Effects of Foliar Application of ZnO Nanoparticles on Secondary Metabolite and Micro-elements of Camelina (Camelina sativa L.) Under Salinity Stress
Автор: Torfeh Akhavan Hezaveh, Fatemeh Rahmani, Hadi Alipour, Latifeh Pourakbar
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 4 т.16, 2020 года.
Бесплатный доступ
The present study investigated the biochemical impact of ZnO nanoparticles (NPs) and salinity stress in Camelina (Camelina sativa L.) plants. ZnO NPs were applied at 0, 20, 40 and 80 mgL-1 and salinity stress (NaCl) were at 0, 50, and 100 mM. Salinity stress significantly enhanced total phenolic compounds, anthocyanin, carotenoid and ability to scavenge the DPPH radical in shoots and roots. We demonstrate that the ZnO NPs, plant carotenoid content is profoundly affected by salinity and ZnO NPs. Salinity stress decreased zinc concentration in shoots and root, but increased macro-element such as calcium (Ca) and phosphorous (P). Application of 20 mgL-1 ZnO NPs gave the most impact contents of calcium (Ca), phosphorous (P). With increasing ZnO NPs concentration, zinc (Zn) content was increased. Foliar application of ZnO NPs did not show any significant effect on flavonoid content in Camelina but caused increases in DPPH radical scavenging capacity in shoot and root. We found that the amount of phenolic compounds in shoot was decreased at 20 mgL-1 of ZnO NPs concentration. ZnO NPs at 20 mgL-1 was more provocative to reduce the effects of NaCl. Therefore, the results suggest that the appropriate concentration of ZnO NPs (20 mgL-1) could overcome the negative effects of salt stress in Camelina.
Camelina, Elements, Salinity, Secondary metabolites, ZnO NPs
Короткий адрес: https://sciup.org/143173862
IDR: 143173862
Текст научной статьи Effects of Foliar Application of ZnO Nanoparticles on Secondary Metabolite and Micro-elements of Camelina (Camelina sativa L.) Under Salinity Stress
Camelina sativa (camelina, false fax or gold of pleasure) is an oilseed crop of the Brassica (Cruciferae) family (Hunsaker et al. , 2013) that contains other bioactive compounds such as flavonoids and phenolic products (Cherian, 2012) and considers as a new source of essential fatty acids, particularly omega-3 fatty acids (Ibrahim and Habbasha, 2015). Camelina has enhanced drought, salinity and cold tolerance, displays early maturation, and requires fewer inputs compared to other oilseed crops (Heydarian et al. , 2018). Ability to avoid drought by developing deep root systems or making metabolic adjustments is a common feature of both drought and salt tolerance (Heydarian et al. , 2018).
Among the abiotic stresses, salinity is one of notable challenge factor impairing agricultural productivity worldwide which causes impairment and inhibition of the development and growth crops (Khalid et al. , 2015). It causes the disruption of the homeostatic balance of water potential and resulting in decreased availability of water to root cells and the plants tend to accumulation of cytotoxic ions which decreases metabolism and imposes premature senescence and ultimately kills cell in their vacuoles to protect their cytoplasmic water potential (Vahdati and Lotfi, 2013). These metabolic imbalances cause oxidative stress.
It is crucial to understand how ROS disturb the balance of biological processes, which is currently a great challenge. Also, secondary metabolites are the main sections of plants with a great pharmaceutical value (Kreslavski et al. , 2013).
Accordingly, secondary metabolites are critical for the environmental adaptation and stress alleviation in plants. Abiotic environmental factors include the heavy metals, light, drought, and salt that can affect plant growth and secondary metabolite production (Akula and Ravishankar, 2011).
‘Elicitor’ as a substance, initiates or improves the biosynthesis of specific compounds in living cell system when applied at trace amounts. Moreover, the change in the concentration of micronutrient nanoparticles in plants, as Elicitor, may be shown to be effective on secondary metabolites production. Although zinc is one of the most abundant elements on Earth, due to low solubility of zinc containing minerals, it is in low concentration or not accessible for plants and microorganisms, specifically in dry areas with alkaline and saline soils. In this regard, a strategy for solving zinc deficiency is the application of nanoparticles (Marslin et al., 2017). Sprays are one of the direct routes of ZnO NPs into the environment, which are also present in agricultural spraying as a protector material against UV radiation (Gogos et al., 2012).
In recent years, the increasing number of evidences has substantiated the role of secondary metabolites as antioxidants and antiradicals, which help the plants in alleviating oxidative stress in hostile environments. Synthesis and accumulation of polyphenol are generally induced in response to biotic and abiotic stresses (Arbona et al. , 2013; Bartwal et al. , 2013). The major advantage of plants is the synthesis of bioactive secondary metabolites. In addition, the array of secondary metabolites is specific to plant species, and also their biosynthesis is strictly regulated at the developmental stage, by tissue or cell groups, and of course by several stress factors (Munns et al. , 2008; Karowe and Grubb, 2011). Moreover, secondary metabolites play an important role in the adaptation and defense of plants facing adverse environmental conditions. Accordingly, flavonoids are known as secondary plant metabolites with a wide variety of possible functions including antioxidative activity (Keilig and Ludwig-Müller, 2008). Carotenoids are also considered as non-enzymatic anti-oxidants (Telesinski et al. , 2008). Therefore, the purpose of this study was to investigate the changes in the chemical composition of the shoot parts and roots of camelina plants affected by salinity and the spraying of ZnO NPs In this regard, it has been found that, the foliar application of nano-Zn decreased the negative effects of salinity stress on sunflower (Torabian et al. , 2016) and cotton (Hussein and Abou-Baker, 2018) plants. Also, ZnO NPs increased the yield and grow parameters of cotton under the stress conditions.
The present study investigated the effects of ZnO NPs at salinity concentrations on secondary metabolites production in camelina using these nano, which maybe stimulate secondary metabolite synthesis.
MATERIALS AND METHODS
Seeds of Camelina were supplied by Strictly Medicinal Seeds Newsletter of Oregan province state, USA. Also, the effects of salinity and ZnO NPs foliar spray on the biochemical and physiological responses of the plants were experimentally investigated in a greenhouse during the growing seasons in 2016. At first, ten seeds were planted in perlite/soil mixture (1:2) filled pots with soil texture of clay-loamy. Afterward, the soil samples were taken from a farmland at depth between 0 and 30 cm. next, chemical and physical characteristics of soil were evaluated using Hydrometer method, as shown in tables 1 and 2. At 4-leaf stage, three of the highest grown plants with homogenous seedlings were identified in each pot and the other plants were then removed. The pots were kept in a greenhouse under a photoperiod of16/8 h at 20±3°C and 18±3°C during days and nights for one month, respectively. Then, they were treated with different concentrations of NaCl and ZnO NPs.
A Factorial experiment with three salinity (0, 50, 100 mM) and four ZnO NPs concentrations (0, 20, 40, 80 mg L-1) concentrations was performed based on the completely randomized design (CRD) with three replications. Subsequently, NaCl was added to irrigation water in step-wise aliquots of 50 mM up to 100 mM. Foliar application of ZnO NPs was applied twice as follows: the first one was immediately used after the end of six-leaf stage and the second foliar application was carried out one week later. Plants were sprayed until the leaves were completely wet. The control plants were also sprayed with the same quantity of distilled water.
NP solutions were prepared by suspending ZnO NP in de-ionized water through ultrasonication (100 W and 40 KHz for 30 minutes) (Prasad et al., 2012). After dispersion, solution pH was adjusted at 6.7. The scanning electron microscope (SEM) images of ZnO NPs are shown in Figure 1. The particles were white in color with almost spherical morphology with diameter 10 - 30 nm, density 5.60 g/cm3 and purity 99%.
In each pot, one plant was selected for further growth and performing the biochemical and physiological analyses and the remaining two plants were left to reach the seed stage. Once the fresh biomass of plants was recorded, they were dried in an oven for two days at 70°C and their dry biomass was measured to determine the plant growth.
Preparation of extracts
Portions of dried plant shoots and roots (0.1 g) were extracted with chloroform, methanol and ethyl acetate using soaking method for 48 h followed by shaking for 30 min, filtering through anhydrous sodium sulfate, and performing vacuum-evaporation. Then, extracts were stored at 4 °C in sealed vials until test.
Total content of phenolic compounds
Total phenolics contents in methanolic extracts were measured by Folin-Ciocalteu method. (0.2 cm3) aliquots of methanolic extracts were placed in (10 cm3) volumetric flasks and diluted with (0.5 cm3) Folin-Ciocalteu reagent. After three min, 1 cm3saturated sodium carbonate was added and flasks were filled with water to (10 cm3). After 2 h, absorbance of samples was measured on a UV-Vis spectrophotometer (HALO XB-10) at ʎ max 725 nm against a reagent blank and were reported as total phenols in micromoles of Gallic acid equivalents (GAE) per gram of fresh weight.
DPPH free radical scavenging activity
Briefly, a 0.1 mM solution of DPPH° in methanol was prepared and 0.3 ml of this solution was added 0.5 ml of samples at different concentrations (10–80 µg/ml). Mixtures were vigorously shaken and left for 30 min at room temperature. Then, their absorbance was recorded at 517 nm on a spectrophotometer. Lower absorbance of reaction mixture indicated higher activity of free radical-scavenging (Ak and Gulcin, 2008). DPPH° radical scavenging capacity was calculated using the following equation:
DPPH° scavenging effect % = (1- AC ) ×100
Where AC is control absorbance and AS is the absorbance in the presence of samples or standards (Elmastas et al., 2006). Scavenging activity was reported as percentage decrease in absorbance with time.
Determination of flavonoid content
Flavonoid contents of plant extracts were measured using spectrophotometric method (Staszczak, 2008). Samples were consisted of 1 ml extract methanol solution and 1 ml 2% AlCl 3 solution dissolved in methanol and were incubated for 1h at room temperature. Samples absorbance was recorded in triplicates using spectrophotometer at ʎ max = 415 nm and average absorbance value was reported. Similar procedure was employed for standard solutions and a calibration curve was drawn and the concentrations of flavonoids in extracts were reported in terms of routine equivalent (µm/g in 0.1 g dry sample).
Total anthocyanin content
To determine total monomeric anthocyanin concentration by pH differential method, a simple and rapid spectrophotometric method based on the structural transformation of anthocyanin and pH change (colored at pH 1.0 and colorless at pH 4.5) (Lee et al. , 2005), 3 ml extract sample was diluted in 5 ml of two different buffers: 0.025 M potassium chloride at pH 1.0 and 0.4 M sodium acetate at pH 4.5. After incubation for 30 min at room temperature, absorption (A) was measured at ʎ = 510 and ʎ = 700 nm (spectrophotometer UV-visible HALO XB-10). All experiments were performed in duplicate.
The following results were obtained according to Lee et al. (2005):
Asp = (A510 – A700) pH1.0 – (A510 – A700) pH 4.5
Total anthocyanin (TA) was determined using the following equation:
TA = (Abs pH 1 - Abs pH 4.5 ) × 484.82 ×1000/2485× ʎ × DF where 484.42 and 2482 molecular weight and molar absorption coefficient (cyanidin3- glucoside molecule), ʎ is cuvette optical path length (1 cm) and DF is dilution factor. Total anthocyanin content was reported as mg of anthocyanin in 0.1 g dry sample.
Carotenoid assays
Carotenoid was extracted using 0.1 g fresh material in 10 ml 80% aqueous acetone. After filtering, carotenoid content was measured on a spectrophotometer (Shimadzu, Japan) at 663.2 nm and carotenoid concentration was calculated according to the method of Lichtenthaler (1987).
Measurement dissolved metal ion content
To investigate the roles of the dissolved metal ions in causing phytotoxicity, Zn+2 content of metal oxide NPs suspensions was determined. Firstly, ZnO NPs suspensions at (0, 20, 40, 80 mgL-1) was centrifuged at 15,000 rpm for 30 min after dispersal Then, the supernatant was filtered through 0.2 m glass filters, and the content of Zn element was analyzed by ICP-OES method (730-ES Varian company, USA) at Sharif Industrial Laboratory Service Center (206 nm) (Table 2). Sample preparation
One-gram sample of shoots and roots dried in an oven were grounded in mortar. Then, the obtained powder was stored in a dry and dark place at room temperature in the polyethylene bags till further analyses.
Elemental analysis
Dried samples (0.1 g) were digested by concentrated H2 SO4 (99.7%). Concentration of Zn and Ca was determined using atomic absorption spectrophotometer (model Aa680, Shimadzu, Japan) according to method described (Allen et al. , 1984, Ebrahimian et al. , 2017) Total P content was obtained by complete digestion of the samples (500 mg FW, in triplicate) with nitric acid (100%) and perchloric acid (100%) (1:1 v/v) and subsequent colorimetric quantification using the molybdate method (Gomes et al. , 2017).
Statistical analysis of data
The data were tested for normality of residuals using the Kolmogorov-Smirnov. Analysis of variance (ANOVA) was performed using PROC GLM in SAS 9.2. Means were separated using Tukey’s test and each average was presented with the standard error. Significant differences between means were denoted with letters and different letters denote statistical significance at p≤0.05.
RESULTS
Phenol content
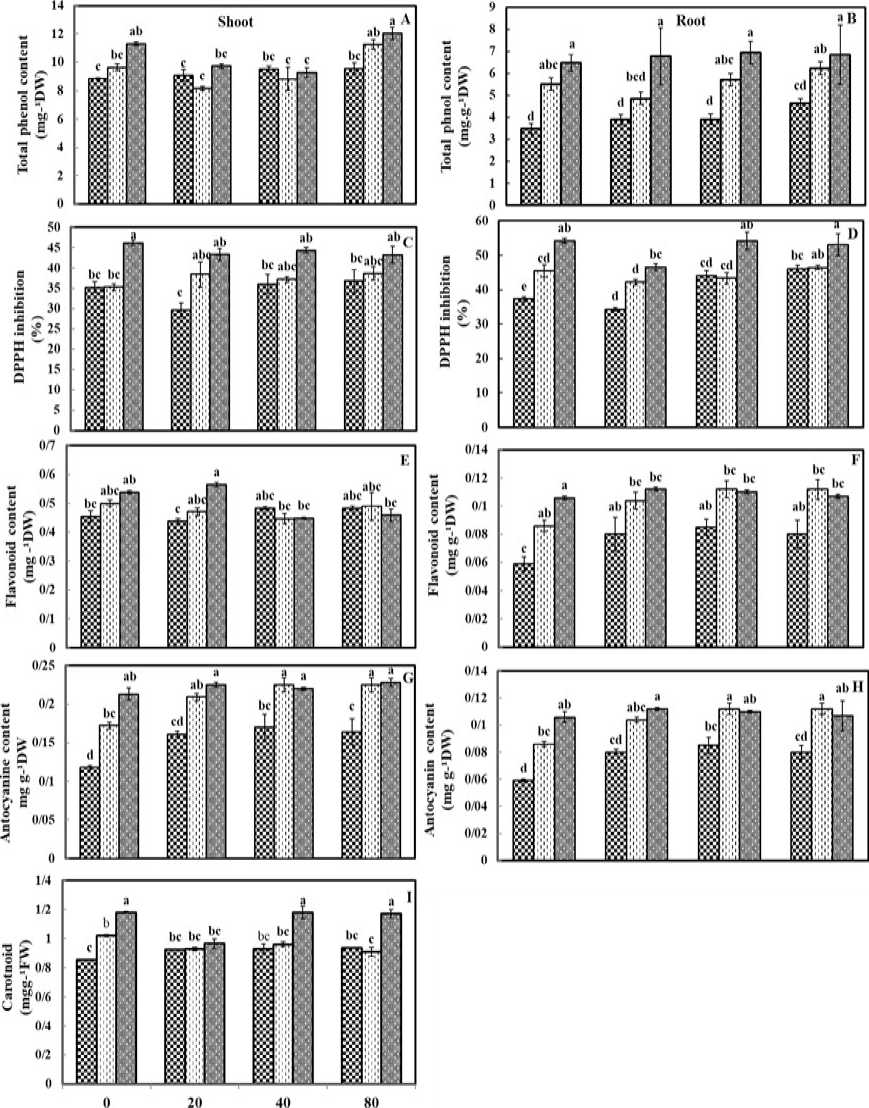
Phenol content increased in shoot and root at 100
mM of salinity stress that were observed to be 14.48% and 69.84%, respectively, as compared to the control plants (Figures 2A and B). Also, foliar application of ZnO NPs at 80 mgL-1 concentration increased the total phenol content in both shoot and root (8.25%) and root (32.75%) as compared to the control plants (Figures2-A and B). The maximum total phenol contents of shoot and root were obtained at 100 mM of salinity stress and 80 mgL-1 of ZnO NPs in shoot (36.19%) and in root (96.83%) compared to the control plants (Figures 2A and 2B). However, ZnO NPs at all the concentrations with NaCl (100 mM) increased the amount of phenol in the root (a relatively stable increase) (Figure 2B).
DPPH radical scavenging activity
Total anti-oxidative capacities of shoot and root has also increased along with the increase of salt stress (Figures 2C and 2D). The highest total anti-oxidative capacity was obtained in shoot (28.52%) (Figure 2C), and root (28.63 %) under 100 mM of salinity stress (Figure 2D). The lowest decrease in DPPH radical scavenging activity was observed in shoot (16%) and root (7.84%) due to applying ZnO NPs at 20 mgL-1 (Figures 2C and 2D).
The obtained results show that, the lowest antioxidant capacity of root was obtained for the combined treatment of salinity stress at 100 mM and ZnO NPs at 20 mgL-1 with a 6.36% reduction compared to those plants treated by only NaCl at 100 mM (Figure 2D). Moreover, the plants treated with NaCl 50 mM and ZnO NPs (20 mgL-1) had shown the lowest DPPH radical scavenging activity (7.19%) of root (Figure 2D).
Flavonoid content
The significantly highest flavonoid content of shoot was evidenced under salinity imposition NaCl 100 mM (8.69%) as compared to the non-stressed plants (Figure 2E). In addition, flavonoid content of root indicated no significant alteration under salt stress (Figure 2F). Moreover, no meaningful change was observed in flavonoid contents of shoot and root by the foliar application of ZnO NPs. The lowest total flavonoid content of shoot was found in the combined treatment of NaCl (50 mM) and ZnO NPs (40 mgL-1) (10.6%) also, the plants treated with NaCl (100 mM) and ZnO NPs (40
mgL-1). contained the lowest shoot flavonoid level (32.72%) (Figure 2E).
Anthocyanin
Application of 100 mM NaCl enhanced the anthocyanin contents to 46.66% and 57.14% in shoot and root as compared to the control plants, respectively (Figures 2G and 2H). The anthocyanins level showed steady increases in shoot and root under the applications of40 and 80 mgL-1 ZnO NPs applications (Figures 2G and 2H). The highest amount of anthocyanin was observed under the concomitant treatment of NaCl (100 mM) and ZnO NPs (80 mgL-1) in shoot (93.22%) as compared to the control plant. In root, the highest anthocyanin content was detected with the combination treatment of salinity (50 mM) and ZnO NPs (40 mgL-1) (1.15 fold) as compared to the control plants (Figures 2G and H).
Carotenoid
Carotenoid content has significantly increased at (21.9%) at 100 mM. NaCl exposure as compared to the normal conditions (Figure 2I).The lowest carotenoid content was observed under ZnO NPs (20 mgL-1) application by a 7.84% decrease as compared to the control plants(Figure 2I). The combined treatment of ZnO NPs at all concentrations with NaCl (50 mM) induced the lower carotenoid contents compared to NaCl (50 mM) without applying ZnO NPs (Figure 2I). The highest carotenoid content was observed under the combined treatment of (100 mM) and ZnO NPs (40 mgL-1) with 38.057% increase.
Zn, P, Ca Concentrations
NaCl affected the elements content of shoots and roots in camelina. The calcium calcium (Figures 2A and 2B) and zinc (Figures 3C and 3D) and zinc decreased in shoots and roots with the increasing salinity to 100 mM. Application of 100 mM NaCl that decreased calcium content to the lowest level as reported to be 27.90% in shoot (Figure 3A) and 43.18% in root (Figure 3B) in root in comparison to the control plants. The amount of zinc decreased in shoot to 39.90% (Figure 2C) and root to 29.57% (Figure 3D) at 100 salinity stress.
However, an increase in NaCl level to100 mM NaCl, and a decline in phosphorus content of roots to (89.09%) were observed (Figure 3E).
On the contrary, the maximum amount of phosphorus was observed under0 mM NaCl in shoot (control plants) (Figure 2F). Also, application of100 mM. NaCl, decreased the phosphorus contents of shoot (50.71 %) in comparison to the control plants (Figure 3E).
The plants treated with 20 mgL-1 ZnO NPs experienced non-significant increase of calcium in shoots (Figure 3A). In the root, a significant reduction was observed in calcium as 26.96%, due to the spraying of80 mgL-1 ZnO NPs (Figure3- B). In shoot, accumulation of zinc was observed under all ZnO NPs applications, with 20.07% and 13.02% induction under 40 and 80 mgL-1, respectively (Figure 3C).
Moreover, in this study, zinc content has significantly enhanced under all the concentrations of ZnO NPs in root (Figure 3D). In shoot tissues, the lowest amount of phosphorus with a 76.39% reduction was observed by applying ZnO NPs (80 mgL-1) (Figure 3E). As well as in the root, a 2.4-fold decrease was observed in phosphorus under 80 mgL-1 ZnO NPs treatment (Figure 3F).
Table 1. Effect of methyl salicyate (MeSA)on SOD (U/mg FW) activity of three rice genotypes.
Concentration
ZnO NPs mgL-1
Zn (ppm)
20 40 80
0.55±0.80 0.89±1.40
1.45±0.50
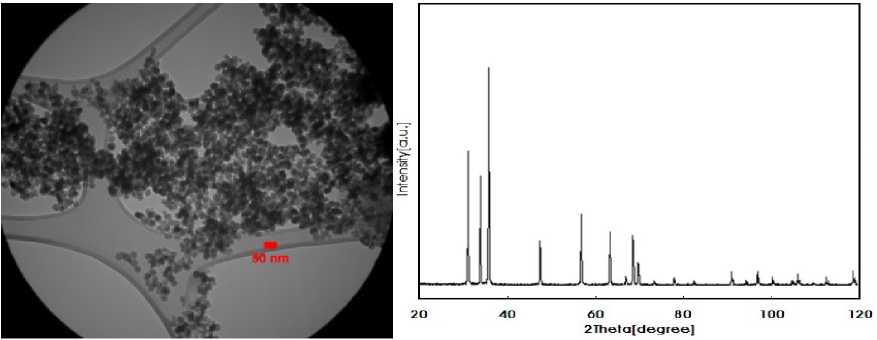
Figure1. Graph and images by microscope SEM. (Scanning electron microscope) (by Akhavan Hezaveh et
Table 2. The concentration of the amount used in the suspension of Zn used in this experiment.
al. , 2020)
0OmMN«Cl QSOmMNuCI OlOOmMNwCI
ZnO NP» (mgb'i
Figure 2. Effects of different concentrations of NaCl, and ZnO NPs on A) PhSh: Phenol content of shoot; B) PhR: Phenol content of root C) DPPHSh: DPPH inhibition of shoot; D) DPPHR: DPPH inhibition of root E); FSh: Flavonoid content of shoot; F) FR: Flavonoid content of root; G) AntoSh: Anthocyanin content of Shoot; H) AntoR: Anthocyanin content of root; I) Carot: Carotenoid. Means followed by different letters differ significantly when determined by the Tukey’s test
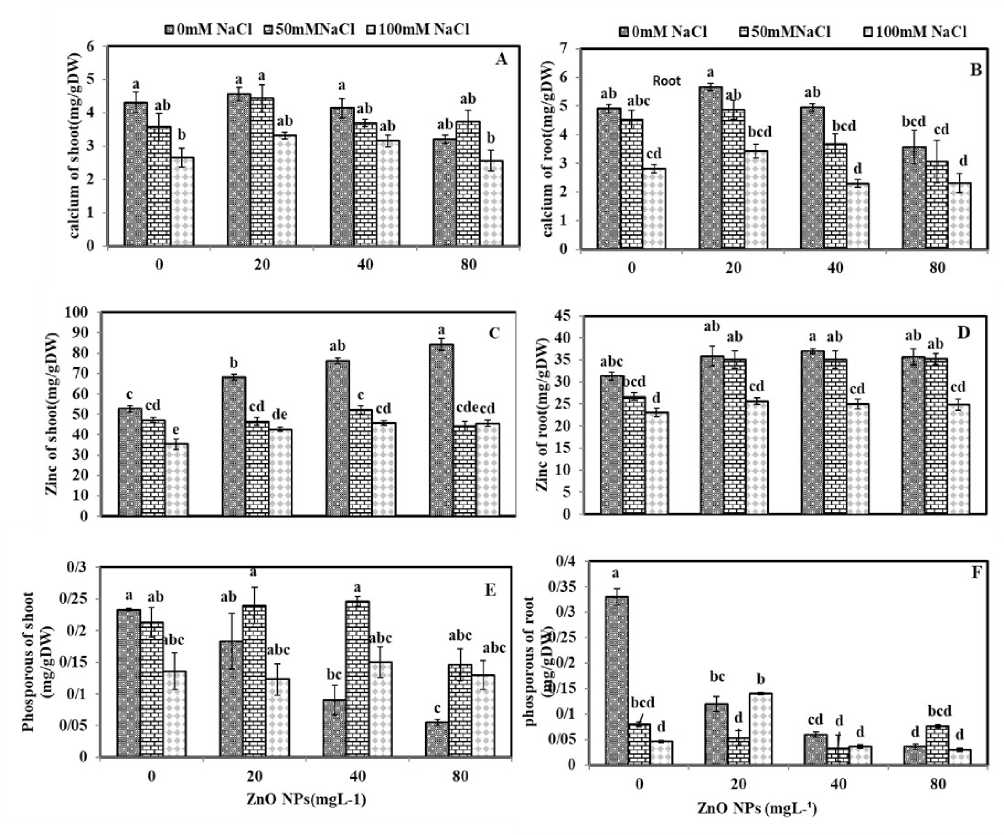
Figure 3. Effects of different concentrations of NaCl, and ZnO NPs on A) calcium of shoot; B) calcium of root; C) zinc of shoot; D) zinc of root E); phosphorus of shoot; F) phosphorus of root;. Means followed by different letters differ significantly when determined by the Tukey’s test
Minimum quantities of calcium (2.56 mg/g DW) (Figure 2A) and zinc (35.5 µg/gDW) (Figure 3C) were detected in shoot at 100 mM NaCl stress and 80 mgL-1 ZnO NPs. In roots, the lowest value of calcium content of 2.30 (mg/g DW) was found in the plants treated with 100 mM NaCl and ZnO NPs (0 mgL-1) (Figure 2B). Also, application of 100 mM NaCl with ZnO NPs (80 mgL-1) altered the zinc content to 23.03 (µg/gDW) in roots (Figure 3D). The highest amount of phosphorus was evidenced under the treatment of NaCl (100 mM) and ZnO NPs (40 mgL-1) by 3.47 times more than the control plants (Figures 3E). In the root tissues, the highest increase was observed to be 8.16 times at50 mM NaCl and 20 mgL-1 ZnO NPs with 6.18 as compared to the control plants (Figure 3F).
DISCUSSION
Total phenol
The result of this study show that, the total phenol contents of shoots and roots have significantly increased under NaCl(100 mM). In fact, by increasing salinity concentration, an enhancement was observed in secondary metabolite as well as toxicity symptoms. The result of the present study revealed very slight and significant changes in phenolic and anthocyanin contents under ZnO NPs exposure. When plant experiences metal stress, phenolic compounds can act as either metal chelators or ROS scavengers (Michalak,
2006). Accordingly, the role of zinc in the application of carbon to produce phenolic compounds in shikimic acid cycle and acetate can be considered as one of the reasons of this increase (Misra et al. , 2006). Hamid et al. (2010) and Vinod et al. (2012) reported an increase in the total phenolic content in wheat plants under Cu and Zn stress. Zinc induces oxidative stress (80 mgL-1) and also produces free radicals in plants, and also enables non-enzymatic antioxidant defense system (such as carotenoids and phenolic compounds) (Misra et al. , 2006). In addition, the application of ZnO NPs with salinity did not only help the salinity improvement, but also induced oxidative stress by generating free radicals and reactive oxygen species (ROS). Excessive ZnO NPs at 80 mgL-1 in interaction with salinity could profoundly affect the normal ionic homeostatic systems by intervening with the uptake, transport, osmotic adjustment of essential ions, which result in the format of metabolic processes such as transpiration, photosynthesis and enzyme activities related to metabolism (Mahajan et al. , 2011). Notably, there have been numerous reports on the induced accumulation of the phenolic compounds and defense systems in the plants under stress. In plants, phenolic compounds such as flavonols and phenyl propanoids act as the potential antioxidant compounds by donating electrons to guaiacol peroxidases (GPX) to detoxify the high amounts of H 2 O 2 produced under the stress conditions (Bartwal et al. , 2013). The obtained results showed that the enhancement in phenolic biosynthesis of the Zn treated (80 mgL-1) groups might be due to a higher expression of genes in the phenolic biosynthetic pathway
DPPH
DPPH scavenging assay is a reliable and easy technique to investigate antioxidant properties. DPPH scavenging has been employed on studying the antioxidant properties of wheat, vegetables, herbs, edible seed oils, conjugated linoleic acids, and flours in various organic solvents such as ethanol, aqueous acetone, methanol, aqueous alcohol, and benzene (Zhou et al., 2005). DPPH radical scavenging activity (I %) has significantly elevated under salinity stress. Also, the enhancement of I% means that more antioxidants have been produced. Moreover, the treatment with ZnO NPs (20 mgL-1) decreased I% levels in the treated plants.
It was turned out that, the plant responses to the salt stress are complex and involve both osmotic and ionic homeostasis, as well as cell detoxification. An increase has been reported in free radical degradation activity of DPPH for Cakile maritime under salinity stress (Ksouri et al., 2007). Also, antioxidants could interfere with the oxidation process induced by various stresses acting as oxygen scavengers; therefore, the tolerance to salinity stress might be correlated with an increase in the antioxidant potential (Zhou et al. , 2018).
General conclusions in our study on the effects of ZnO NPs have indicated that, it has no significant effects on DPPH activity. Our results suggest that, all camelina extracts (both plant organs) had antioxidative properties; however, the scavenging activity was the highest that was obtained from roots subjected to salinity stress. An indirect relationship was found between the nutritive effect of zinc on DPPH radical scavenging activity and the role of zinc in increasing the amount of phenolics, which is in agreement with our results. Also, a direct relationship has been reported between the antioxidant capacity and amount of phenolic compounds (George et al. , 2005s; Marreiro et al. , 2017). Total antioxidant activity was declined at two levels of salinity in combination with ZnO NPs (20 mgL-1), which can be due to the positive effects of zinc on reducing the percentage of radical degradation ZnO NPs (20 mg L-1 in root). Hence, by increasing salinity, zinc plays an antioxidant role; therefore, it produces less antioxidants. Also, an enhancement has been detected in the DPPH activity of methanolic extracts of M. vulgare. under 100 mM. NaCl (Rezgui et al. , 2017).
Flavonoid
Accumulation of flavonoids due to salinity may indicate that, camelina is relying on large amounts of flavonoid to cope with the harmful impacts of salinity. Plants respond to salt stress by simulating phenylalanine ammonia lyase PAL) (Gao et al., 2008), which is involved in the phenylpropanoid pathway enhancing the phenolic production (Lim et al., 2012). One reason for the flavonoid content induction is restricting the photosynthetic electron transfer during stress causing metabolic changes in the plant. Flavonoids make membranes resistant to oxidative factors by reducing their fluidity as well as prevention of free radical’s release. Moreover, flavonoids are frequently induced by abiotic stress and play a role in plant protection (Grace and Logan, 2000: Mierziak et al., 2014). Accordingly, they are accumulated in the plant tissues, which can protect them from damaging effects due to their free radical scavenging activity induced by the hydroxyl groups. Besides, stress factors can induce the increased generation of toxic reactive oxygen species (ROS). Flavonoids are strictly controlled by the plant antioxidative system ROS (Mierziak et al., 2014). Due to the positive effects of zinc on stress reduction, it may reduce flavonoids in the same treatments.
According to our results, the increased ZnO NPs concentration elevated the amount of flavonoid in shoots and roots compared to the control plants. Decreasing stress effects by flavonoids can be attributed to the attachment of phenolic to heavy metal ions (Arbona et al. , 2013). Moreover, flavonoids decrease the production of reactive oxygen species (ROS) through suppressing singlet oxygen and inhibiting the enzymes that generate ROS ( Mierziak et al. , 2014). Therefore, it was concluded that, concentration of bioactive secondary metabolites such as flavonoids in edible parts of plants is severely affected under stress conditions, altering health, and organoleptic properties of the plants. Previous studies also reported the induction of flavonoids under an extra heavy metal stress (Kim et al. , 1999; Michalak, 2006). Also, adjunct of Heavy metals for example zinc affect phenylpropanoid, flavonoids, and phenol metabolisms (Michalak, 2006). Increase in the flavonoid content by additional heavy metals e.g Fe+2, Fe+3, Cu+3, Zn+2, Al+3 and Mg+2 cations, supports the results obtained Shweta and Agrawal (2006) for spinach( Spinacia oleracea L.), by Hilal et al. (2004) for quinoa ( Chenopodium quinoa Willd) and by Rathore et al. (2003) for wheat ( Triticum aestivum L.).
Although in this study, flavonoid content of shoot was not significantly increased by increasing of ZnO NPs, in the root, it was the reverse. Furthermore, flavanols are among the metabolites of anthocyanin biosynthetic pathway at camelina and upstream enzymes in the flavonoids biosynthetic pathway that seems to enter anthocyanidins biosynthetic pathway (Dai et al., 2016).
Anthocyanin
Anthocyanins play significant roles in alleviating abiotic and biotic stresses in plants. One of the factors indicating that extra metals (Posmyk et al. , 2009) and NaCl (Wahid and Ghazanfar, 2006) lead to oxidative stress is considered to be the increase in the amount of anthocyanin. Anthocyanin has antioxidant properties, acts as free radical receptor, and protects plants against oxidative stress (Elisia, 2006).
Under the salinity stress conditions, anthocyanin can act as an osmotic regulator. In this regard, anthocyanin can also become an osmotic compatible solution under the aqueous or salinity stress conditions. Their synthesis and localization in roots, stems, and especially in leaf tissues make them possible for the plant to develop resistance against several environmental stresses. Also, an increase in anthocyanin content indicates its protective role in handling the stress and possibly reduces the damage caused by salinity (Wang et al. , 2009). Anthocyanin accumulation is stimulated by an increase in the salinity concentration in various plant organs (Eryılmaz, 2006).
In this regard, it is still unknown that whether the induction of anthocyanin is just a response to stress or is a defensive system against the biological damages (Asad et al. , 2015). However, a higher anthocyanin level can decrease oxidative stress (Elisia, 2006).
In this work, we have found an increase in Anthocyanin under saline soils. Generally, salinity and/or zinc can increase the photosensitivity through disturbing the structure of chloroplast, so the plant produces the light protective pigments like anthocyanin that protect the plant against light-induced oxidative damage (Jahangir et al. , 2009). It is still unknown that, whether the induction of anthocyanin is just a response to stress or is a defensive system against biological damages (Asad et al. , 2015). One of the factors indicating that heavy metals (e.g. zinc) lead to oxidative stress is the increase in the amount of anthocyanin (Posmyk et al. , 2009).
Carotenoids
Carotenoids are soluble antioxidant compounds, which manage to reduce the oxidative damage to the plant through the non-enzymatic pathway. Also, they are one of the essential components for salt tolerance in plant species (Abedini, 2016). In addition, carotenoids raise antioxidant capacity of plant, to protect the photosynthetic systems (Abedini, 2016). Elevation in the carotenoid content under salinity can dissipate excessive energy of photosystems I and II as heat or in non-harmful chemical reactions and can also fix chloroplast membrane (Havaux et al. , 2003; Coskun et al. , 2010).
Application of ZnO NPs slowly and constantly increased the carotenoid content. Zn due to detoxification is able to prevent the photosynthetic membranes degradation. Increasing carotenoids in stressed plants, with a positive effect on the fluidity of thylakoid membranes, can reduce the permeability of membranes against reactive oxygen species (Candan et al. , 2003). As a result, photosynthetic systems would be protected against the oxidative damages. In addition, ZnO NPs foliar application increased carotenoids that cause a reduction in the amount of lipids peroxidation; more protection of cells, chloroplasts, and photosynthesis pigments; and chlorophyll catabolism prevention. It seems that, a certain amount of zinc induces oxidative stress and also causes the synthesis of carotenoids.
The concomitant application of ZnO NPs (20 mg L-1) and NaCl (100 mM) exerted a significant decrease in the carotenoid content and then showed a steady increase along with the Nano increasing. Reductions in the interaction between salinity and ZnO NPs may be due to the positive effect on zinc. Notably, improvement of zinc effect on salinity on pigments has already been reported in (Weisany et al. , 2011) and tomatoes (Askari et al. , 2015). The inhibitory effect of the sufficient concentrations of Zn application on the production of these components in saline conditions has also been reported by Weisany et al. (2012).
Calcium, Zinc and Phosphorus
The obtained results reveal that, salinity stress effects on the content elements of camelina and ZnO
NPs spraying caused a decrease in calcium, zinc, and phosphorus contents of shoot and root. In this regard, phosphorus is the most important element interfering with zinc uptake by plants. Mateos-Naranjo et al. (2008) demonstrated that, zinc can affect the tissue phosphorus concentrations of S. densiflora . Foliar applications of ZnO NPs resulted in the increased amount of zinc in shoot and root decadence of the phosphorus in shoot and root. It has been reported that, along with increasing of the zinc concentrations, zinc concentration in the root and shoots of corn increases; therefore, its amount is going to be more in the shoot than the root (Hong and Ji-Yun, 2007). Also, Zinc is an active element in biochemical processes, which has chemical and biological interactions with some other elements (Mousavi et al. , 2012). The differences in shoot and root for zinc could be due to the efficient zinc transport through the shoots cortex and its translocation via the xylem occurring in camelina. The low zinc translocation that was measured (87) here is comparable to that observed by Sofo et al. (2013), who exposed Arabidopsis plants to 150 μM. ZnSO4 for 14 days. The High concentrations of ZnO NPs spray due to the decreased concentration of phosphorus can reduce the availability of zinc (Taheri et al. , 2012). The highest rate (80 mg L-1) of ZnO NPs resulted in the lowest amounts of increases of Phosphorus and Calcium. It was recorded that, zinc can affect the tissue Calcium, N, and Phosphorus concentrations (Mateos-Naranjo et al., 2008). One of the cases of salt tolerance in the plant is calcium accumulation, which plays a role in stress and interferes with the salinity correction (Posmyk et al. , 2007). In this regard, several studies have been conducted on the interaction of zinc and phosphorus, all of which confirm the zinc and phosphorus imbalance in the plant. As a result, metabolism defects can appear in the plant cells due to zinc and phosphorus imbalance (Khorgamy and Farnis. 2009; Das et al. , 2005).
Concentrations of phenolic compounds kept increasing, up to the concentration of 80 mgL-1Concentrations 20 mgL-1 caused significant reductions in the anthocyanin levels; however, Anthocyanins had their maximum activity at the concentration of 40 mgL-1 ZnO NPs, above which the activity remained constant.
The activities of carotenoids had firstly decreased with a mild slope up to the concentration of 80 mgL-1, and above that, they remained constant. Also, DPPH of root showed its highest activity at the concentration of 80 mgL-1 and salinity of 100 mM Therefore, in this plant, the phenolic compounds played the most important role in the plants' defense system, which was followed by carotenoids and anthocyanin, and finally DPPH came in third place. These results suggest that, the application of ZnO NPs may have a better efficiency compared to the conventional ZnSo4 fertilizers in overcoming the negative effects of salt stress on camelina in 20-40 mgL-1 due to the rapid overcoming on deficient, ease of use, and reducing the toxicity, which can be considered as a more effective defense mechanism on salt-induced O-2 generation in these concentrations of ZnO NPs. Addition of ZnO NPs under salt stress resulted in additional decrease in flavonoid, phenol, carotenoid, and DPPH of root. Furthermore, it was confirmed that, the toxic effects observed in ZnO NPs treated plants were not due to the Zn+2 release by ZnO NPs into the supernatants. Our results show that, if Nano metal concentration increases, Nano metal itself can be considered as a plant stress and does not only reduce the effects of salinity, but also exacerbates the toxicity of salinity through a high accumulation of toxic ions. When zinc amount is excessive, it can cause toxicity in plants. Therefore, it can be deduced that, zinc spray application leads to an increase in the photosynthesis of the plant. Zn application markedly increased Zn and also decreased Na and Cl concentrations in rice (Saleh and Maftoun, 2010).
Zinc is the micronutrient playing a major role in the biochemical and physiological processes underlying plant growth and carbohydrate metabolism, which have shown a beneficial effect on the other essential micronutrients on plant (Askary et al., 2017). Notably, Zinc is an essential intervening with various enzymes or can be considered as a functional structural or regulatory cofactor (Farahat et al., 2007). Zinc is also restricting excessive Na+ and Cl- uptake (Siddiqui et al., 2015). The selectivity of plant cells for ionic nutrient transport is well known; however, Zn can immediately enter because of its lower affinity to poly galacturonic acid, compared to other elements such as Pb, Cr, Cu or Ca (Chilian et al., 2015).
CONCLUSION
According to our results, foliar application of ZnO NPs (20 mgL-¹) could improve the physiological and biochemical performances of camelina plants in terms of increasing the antioxidant compounds.
In this study, ZnO NPs (80 mgL-¹) with salinity (100 mM) significantly disturbed the normal ionic homeostatic systems in plants by interfering with the uptake, transport, osmotic, and regulation of essential ions, which in turn result in the disruption of metabolic processes. However, ZnO NPs (20 mg L-1) decreased the harmful effect of soil salinity and played an important role in antioxidative system and improvement of plant nutrition under the saline conditions.
The increase in secondary metabolites and Anthocyanin accumulation are thought to enhance the ROS scavenging efficiency, thereby improve the Camelina tolerance to salt stress.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Список литературы Effects of Foliar Application of ZnO Nanoparticles on Secondary Metabolite and Micro-elements of Camelina (Camelina sativa L.) Under Salinity Stress
- Abdel-Wahed, M., S., A., Amin, A., A., and El Rashad, Abedini, M. (2016). Physiological responses of wheat plant to salinity under different concentrations of Zn. Acta Biologica Szegediensis, 60(1), 9-16.
- Ak, T. and Gülçin, İ. (2008). Antioxidant and radical scavenging properties of curcumin. Chemicobiological interactions, 174(1), 27-37.
- Akhavan Hezaveh, T., Pourakbar, L., Rahmani, F., & Alipour, H. (2020). Effects of ZnO NPs on phenolic compounds of rapeseed seeds under salinity stress. Journal of Plant Process and Function, 8(34), 11-18.
- Akula, R. and Ravishankar, G.A. (2011). Influence of abiotic stress signals on secondary metabolites in plants. Plant signaling and behavior, 6(11), 1720-1731.
- Akula, R. and Ravishankar, G.A. (2011). Influence of abiotic stress signals on secondary metabolites in plants. Plant signalling and behaviour, 6(11), 1720-1731.
- Allen, S.F. Grimshaw, H.F. Rowl, A.B. (1984). Chemical Analysis. In Moor, P.D. Chapman, S.B. (Eds.) Methods in plant ecology. Blackwell, Oxford, pp. 185‒344.
- Arbona Mengual, V., Manzi, M., De Ollas Valverde, C.J. and Gómez Cadenas, A. (2013). Metabolomics as a Tool to Investigate Abiotic Stress Tolerance in Plants. International Journal Molecular Sciences 14 (3), 4885-4911.
- Askari, M., Amini, F. and Jamali, F. (2015). Effects of zinc on growth, photosynthetic pigments, proline, carbohydrate and protein content of Lycopersicum esculentum L. under salinity. JPPF 3, 45-58.
- Bartwal, A., Mall, R., Lohani, P., Guru, S.K. and Arora, S. (2013). Role of secondary metabolites and brassinosteroids in plant defense against environmental stresses. Journal of Plant Growth Regulation, 32(1), 216-232.
- Candan, N. and Tarhan, L. (2003). Changes in chlorophyll-carotenoid contents, antioxidant enzyme activities and lipid peroxidation levels in Zn-stressed Mentha pulegium. Turkish Journal of Chemistry, 27(1), 21-30.
- Cherian, G. (2012). Camelina sativa in poultry diets: opportunities and challenges. Biofuel co-products as livestock feed: opportunities and challenges. Rome: FAO, 303-310.
- Chilian, A., Bancuta, R.O., Bancuta, I., Setnescu, R., Ion, R.M., Radulescu, C., Setnescu, T., Stihi, C., Gheboianu, A.I. and Chelarescu, E.D. (2015). Study of the influence of Zn concentration on the absorption and transport of Fe in maize by AAS and EDXRF analysis techniques. Romanian Reports in Physics, 67(3), 1138-1151.
- Coskun, D., Britto, D.T. and Kronzucker, H.J. (2010). Regulation and mechanism of potassium release from barley roots: an in planta 42K+ analysis. New Phytologist, 188(4), 1028-1038.
- Dai, L.P., Xiong, Z.T., Huang, Y. and Li, M.J. (2006). Cadmium‐induced changes in pigments, total phenolics, and phenylalanine ammonia‐lyase activity in fronds of Azolla imbricata. Environmental Toxicology: An International Journal, 21(5), 505-512.
- Ebrahimian, E., Bybordi, A. and Seyyedi, S.M., (2017). How nitrogen and zinc levels affect seed yield, quality, and nutrient uptake of canola irrigated with saline and ultra-saline water. Communications in Soil Science and Plant Analysis, 48(3), pp.345-355.
- Elisia, I. (2006). The protective effect of blackberry anthocyanins against free radical-induced oxidation and cytotoxicity in multiple cell lines (Doctoral dissertation, University of British Columbia).
- Elmastaş, M., Dermirtas, I., Isildak, O. and Aboul‐Enein, H.Y. (2006). Antioxidant activity of S‐carvone isolated from spearmint (Mentha Spicata L. Fam Lamiaceae). Journal of liquid chromatography & related technologies, 29(10), 1465-1475.
- Eryllmaz F. (2006). The relationships between salt stress and anthocyanin content in higher plants. Biotechnology & Biotechnological Equipment, 20(1), 47-52.
- Farahat, M.M., Ibrahim, M.S., Taha, L.S. and El-Quesni, E.F. (2007). Response of vegetative growth and some chemical constituents of Cupressus sempervirens L. to foliar application of ascorbic acid and zinc at Nubaria. World Journal of Agricultural Sciences, 3(4), 496-502.
- Gao, S., Ouyang, C., Wang, S., Xu, Y., Tang, L. and Chen, F. (2008). Effects of salt stress on growth, antioxidant enzyme and phenylalanine ammonialyase activities in Jatropha curcas L. seedlings. Plant Soil Environ, 54(9), 374-381.
- Georgé, S., Brat, P., Alter, P. and Amiot, M.J. (2005). Rapid determination of polyphenols and vitamin C in plant-derived products. Journal of Agricultural and food chemistry, 53(5), 1370-1373.
- Gogos, A., Knauer, K. and Bucheli, T.D. (2012). Nanomaterials in plant protection and fertilization: current state, foreseen applications, and research priorities. Journal of agricultural and food chemistry, 60(39), 9781-9792.
- Gomes, M.A.D.C., Pestana, I.A., Santa-Catarina, C., Hauser-Davis, R.A. and Suzuki, M.S. (2017). Salinity effects on photosynthetic pigments, proline, biomass and nitric oxide in Salvinia auriculata Aubl. Acta Limnologica Brasiliensia, 29, e9.
- Grace, S.C. and Logan, B.A. (2000). Energy dissipation and radical scavenging by the plant phenylpropanoid pathway. Philosophical Transactions of the Royal Society of London B: Biological Sciences, 355(1402), 1499-1510.
- Hamid, N., Bukhari, N. and Jawaid, F. (2010). Physiological responses of Phaseolus vulgaris to different lead concentrations. Pakistan Journal of Botany, 42(1), 239-246.
- Havaux, M., Lütz, C. and Grimm, B. (2003). Chloroplast Membrane Photostability in chlPTransgenic Tobacco Plants Deficient in Tocopherols. Plant Physiology, 132(1), 300-310.
- Heydarian, Z., Yu, M., Gruber, M., Coutu, C., Robinson, S.J. and Hegedus, D.D., 2018. Changes in gene expression in Camelina sativa roots and vegetative tissues in response to salinity stress. Scientific reports, 8(1), pp.1-22.
- Hong, W.A.N.G. and Jin, J.Y. (2007). Effects of zinc deficiency and drought on plant growth and metabolism of reactive oxygen species in maize (Zea mays L). Agricultural Sciences in China, 6(8), 988-995.
- Hussein, M.M. and Abou-Baker, N.H. (2018). The contribution of nano-zinc to alleviate salinity stress on cotton plants. Royal Society open science, 5(8), 171809. 1-11
- Ibrahim, F. M. and El Habbasha, S. F. (2015). Chemical composition, medicinal impacts and cultivation of Camelina (Camelina sativa). Int. J. PharmTech Res, 8, 114-122
- Jahangir, M., Abdel-Farid, I.B., Kim, H.K., Choi, Y.H. and Verpoorte, R.(2009). Healthy and unhealthy plants: The effect of stress on the metabolism of Brassicaceae. Environmental and Experimental Botany, 67(1), 23-33.
- Karowe, D.N. and Grubb, C. (2011). Elevated CO2 increases constitutive phenolics and trichomes, but decreases inducibility of phenolics in Brassica rapa (Brassicaceae). Journal of chemical ecology, 37(12), 1332-1340.
- Keilig, K. and Ludwig-Mueller, J. (2009). Effect of flavonoids on heavy metal tolerance in Arabidopsis thaliana seedlings. Botanical Studies, 50(3), 311-318.
- Khalid, H., Kumari, M., Grover, A. and Nasim, M. (2015). Salinity stress tolerance of camelina investigated in vitro.Scientia agriculturae bohemica, 46(4), 137-144.
- Khorgamy, A., & Farnia, A. (2009). Effect of phosphorus and zinc fertilisation on yield and yield components of chick pea cultivars. In 9th African Crop Science, Conference Proceedings, Cape Town, South Africa, 28 September-2 October 2009 (pp. 205- 208). African Crop Science Society.
- Kim, M.S., CHUL, K., Do Hyun, J.O. and Yeon, W.R. (1999). Effect of fungal elicitor and heavy metals on the production of flavonol glycosides in cell cultures of Ginkgo biloba. Journal of microbiology and biotechnology, 9(5), 661-667.
- Kreslavski, V.D., Lyubimov, V.Y., Shirshikova, G.N., Shmarev, A.N., Kosobryukhov, A.A., Schmitt, F.J., Friedrich, T. and Allakhverdiev, S.I. (2013). Preillumination of lettuce seedlings with red light enhances the resistance of photosynthetic apparatus to UV-A. Journal of Photochemistry and Photobiology B: Biology, 122, 1-6.
- Ksouri, R., Megdiche, W., Debez, A., Falleh, H., Grignon, C. and Abdelly, C.(2007). Salinity effects on polyphenol content and antioxidant activities in leaves of the halophyte Cakile maritima. Plant Physiology and Biochemistry, 45(3-4), 244-249.
- Kumari, M., Khan, S.S., Pakrashi, S., Mukherjee, A. and Chandrasekaran, N. (2011). Cytogenetic and genotoxic effects of zinc oxide nanoparticles on root cells of Allium cepa. Journal of hazardous materials, 190(1-3), 613-621
- Lee, J. Durst, R. W., & Wrolstad, R. E. (2005). Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differentiamethod: collaborative study. Journal of AOAC international, 88(5), 1269-1278.
- Lichtenthaler, H.K. (1987). Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 148, 350-382
- Lim, J.H., Park, K.J., Kim, B.K., Jeong, J.W. and Kim, H.J. (2012). Effect of salinity stress on phenolic compounds and carotenoids in buckwheat (Fagopyrum esculentum M.) sprout. Food chemistry, 135(3), 1065-1070.
- Mahajan, P., Dhoke, S.K. and Khanna, A.S. (2011). Effect of nano-ZnO particle suspension on growth of mung (Vigna radiata) and gram (Cicer arietinum) seedlings using plant agar method. Journal of Nanotechnology, 2011, Article ID 696535.
- Marreiro, D., Cruz, K., Morais, J., Beserra, J., Severo, J. and de Oliveira, A.(2017). Zinc and oxidative stress: current mechanisms. Antioxidants, 6(2), 24.
- Marslin, G., Sheeba, C.J., and Frankin, G. (2017). Nanoparticles alter secondary metabolism in plant via ROS burst. Frontiers in plant science, 8, 832.
- Mateos‐Naranjo, E., Redondo‐Gómez, S., Cambrollé, J., Luque, T. and Figueroa, M.E. (2008). Growth and photosynthetic responses to zinc stress of an invasive cordgrass Spartina densiflora. Plant Biology. 10, 754–762.
- Michalak, A. (2006). Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Polish Journal of Environmental Studies, 15(4), 523–530.
- Mierziak, J., Kostyn, K. and Kulma, A. (2014). Flavonoids as important molecules of plant interactions with the environment. Molecules, 19(10), 16240-16265.
- Misra, A., Dwivedi, S., Srivastava, A.K., Tewari, D.K., Khan, A. and Kumar, R. (2006). Low iron stress nutrition for evaluation of Fe-efficient genotype physiology, photosynthesis, and essential monoterpene oil(s) yield of Ocimum sanctum. Photosynthetica, 44(3), 474-477.
- Munns, R. and Tester, M. (2008). Mechanisms of salinity tolerance. Annu. Rev. Plant Biol., 59, 651-681.
- Posmyk, M.M., Kontek, R. and Janas, K.M. (2009). Antioxidant enzymes activity and phenolic compounds content in red cabbage seedlings exposed to copper stress. Ecotoxicology and Environmental Safety, 72(2), 596-602.
- Prasad, T.N.V.K.V., Sudhakar, P., Sreenivasulu, Y., Latha, P., Munaswamy, V and et al. (2012). Effect of nanoscale zinc oxide particles on the germination, growth and yield of peanut. Journal of plant nutrition, 35(6), 905-927.
- Rathore, D., Agrawal, S.B. and Singh, A. (2003). Influence of supplemental UV-B radiation and minerals on biomass, pigments and yield of two cultivars of wheat (Triticum aestivum L.). International Journal of Biotronics, 32, 1-5.
- Rezgui, M., Majdoub, N., Ben-Kaab, S., Marzouk, B., Gouia, H., Araújo, M.E.M. and Ben-Kaab, L.B., (2017). How Salt Stress Represses the Biosynthesis of Marrubiin and Disturbs the Antioxidant Activity of Marrubium Vulgare L. Polish Journal of Environmental Studies, 26(1), 267-277.
- Saleh, J. and Maftoun, M. (2010). Interactive effects of NaCl levels and zinc sources and levels on the growth and mineral composition of rice. Journal of Agricultural Science and Technology, 10, 325-336.
- Shweta, M., & Agrawal, S. B. (2006). Interactive effects between supplemental ultraviolet-B radiation and heavy metals on the growth and biochemical characteristics of Spinacia oleracea L Brazilian Journal of Plant Physiology, 18(2), 307-314.
- Siddiqui, S.N., Umar, S. and Iqbal, M. (2015). Zincinduced modulation of some biochemical parameters in a high-and a low-zinc-accumulating genotype of Cicer arietinum L. grown under Zndeficient condition. Protoplasma. 252, 1335–1345.
- Sofo, A., Vitti, A., Nuzzaci, M., Tataranni, G., Scopa, A., Vangronsveld, J. ... & Sanità di Toppi, L. (2013). Correlation between hormonal homeostasis and morphogenic responses in Arabidopsis thaliana seedlings growing in a Cd/Cu/Zn multi‐ pollution context. Physiologia plantarum, 149(4), 487-498.
- Staszczak, M. (2008). The role of the ubiquitinproteasome system in the response of the ligninolytic fungus Trametes versicolor to nitrogen deprivation. Fungal Genetics and Biology, 45(3), 328-337.
- Taheri, N., Sharif-Abad, H. H., Yousefi, K., & Roholla-Mousavi, S. (2012). Effect of compost and animal manure with phosphorus and zinc fertilizer on yield of seed potatoes. Journal of soil science and plant nutrition, 12(4), 705-714.
- Telesiñski, A., Nowak, J., Smolik, B., Dubowska, A. and Skrzypiec, N. (2008). Effect of soil salinity on activity of antioxidant enzymes and content of ascorbic acid and phenols in bean [Phaseolus vulgaris L.] plants. Journal of Elementology, 13(3), 401-409
- Torabian, S., Zahedi, M., & Khoshgoftarmanesh, A. (2016). Effect of foliar spray of zinc oxide on some antiox idant enzymes activity of sunflower under salt stress. J. Agr. Sci. Tech. 18, 1013-1025
- Vahdati, K., & Lotfi, N. (2013). Abiotic stress tolerance in plants with emphasizing on drought and salinity stresses in walnut. Abiotic Stress–Plant Responses and Applications in Agriculture, 10, 307-365.
- Vinod, K., Awasthi, G. and Chauchan, P.K. (2012). Cu and Zn tolerance and responses of the biochemical and physiochemical system of wheat. Journal of Stress Physiology and Biochemistry, 8(3). 203-213
- Wahid, A. and Ghazanfar, A. (2006). Possible involvement of some secondary metabolites in salt tolerance of sugarcane. Journal of plant physiology, 163(7), 723-730.
- Wang, C., Zhang, S.H., Wang, P.F., Hou, J., Zhang, W.J., Li, W. and Lin, Z.P.(2009). The effect of excess Zn on mineral nutrition and antioxidative response in camelina seedlings. Chemosphere, 75(11), 1468-1476.
- Weisany, W., Sohrabi, Y., Heidari, G., Siosemardeh, A. and Ghassemi-Golezani, K. (2011). Physiological responses of soybean (Glycine max L.) to zinc application under salinity stress. Australian Journal of Crop Science 5, 1441-1447.
- Weisany, W., Sohrabi, Y., Heidari, G., Siosemardeh, A. and Ghassemi-Golezani, K. (2012). Changes in antioxidant enzymes activity and plant performance by salinity stress and zinc application in soybean (Glycine max L.). Plant Omic 5, 60-67.
- Zhou, J., Diao, X., Wang, T., Chen, G., Lin, Q., Yang, X., & Xu, J. (2018). Phylogenetic diversity and antioxidant activities of culturable fungal endophytes associated with the mangrove species Rhizophora stylosa and R. mucronata in the South China Sea. PloS one, 13(6)


