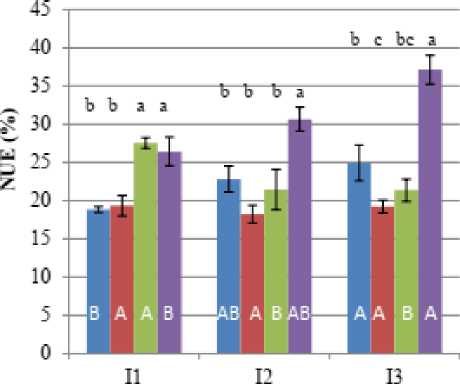
A and i+ A treatments, respectively. The efficient use of N fertilizer (%NUE) ranged between 19% in the control (i.e., no foliar application under high water stress) and 37% in well irrigated plants of i+ A treatment (Fig. 3). The synergistic effects of i and A application on %NUE were greater than that of i or A applied separately. These results are similar to findings reported by other workers demonstrating that foliar application of i or A improved plant growth under stresses by stimulating the accumulation of mineral elements including nitrogen (Gunes et al. 2005; Yildirim et al. 2008; Al-Chammaa et al. 2019; Kurdali and Al-Chammaa 2013). Etesami and Jeong (2023) reported that silicon interferes with the absorption of essential nutrients by plants and could affect the nutrient availability by optimizing soil fertility, making nutrients more available to plants. Guo et al. (2006) reported that the increase in root growth with i application would have the potential to enhance the root’s absorptive capacity and nutrient uptake. Likewise, the enhancement of endogenous abscisic acid (ABA) concentration in i-fed plants could lead to lateral root growth stimulation (Agarie et al. 1992) which in turn increase nutrient uptake. On the other hand, there are some evidences that A can induce the synthesis of nitrite reductase enzyme, which plays a key role in nitrogen metabolism and assimilation (Ghasemzadeh and Jaafar, 2013). Al-Chammaa et al. (2019) reported that applied A to water-stressed chickpea resulted in increments of soil nitrogen uptake (Ndfs) as well as amount of N derived from fertilizer (Ndff) and its use efficiency (%NUE). In this study, i and/or A treated plants under high water stress resulted in significant greater root DM than the control, with the highest value being obtained in i+ A treatment (Table 1). Consequently, the enhanced soil nitrogen uptake by water-stressed plants following i+ A applications may improve biomass accumulation and nitrogen yield. Thus, the co-application of these two compounds (i.e., i+ A) showed positive effects on soil N uptake and %FUE more than separate application indicating that i and A supports each other’s in improving N uptake under different watering regimes.
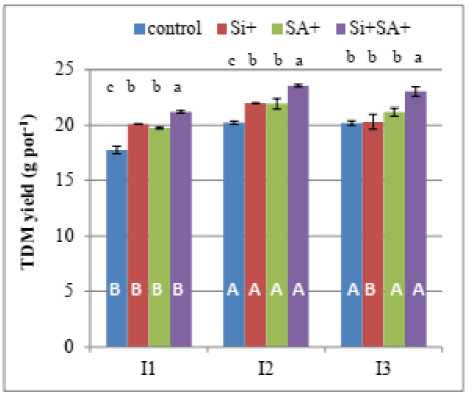
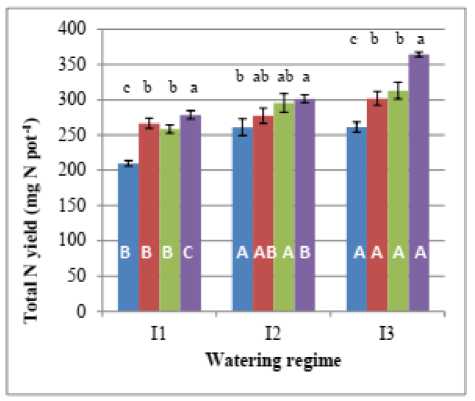
Figure. 1. Dry matter (g pot-1) and amounts (mg N pot-1) of nitrogen in the whole plant of barley grown under different water regimes (I1, high stress; I2, mild stress and I3, well-watering) as affected by foliar spraying with salicylic acid ( A) and/or silicic acid ( i). Columns followed by the same letter are not significantly different ( <0.05). mall letters indicate comparisons amongst treatments for each water regime. Capital letters denote comparison amongst water regimes for each applied treatment
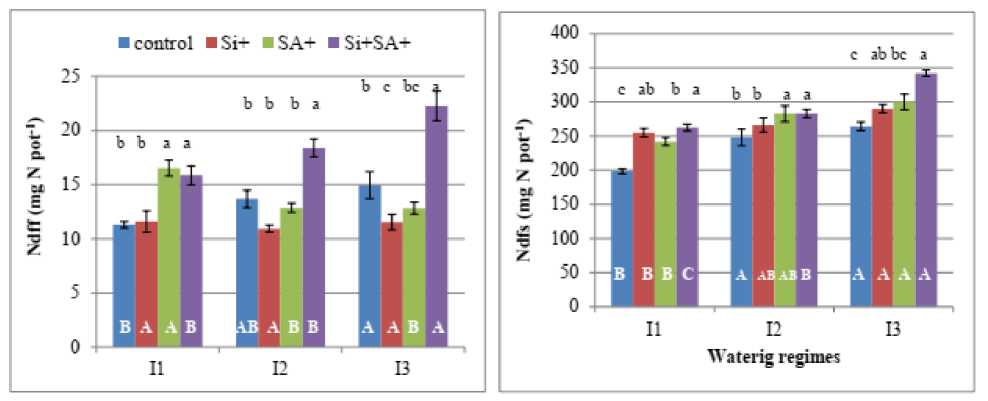
Figure. 2. Amounts (mg N pot-1) of nitrogen derived from soil (Ndfs) and fertilizer (Ndff) in the whole plant of barley grown under different water regimes (I1, high stress; I2, mild stress and I3, well-watering).as affected by foliar spraying with salicylic acid ( A) and/or silicic acid ( i). Columns followed by the same letter are not significantly different ( <0.05). mall letters indicate comparisons amongst treatments for each water regime. Capital letters denote comparison amongst water regimes for each applied treatment.
■ control ■ Si+ ■ SA- ■ S1+SA+
Watering regimes
Figure. 3. Nitrogen use efficiency of added fertilizer (%) in the whole plant of barley grown under different water regimes (I1, high stress; I2, mild stress and I3, well-watering).as affected by foliar spraying with salicylic acid ( A) and/or silicic acid ( i). Columns followed by the same letter are not significantly different ( <0.05). mall letters indicate comparisons amongst treatments for each water regime. Capital letters denote comparison amongst water regimes for each applied treatment
Table 1: Dry matter yield (g pot-1) and nitrogen uptake (mg N pot-1) in shoots and roots of barley plants grown under different water regimes as affected by foliar spraying with salicylic acid ( A) and/or silicic acid ( i).
|
Dry matter yield (g pot-1) |
|||||
|
Shoots |
|||||
|
Treatments |
W (Si-SA-) |
Si+ |
SA+ |
Si+SA+ |
L.S.D |
|
I1 |
11.53±0.26c, B |
13.10±0.06b,B |
12.97±0.09b,C |
13.90±0.06a,C |
0.46 |
|
I2 |
14.30±0.68c,A |
15.67±0.33b,A |
16.30±0.33b,A |
17.90±0.06a,A |
1.35 |
|
I3 |
14.03±0.15b,A |
13.97±0.55b,B |
14.97±0.03b,B |
16.57±0.30a,B |
1.05 |
|
LSD 0.05 |
1.48 |
1.29 |
0.69 |
0.61 |
|
|
Roots |
|||||
|
I1 |
6.23±0.35cA |
6.97±0.03b,A |
6.77±0.13, b, A |
7.30±0.15a,A |
0.36 |
|
I2 |
5.93±0.18ab,A |
6.30±0.20a, B |
5.60±0.20b, B |
5.67±0.12ab,C |
0.68 |
|
I3 |
6.17±0.36a,A |
6.37±0.13a,AB |
6.20±0.36a, AB |
6.47±0.17a,B |
N.S |
|
LSD 0.05 |
N.S |
0.66 |
0.87 |
0.51 |
|
|
N-uptake (mg N pot-1) |
|||||
|
Shoots |
|||||
|
I1 |
160.6±4.4b,B |
218.4±8.98a,B |
208.7±0.46a,B |
208.5±7.4a,C |
20.23 |
|
I2 |
205.4±9.85b,A |
232.4±9.5ab,AB |
243.7±13.8a,A |
258.4±1.3a,B |
31.76 |
|
I3 |
218.9±0.97c,A |
260.6±9.2,b |
267.4±7.8b,A |
308.9±4.3a,A |
20.93 |
|
LSD 0.05 |
21.60 |
31.92 |
31.69 |
17.20 |
|
|
Roots |
|||||
|
I1 |
49.2±0.96b,A |
48.1±5.5b,A |
49.60±5.9b,A |
69.85±3.6a,A |
14.49 |
|
I2 |
56.1±4.6a,A |
44.8±2.1a,A |
51.81±4.0a,A |
43.03±6.6a,B |
N.S |
|
I3 |
42.3±6.6ab,A |
40.5±3.5b,A |
45.39±3.9ab,A |
55.65±2.8a,AB |
14.7 |
|
LSD 0.05 |
N.S |
N.S |
N.S |
16.06 |
|
Means ± E within a column (capital letter) and within a row (small letter) followed by the same letter are not significantly different ( <0.05). I: water regimes, expressed as % of field capacity (I1, high stress 45-50%; I2, mild stress 55-60% and I3, well-watering 75–80%). W: spraying with demineralized water as a control ( i- A-); A+: spraying with salicylic acid at 10-5 Mol l-1; i+ : spraying with 100 mg silicic acid (H 4 iO 4 )/L.
CONCLUSION
-
• Exogenous foliar application of i and/or A markedly improved the overall growth parameters (DM, NY, Ndfs, Ndff and %NUE) of barley plants under both stressed and non-stressed conditions.
-
• ynergistic effects of i + A application were apparent compared with i or A applied separately indicating that i and A underpins each other’s in improving plant performance which was more pronounced under high water-deficit than the other watering regimes.
-
• A proper application of i and A might result in increased production of barley, particularly in the semi-arid areas under rain-fed conditions.
ACKNOWLEDGEMENT
I would like to thank rofessor Ibrahim Othman, General Director of the Atomic Energy Commission of yria (AEC ) for his support. The technical assistance of Al-Chammaa M and Al-Ain F along with the staff at the department of agriculture is greatly acknowledged.
CONFLICT OF INTERESTS
The author declares that he has no competing interests.
Список литературы Effects of exogenous silicic (Si) and salicylic acid (SA) applied individually or in combination on barley growth and nitrogen uptake under various watering regimes
- Agarie S., Agata W., Kubota F. and Kaufman P.B. (1992). Physiological roles of silicon in photosynthesis and dry matter production in rice plants. I. Effect of silicon and shading treatments. Japanese Journal of Crop Science 61(2): 200-206. doi :10.1626/jcs.61.200.
- Ahmad Z., Waraich E.A.,Tariq R.M.S. and Iqbal M.A. (2021). Foliar applied salicylic acid ameliorates water and salt stress by improving gas exchange and photosynthetic pigments in wheat. Pakistan Journal of Botany 53(5): doi : http://dx.doi.org/10.30848/PJB2021-5(17).
- Al-Chammaa M., Al-Ain F. and Kurdali F. (2019). Effect of salicylic acid on growth, nodulation and N2-fixation in water stressed chickpeas using 15N and 13C. Advances in Horticultural Science 33(3): 391-401. https://doi.org/10.13128/10.13128/ahs-23289 .
- Aldoude A., Al-Shehadah E., Shoaib A., Jawhar M. and Arabi M.I.E. (2019). Salicylic acid pathway changes in barley plants challenged with either a biotrophic or a necrotrophic pathogen. Cereal Research Communications. 47 (2): 324-333. doi: 10.1556/0806.47.2019.004.
- Badea A and Wijekoon C. (2021). "Benefits of barley grain in animal and human diets." cereal grains. Volume 1, December. IntechOpen. doi:10.5772/intechopen.97053.
- Bandurska H. and Stroinski A. (2005). The effect of salicylic acid on barley response to water deficit. Acta Physiologiae Plantarum. 27(3):379-386. https://doi.org/10.1007/s11738-005-0015-5.
- Barros T.C., de Mello Prado R., Roque C.G., Arf M.V. and Vilela R.V. (2019). Silicon and salicylic acid in the physiology and yield of cotton, Journal of Plant Nutrition, 42(5): 458-465. DOI: 10.1080/01904167.2019.1567765
- Cai K., Chen X., Han Z., Wu X., Zhang S., Li Q., Nazir M.M., Zhang G. and Zeng F. (2020). Screening of worldwide barley collection for drought tolerance: the assessment of various physiological measures as the selection criteria. Frontiers in Plant Science. 11:1159. doi: 10.3389/fpls.2020.01159.
- de Souza Junior J.P., Frazao J.J., de Morais T.C.B., Espoti C.D., dos Santos Sarah M.M. and de Mello Prado R. (2021). Foliar spraying of silicon associated with salicylic acid increases silicon absorption and peanut growth. Silicon 13: 1269-1275. https://doi.org/10.1007/s12633-020-00517-y.
- El-Moukhtari A., Lamsaadi N., Oubenali A., Mouradi M., Savoure A., and Fariss M., (2022). Exogenous silicon application promotes tolerance of legumes and their N2 fixing symbiosis to salt stress. Silicon 14, 65176534. https://doi.org/10.1007/s12633-021-01466-w.
- Etesami H. and Jeong B.R. (2023). How does silicon help alleviate biotic and abiotic stresses in plants?. Mechanisms and future prospects. Editor(s): Ghorbanpour M, Shahid MA, Plant Stress Mitigators, Press Chapter 22, Academic, 359-402, ISBN 9780323898713, https://doi.org/10.1016/B978-0-323-89871-3.00031-8.
- Ghasemzadeh A. and Jaafar H.Z.E. (2013). Interactive effect of salicylic acid on some physiological features and antioxidant enzymes activity in ginger (Zingiber officinale Roscoe). Molecules, 18(5):5965-5979. doi: 10.3390/molecules18055965.
- Gorni P.H., Cornelissen B., da S. and Pereira A.A. (2021). Exogenous salicylic acid and ferulic acid improve growth, phenolic and carotenoid content in tomato. Advances in Horticultural Science, 35(4): 335-341. https://doi.org/10.36253/ahsc-8295.
- Gunes A., Inal A., Alpaslan M., Cicek N., Guneria E., Eraslana F. (2005). Effects of exogenously applied salicylic acid on the induction of multiple stress tolerance and mineral nutrition in maize (Zea mays L.). Archives of Agronomy and Soil Science, 51: 687695. https://doi.org/10.1080/03650340500336075
- Guo Z.G., Liu H.X., Tian F.P., Zhang Z.H and Wang S.M. (2006). Effect of silicon on the morphology of shoots and roots of alfalfa (Medicago sativa). Australian Journal of Experimental Agriculture 46(9): 11611166. https://doi.org/10.1071/EA05117.
- Habibi G. (2012). Exogenous salicylic acid alleviates oxidative damage of barley plants under drought stress .Acta Biologica Szegediensis. 56(1):57-63. http://www.sci.u-szeged.hu/ABS.
- Khan A., Kamran M., Imran M., Al-Harrasi A., Al-Rawahi A., Al-Amri I., Lee I. and Khan A. (2019). Silicon and salicylic acid confer high-pH stress tolerance in tomato seedlings. Scientific Reports 9, 19788. https://doi.org/10.1038/s41598-019-55651-4.
- Kurdali F and Al-Chammaa M. (2013). Growth, carbon isotope discrimination and nitrogen uptake in silicon and/or potassium fed barley grown under two watering regimes. Journal of Stress Physiology & Biochemistry. 9(1):14-27. ISSN 1997-0838 .
- Kurdali F., Al-Chammaa M. and Al-Ain F. (2019). Growth and N2-fixation in saline and/or water stressed Sesbania aculeata plants in response to silicon application. Silicon 11: 781-788. https://doi.org/10.1007/s12633-018-9884-2.
- Li Q.F., Ma C.C., Ji J. (2009). Effect of silicon on water metabolism in maize plants under drought stress. Acta Ecologica Sinica. 29, 4163-4168.
- Liu J., Qiu G., Liu C., Li H., Chen X., Fu Q., Lin Y., Guo B. (2022). Salicylic acid, a multifaceted hormone, combats abiotic stresses in plants. Life (Basel). 12(6):886. doi: 10.3390/life12060886. PMID: 35743917; PMCID: PMC9225363.
- Maghsoudi K., Emam Y., Ashraf M., and Arvin M.J. (2019). Alleviation of field water stress in wheat cultivars by using silicon and salicylic acid applied separately or in combination. Crop and Pasture Science. 70(1): 36-43. https://doi.org/10.1071/CP18213 .
- Marschner H. (1995) Mineral nutrition of higher plants. Second edition. Academic press limited. London U.K. NW1 7DX
- Maruri-Lopez I., Aviles-Baltazar N.Y., Buchala A. and Serrano M. (2019). Intra and extracellular journey of the phytohormone salicylic acid. Frontiers in Plant Science. 10:423. doi: 10.3389/fpls.2019.00423.
- Mir R.A., Aryendu and Somasundaram R. (2021) Salicylic acid and salt stress tolerance in plants: A Review. Journal of Stress Physiology & Biochemistry 17 (3): 32-50: ISSN 1997-0838.
- Pirasteh-Anosheh H., Emam Y., Sepaskhah A.R. (2015). Improving barley performance by proper foliar applied salicylic acid under saline conditions. International Journal of Plant Production 9(3): 467-486. ISSN: 1735-6814.
- Samarah, H.N. (2005). Effects of drought stress on growth and yield of barley. Agronomy for Sustainable Development. 25, 145-149. doi: 10.1051/agro:2004064.
- Torun H., Novak O., Mikulik J., Pencik A., Strnad M. &Ayaz F.A. (2020). Timing-dependent effects of salicylic acid treatment on phytohormonal changes, ROS regulation, and antioxidant defense in salinized barley (Hordeum vulgare L.). Scientific Reports 10:13886. https://doi.org/10.1038/s41598-020-70807-3 .
- Yildirim E., Turan M., Guvenc I. (2008). Effect of foliar salicylic acid applications on growth, chlorophyll, and mineral content of cucumber grown under salt stress. Journal of Plant Nutrition. 31(3): 593-612. DOI: 10.1080/01904160801895118 .
- Zapata F. (1990). Isotope techniques in soil and plant nutrition studies. In: Hardarson G. (ed.), Use of nuclear techniques in studies of soil-plant relationships. International Atomic Energy Agency (IAEA), Vienna. pp. 61-127.
- Zhang W., Xie Z., Wang L., Li M., Lang D., Zhang X. (2017). Silicon alleviates salt and drought stress of Glycyrrhiza uralensis seedling by altering antioxidant metabolism and osmotic adjustment. Journal of Plant Research 130, 611-624. doi:10.1007/s10265-017-0927-3


