Effects of Food Deprivation and Seasons on Circadian Rhythm in Body Temperature and Bodyweight in Rouen Ducks under Tropical Conditions
Автор: Joseph Olusegu Ayo, Ndazo Salka Minka, Fatima Bukar Hassan, Harold Kuta Makeri
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 3 т.16, 2020 года.
Бесплатный доступ
Background: The present study investigated the effects of four days of fasting on colonic temperature (CT) and body weight responses in 20 adult male Rouen ducks (Anas platyrhynchos domesticus) during the hot-dry (12:12 light/dark cycles) and cold-dry (harmattan) (11:13 light/dark cycles) seasons under natural light/day cycles in tropical conditions. The ambient temperature and relative humidity of the pen and the CT of the ducks were recorded bihourly for four days before fasting and subsequently for another four days during fasting. During the fasting period food was completely withdrawn from the ducks at 06:00 h for four days (96 h), but the ducks were given access to clean drinking water ad libitum throughout the fasting period. Results: A major finding in the present study was that four days of food deprivation, photoperiods, season and duration of fasting did not induce hypothermia or changes in circadian rhythmic pattern of CT in the Rouen ducks. The result, for the first time, suggests that circadian modulation of fasting-induced hypothermia was lacking in Rouen ducks. The mesor and amplitudes of CT obtained during both the harmattan and hot-dry seasons before fasting were not different (P > 0.05) from those obtained during fasting. The acrophases in both seasons before and during fasting were restricted to the photophase at 14:00 h, except for the acrophase in fasted ducks during the hot-dry season, which was delayed to 16:00 h. Body weight of the ducks decrease significantly (P > 0.05) only on the 4th day of fasting. Conclusion: Overall, unlike other birds and mammals, the Rouen ducks showed greater starvation resilience, apparently due to an unknown component of fasting resistance or regional heterothermy. Future studies to elucidate the mechanism by which Rouen ducks were resistant to fasting are still required.
Circadian rhythm, Colonic temperature, Fasting, Hypothermia, Rouen duck
Короткий адрес: https://sciup.org/143173845
IDR: 143173845
Текст научной статьи Effects of Food Deprivation and Seasons on Circadian Rhythm in Body Temperature and Bodyweight in Rouen Ducks under Tropical Conditions
Animals are known to maintain a constant metabolic rate under thermoneutral environment; however, when energy intake is restricted or provided in excess, metabolic rate correspondently reduces or increases to correct for the energy imbalance (Piccione et al ., 2002). The down- regulation (facultative hypothermia) of metabolic rate and body temperature below normorthermic levels, is a widespread physiological mechanism adopted by birds to conserve energy (McKechnie and Lovegrove, 2002; Schleucher, 2004).
Prolong food deprivation causes a decrease in body temperature (hypothermia) of homeotherms, especially in small birds and mammals, and such hypothermia is modulated by circadian system (Piccione et al ., 2002). Generally hypothermia occurs in animals primarily during the inactive period (during the night for diurnal animals and during the day for nocturnal animals). Several research findings in birds and mammals, including humans, demonstrated a decrease in core body temperature as a result of low energy expenditure in animals subjected to food deprivation for prolong period of time (Piccione et al ., 2002). Although hypothermia has been shown to occur in many avian species (McKechnie and Lovegrove, 2002), the cues eliciting this phenomenon are not yet well understood.
Among factors that affect the circadian rhythm of an organism is the environment in which an organism lives (Mrosovsky, 1999; Ben-Hamo et al., 2010). It is to be expected that environmental temperature may affect the oscillatory pattern of core temperature and blood biochemical variables (Giannetto and Piccione, 2009; Piccione et al. 2013). Of recent, studies on quail and ducks deprived of food show that ambient temperature affects the depth of hypothermia (Laurila, 2005; Tattersall et al., 2016) and it is likely that the ‘trigger’ to entering hypothermia is under the control of multiple physiological inputs (Schleucher, 2004), which may even differ among species. Inspite of numerous studies on the responses of animals and humans to food deprivation, such studies were performed majorly in temperate regions of the world under control environment (Piccione et al. 2013; Ayo et al., 2017). Studies on the effect of food deprivation and different seasons on birds under tropical conditions are still limited. The Northern Guinea Savannah zone where this study was conducted has three distinct seasons, viz: the raining (late May to late October), the hot-dry (March to May) and cold-dry or harmattan (early November to late February) seasons. The raining season is characterized by rainfall and relatively moderate ambient temperature (Odekunle, 2004). The hot-dry is characterized by high ambient temperature, intense sunlight with no rain, and the season is known to cause heat stress in birds (Donkoh, 1989; Minka and Ayo, 2016), while the harmattan season is characterized by a very cold-dry and heavy dust-laden wind, blowing northeast and west off the Sahara desert into the Gulf of Guinea, towards the Caribbean and South America (Oladele, 2007; Minka and Ayo, 2014). The cold-dry and dust-laden wind has also been shown to be stressful to animals (Igono et al., 1982; Ayo et al. 1998; Minka and Ayo, 2014). It is hypothesized that food depravation during the stressful hot-dry and harmattan seasons may have negative impact on bird’s physiology. The aim of the present study was to evaluate the effects of food deprivation under the hot-dry and cold-dry (harmattan) seasons on circadian rhythm in core body temperature and body weight of Rouen ducks under natural tropical day/light cycles.
MATERIALS AND METHODS
Study area and environmental conditions
The study was carried out in Livestock Farm of the College of Agriculture and Animal Science, Ahmadu Bello University, Kaduna (11ο 10/N, 07ο 38/ ), located in the Northern Guinea Savannah zone of Nigeria during the hot-dry (March) and cold-dry harmattan (December) periods of the year.
Birds and management
A total of 20 clinically healthy, adult male Rouen ducks (Anas platyrhynchos domesticus), aged 7 mouths and weighing between 1.2 to 1.5 kg served as subjects during the hot-dry and harmattan periods. The ducks were obtained from a commercial farm one month to the experimental period and were housed in individual cages (1 m3) under natural 12:12 L/D (0700-1900) during the hot-dry, and 11:13 L/D (0730-1800 h) during the harmattan periods. The ducks were provided with feeder and water through spaces of 1.30 cm and 0.6 cm, respectively. Before and after the fast, ducks were fed ad libitum with a commercial poultry mash from Vital Feeds PLC, Inc, Kaduna, Nigeria. The ducks were given free access to clean drinking water ad libitum.
Experimental procedures
Two weeks to the start of fasting date the ducks were acclimatized to the experimental procedures, which included repeated handling and measurements of core body temperature, and liveweight. The thermal environmental data and length of photoperiods in both seasons were also recorded using standard procedures. The average values for the physiological and thermal environmental data were later used as reference control values. The experiment was divided into two equal, four-day time periods: feeding and fasting periods. During the fasting periods food was completely withdrawn from the ducks at 06: 00 h for four days (96 h), but clean drinking water was provided throughout the fasting periods (Oismenu et al ., 1992; Tattersall et al ., 2016). The four days of food deprivation followed the welfare guides of CCAC (1991) and O CD (2000). These guides clearly states that bodyweight lost and hypothermia in experimental animals should not be more than 20% and 10% from normal body weight and body temperature, respectively. The AT and RH inside the pen, and the CT of the ducks were recorded bihourly during the 4 days of fasting, starting at 06:00 h on day one during both hot-dry and harmattan periods to the final day (4th day) at 06:00 h.
Experimental measurements
The CT of the ducks was recorded by inserting a thermistor probe (Yellow Spring Instruments, Model 46 TUC, Yellow Spring, OH) to a depth of 3-4 cm into the cloaca. Within the same period of CT measurements, the dry- and wet-bulb temperature values were obtained concurrently with the CT readings at the experimental site, using a dry- and wet-bulb thermometer (Hartmann Company, ngland). Body mass of the ducks were recorded using a digital weighing scale (±0.1 g, Hartmann Company, ngland), just before and immediately after fasting. In other to insure accuracy of measurements, all the instruments used in measurements were calibrated according to manufactures’ guidelines.
Ethics statement
The research is approval by the Research and Seminar thical Committee on Animal Welfare of the Department of Animal Health ( thical No. CAAS/VCN -2518 - 54b - 2018/2019). The management of the ducks were done based on the reviewed Guidelines on Duck Welfare Recommendation (GDWR) (2018) and The Humane Society of the United States (2008) on the Welfare of Animals in the Duck Industry.
Statistical analysis
Cosinor analysis was used to determine the CT daily rhythms of individual ducks. The mean mesor (rhythm-adjusted mean), amplitude (half the range of excursion or a measure of the extent of predictable change within a cycle) and acrophase (time of peak) values of the variables of daily rhythm were calculated for each duck and for each time series of the study period. ach time series was evaluated for rhythmicity by repeated-measures analysis of variance (ANOVA model-3) and by the cosinor procedure (Refinetti et al ., 2007). The effects of fasting, season and their interaction were compared. Data were analyzed using the software STATISTICA 5.5 (StatSoft Inc., USA). The difference was tested by paired Student’s t -test. Values of P < 0.05 were considered significant.
RESULTS
During the hot-dry season the ambient temperatures (AT) of the pens during the study ranged from 22.2°C to 39.5°C, with a mean value of 30.5 ± 0.4°C and during the harmattan period AT ranged from 10.5°C to 34.7°C, with a mean value of 22.4 ± 0.2°C. The relative humidity (RH) ranged from 67% to 80% during the hot-dry and 20% to 45% during the harmattan periods. The photophase during the hot-dry and harmattan periods were 12:12 light/dark (L/D) and 11:13 L/D, respectively.
In fasted ducks, the daily mean CT of 40.3 ± 0.06°C recorded during the harmattan season was not different from 40.4 ± 0.05°C recorded during the hot-dry season. In general, the mean CT did not differ between the seasons and between fasted or fed ducks. The maximum CT value differed (P < 0.05) between seasons, but not between fasted and non-fasted ducks (Table 1).
The circadian rhythm of CT of the ducks recorded for four days before fasting during the harmattan and hot-dry seasons exhibited rhythmic pattern. However, during the hot-dry season the circadian rhythm of CT had higher amplitude during the afternoon hours of the day (Figure 1). Similarly, four days of fasting had no effect on the circadian rhythm of CT of the Rouen ducks during the harmattan and hot-dry seasons (Figures 2 and 3). The duration of fasting did not exert significant effect on the oscillatory pattern of CT in the ducks during the harmattan and hot-dry seasons; although the CT decreased on the fourth day of fasting during the hot-dry season, the decrease was not statistically significant (P >0 .05).
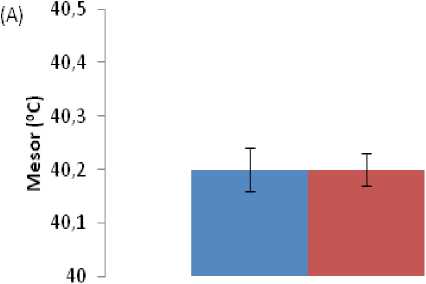
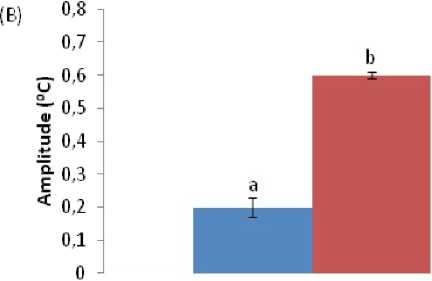
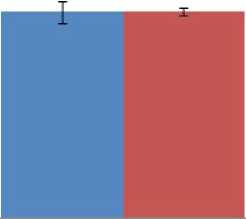
The mesor and amplitude of the CT were not affected by fasting in both seasons, although the amplitude was lower during the harmattan season before and during fasting (Figure 4). During the hot-dry season, the acrophase of CT in fasted ducks was shifted from 14:00 h before fasting to 16:00 h during fasting.
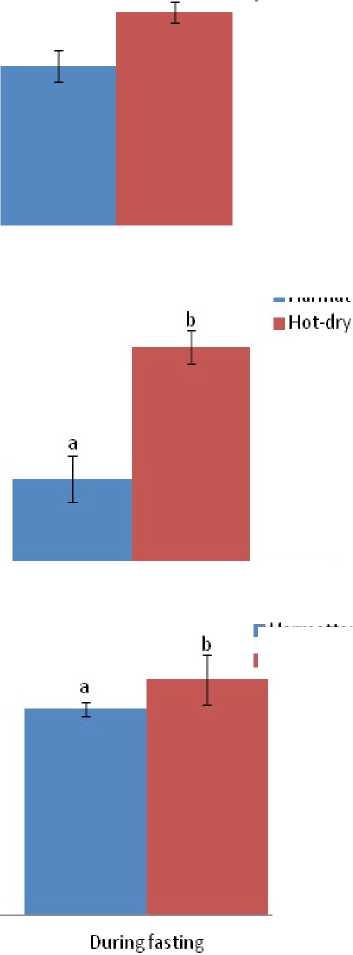
Bodyweight of the ducks was not significantly ((P > 0.05) affected by the duration of fasting from day 1 to day 3, however the effect was significant (P < 0.05) on the 4th day of fasting during both the hot-dry and harmattan seasons (Figure 5).
Table 1. Colonic temperature (0C) of Rouen ducks (n = 20) for four days before and four days during fasting
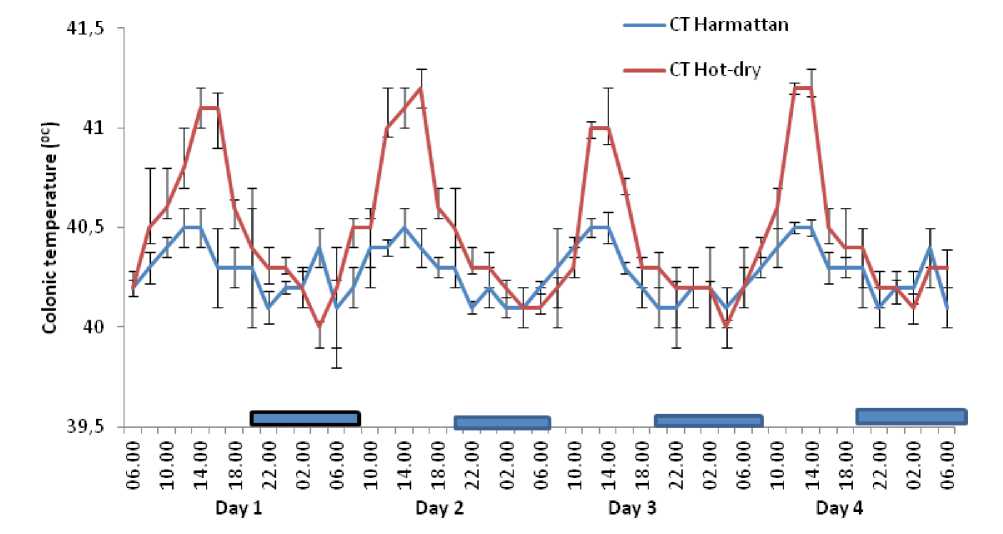
Figure 1. ffects of harmattan and hot-dry seasons on daily rhythms of colonic temperature in non fasted Rouen ducks ach data point represents the mean (±S ) of 20 ducks at each period of measurements. Rhythm characteristics of colonic temperature was analysed using cosinor procedure and the application of a trigonometric statistical model. The horizontal dark bars denote the dark phase of the prevailing light-dark cycle. For each season measurements were made at 2-h intervals for a period of 4 conservative days.
|
Fed ducks Fasted ducks Harmattan Hot-dry Harmattan Hot-dry |
|
|
Mean ± S M |
40.4 ± 0.02a 40.5 ± 0.04 a 40.3 ± 0.06 a 40.4 ± 0.05 a |
|
Maximum |
40.5a 41.2b 40.6a 41.2b |
|
Minimum |
40.1a 40.0a 40.1a 39.9a |
|
Range |
0.4a 1.2b 0.5a 1.3b |
CT = colonic temperature
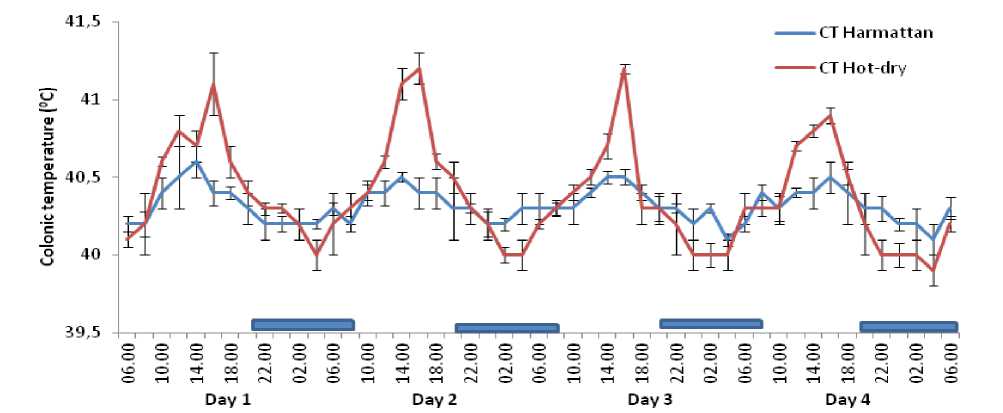
Figure 2. ffects of four days fasting on daily rhythms of colonic temperature in Rouen ducks during the harmattan and hot-dry seasons. ach data point represents the mean (±S ) of 20 ducks at each period of measurements. Rhythm characteristics of colonic temperature was analysed using cosinor procedure and the application of a trigonometric statistical model. The horizontal dark bars denote the dark phase of the prevailing light-dark cycle. For each season measurements were made at 2-h intervals for a period of 4 conservative days. CT = colonic temperature
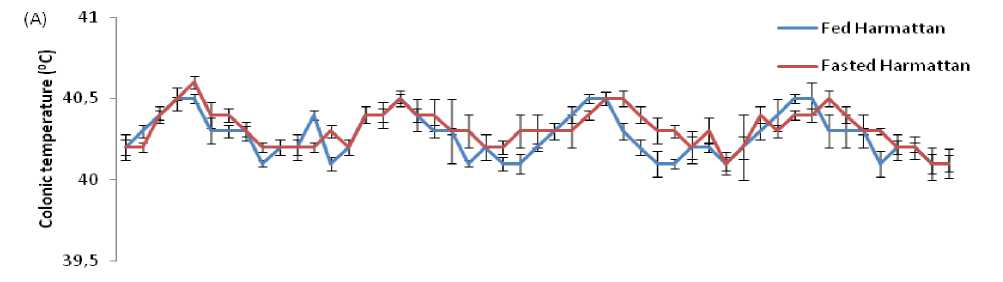
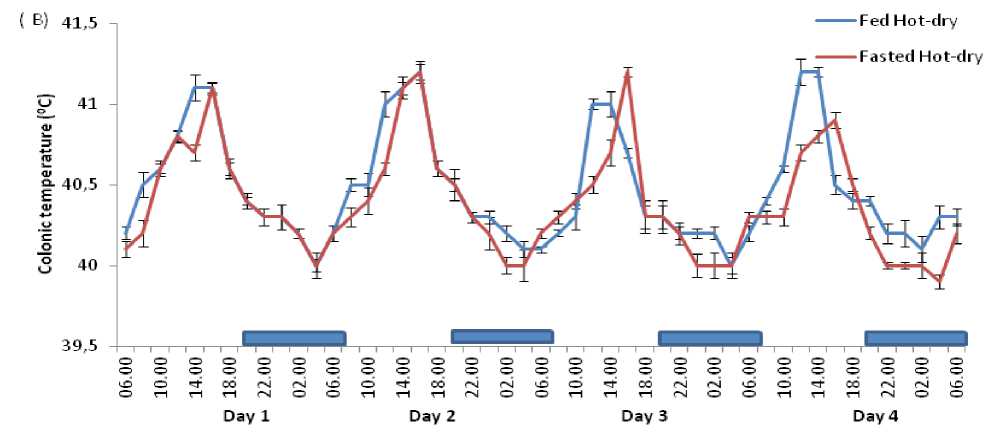
Figure 3. Comparison of circadian rhythm of colonic temperature in Rouen ducks between fed and fasted for four days during the harmattan (A) and hot-dry (B) seasons. ach data point represents the mean (±S ) of 20 ducks at each period of measurements. Rhythm characteristics of colonic temperature was analysed using cosinor procedure and the application of a trigonometric statistical model. The horizontal dark bars denote the dark phase of the prevailing light-dark cycle. For each season measurements were made at 2-h intervals for a period of 4 conservative days.
(С) 19:12
16:48
Before fasting
■ Harmattan
Harmattan Hot-dry
Harmattan
Hot-dry
Figure 4. ffects of ad libitum feeding, fasting, harmattan and hot-dry seasons on characteristic of colonic temperature of Rouen ducks (n = 20) during 96 h record period. For each season measurements were made at 2 h intervals for a period of 4 days. A trigonometric statistical model was used to characterize the main rhythmic parameters according to the single cosinor procedure. Mesor results (A) reflect the daily average colonic temperature. Amplitude (B) reflects the peak or nadir deviation from the mesor. Acrophase (C) reflects the hour of the day when colonic temperature was at its highest. ach bar corresponds to the mean (±S ) of 20 ducks. In each graph, means bearing different letters (a, b) are significantly different from each other (P < 0.05).
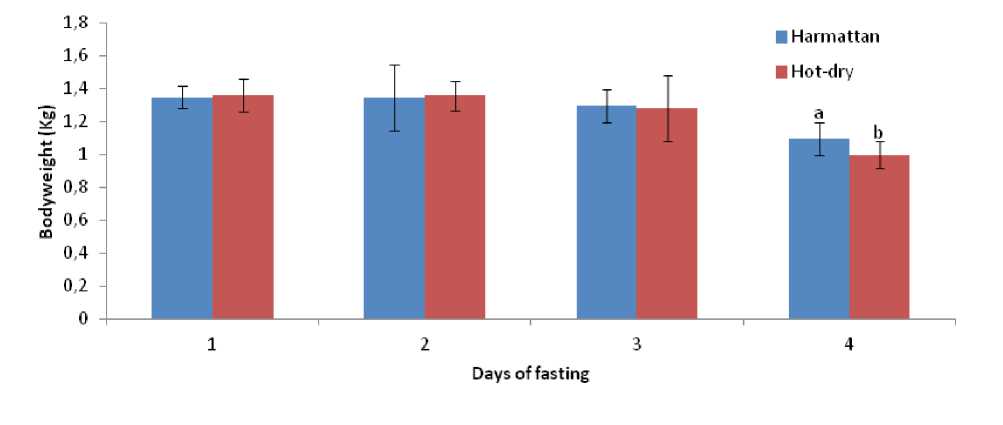
Figure 5. Bodyweight responses of Rouen ducks (n = 20) to the effect of four days fasting during the harmattan and hot-dry seasons. In each graph, means bearing different letters (a, b) are significantly different from each other (P < 0.05).
DISCUSSION
Unique to the present study is the findings that food deprivation for four days under 12:12 h L/D cycle during the hot-dry season and 11:13 h L/D cycle during the harmattan season did not induce hypothermia in ducks during both the photophase and scotophase. The finding is in contrast to several studies performed on birds (Hohtola et al ., 1991; Underwood et al ., 1999; Laurila, 2005; Ben-Hamo et al ., 2010; Tattersall et al ., 2016), mammals (Yoda et al ., 2000) and humans (Martineaud et al ., 2000), where food deprivation for a short period caused a significant decrease in core body temperature below normothermia i.e hypothermia (during the scotophase for diurnal animals and during the photophase for nocturnal animals). Hypothermia was shown to be as a result of low energy expenditure, which consequently serves substantial amount of energy for the bird (Underwood et al ., 1999).
Food-deprived induced hypothermia is observed to be modulated by circadian system, which occurs primarily during the dark phase of the light-dark cycle (Tattersall et al., 2016). In the present study food-deprived induced hypothermia was not modulated by circadian system in the Rouen ducks. Although some studies performed in Pekin ducks reported a relatively stable decrease in core body temperature of food-deprived ducks, these experiments were not rhythmicity studies in nature and were conducted under thermoneutral and controlled laboratory environments (Tattersall et al., 2016). Besides, none of the study was conducted on Rouen ducks under natural day/light conditions. The lack of information on thermoregulation of food-deprived Rouen ducks does not warrant any comparison of our results. Nevertheless, similar resistance to fasting hypothermia was reported in food-deprived king penguin (Fahlman et al., 2005) and in Tegmalm’s owls under controlled thermoneutral and cold environments (Hohtola, 2012). Furthermore, in birds regional heterothermy during fasting was suggested as an explanation for why core body temperature does not vary much with fasting in some species, despite a large metabolic suppression (Hohtola, 2012). Regional heterothermy, apparently, decreases thermal conductance and allows for relatively stable homeothermy (McKechnie and Mzilikazi, 2011). Hohtola (2012) observed that the physiological mechanism that improve fasting endurance include low metabolic rate, low core body temperature, high thermal insulation and capability for hypometabolism.
The low amplitude (0.2 - 0.6°C) of the diurnal CT observed in the Rouen ducks suggests that resting metabolic rate does not vary markedly during the diurnal cycle in ducks. This is especially true during the harmattan season, when amplitude of CT was between
0.1 to 0.2 °C against 0.65 °C recorded during the hot-dry season. Similar low amplitude of core body temperature was reported in Tegmalm’s owls (Hohtola , 2012), and this was suggested as one of the factors responsible for normothermia in these birds during fasting. Our present result on amplitude showed no significant increase in the amplitude of fasted ducks from those of fed ducks. The result is different from that of Tattersall et al . (2016), who reported a significant increase in amplitude of CT of Pekin ducks fasted under thermoneutral conditions and in pigeons fasted under semi-natural conditions (Laurila, 2005). Furthermore, the fact that ducks by nature are aquatic birds and have excellent thermal insulators may account for a low-resting metabolic rate, consequently preventing hypothermia under food deprivation. Good thermal insulation has been reported to be a factor used by owls to sustain normothermia under fasting condition (Hohtola et al ., 1991; Hohtola, 2012). The result of acrophase of CT before fasting in both seasons was similar to those obtained in quails in the zone of study (Minka and Ayo, 2016), however the delayed acrophase reported in fasted ducks suggests that food deprivation has delayed the increase in CT of the ducks.
Of recent, Tattersall et al . (2016) postulated that variability in core body temperature may itself be a source of energetic savings, thus maintaining normothermia during fasting, a concept that has not been formally tested. In fact, birds not showing hypothermia during food deprivation have been classified into a special group (Hohtola, 2012). Unfortunately, to date, research towards this direction has not been expanded (Longo and Mattson, 2014). Although core body temperature rhythm has long been shown to occur as a result of changes in heat production or heat loss (Ben-Hamo et al ., 2010), the present CT results obtained in fasted ducks and other findings by Nagashima et al . (2018) suggest that the body temperature rhythm is not simply a result of circadian change in heat loss or production in the body. Rather, the rhythm is a result of the coordinated thermoregulatory processes that maintain a specific body temperature at a given time of the day. The body temperature rhythm may be generated by the association between the circadian and thermoregulation systems. However, the mechanism is not yet known
(Nagashima et al ., 2018). Thus, more studies are required to timely elucidate the proximate mechanism undelying fasting resistance in Rouen ducks and other birds under natural tropical photoperiods, as this may have clinical significance in the field of medicine and avian biology.
Another postulation on nocturnal hypothermia is the combine effects of fasting and nocturnal release of melatonin. Melatonin released from the pineal glands during the dark phase has been demonstrated to cause a decrease in core body temperature virtually in all mammals, including humans and birds (Zeman et al ., 2001) and its hypothermic effect on core body temperature increases in fasted individuals during the dark phase. In the present study, the level of melatonin was not measured in the Rouen ducks, but the combined effects of fasting and hypothermic effect of melatonin on CT during the dark phase were not clearly observed as in other animals. The reason for the effects was not elucidated in the present study, and it requires further investigation. Nevertheless, findings from study performed on adult ducks demonstrated irregular rhythmicity of melatonin during both photoperiods (Prusik et al ., 2015), and this may be the reason for the failure of melatonin together with fasting to induce hypothermia on CT of Rouen ducks during the dark phase. This resilience ability of the Rouen ducks to withstand fasting without hypothermia suggests that the ducks remained vigilant and alert during starvation, which is a good adaptive mechanism against predators. Nocturnal hypothermia has been shown to decrease vigilance and alertness making the birds’ easy prey (Welton et al ., 2002).
The decrease in bodyweight observed only from day four of fasting differed from several findings in birds and mammals where fasting progressively decreased bodyweight of the animals from day one (Fahlman et al ., 2005; Ben-Hamo et al ., 2010; McCue, 2010).The present result showed that ducks probably have an efficient/different metabolic process as compared to other birds.
CONCLUSION
Fasting Rouen ducks for four days failed to induce hypothermia. The Rouen ducks showed greater starvation resilience, apparently due to a “hidden” component of fasting resistance or regional heterothermy. Further studies are required to elucidate the mechanism by which Rouen ducks show resistance to fasting.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interests.
ACKNOWLEDGMENT
The authors are grateful to Mr. Abraham Mathias, Laboratory Technologist of the College of Agriculture and Animal Science, Ahmadu Bello University, Kaduna, Nigeria for his technical assistance.
Список литературы Effects of Food Deprivation and Seasons on Circadian Rhythm in Body Temperature and Bodyweight in Rouen Ducks under Tropical Conditions
- Ayo J.O., Makeri H.K., Minka N.S. and Aluwong T. (2017) Circadian rhythms of biomarkers of oxidative stress and their characteristics in broiler chickens reared under natural light/dark cycle. Biol. Rhythm Res., 49(1), 119-127.
- Ayo J.O., Oladele S.B., Fayomi A., Jumbo S.D., Hambolu J. (1998) Body temperature, respiration and heart rate in the Red Sokoto goat during the harmattan season. Bull. Anim. Hlth. Prod. Afri., 46, 161–166
- Ben-Hamo M., Pinshow B., McCue M.D., McWilliams S.R. and Bauchinger U. (2010) Fasting triggers hypothermia and ambient temperature modulates its depth in Japanese quail Coturnix japonica. Comp. Biochem. Physiol., 156, 84-91.
- Canadian Council on Animal Care (CCAC). (1991) Guidelines on: choosing an appropriate endpoint in experiments using animals for research, teaching and testing. Ottawa ON: CCAC
- Donkoh A. (1989) Ambient temperature: a factor affecting performance and physiological response of broiler chickens. Int. J. Biometeorol., 33, 259–265.
- Fahlma A., Schmidt A., Handrich Y., Woakes A.Y. and Butler P.J. (2005) Metabolism and thermoregulation during fasting in king penguins, Aptenodytes patagonicus, in air and water. Am. J. Physiol. Regul. Integr. Comp. Physiol., 289, R670–R679.
- Giannetto C. and Piccione G. (2009) Daily rhythms of 25 physiological variables in Bos Taurus maintainedunder natural conditions. J. Appl. Biomed., 7, 55–61.
- Guidelines Duck Welfare Recommendations. (2018) Department for Environment Food and Rural Affairs. UK. https://www.gov.uk
- Hohtola E. (2012) Thermoregulatory adaptations to starvation in birds. In McCue, M.D. (ed.), Comparative physiology of fasting, starvation, and food limitation. Springer, Berlin, Germany, pp. 155–170.
- Hohtola E., Hissa R., Pyörnilä A., Rintamäki H. and Saarela S. (1991) Nocturnal hypothermia in fasting Japanese quail: The effect of ambient temperature. Physiol. Beh., 49, 563-567.
- Igono M.O., Molokwu E.C.I., Aliu Y.O. (1982) Body temperature responses of Savanna Brown goat to the harmattan and hot-dry season. Int. J. Biometeorol., 26, 225–230
- Laurila M., Pilto T. and Hohtola, E. (2005) Testing the flexibility of fasting-induced hypometabolism in birds: effect of photoperiod and repeated food deprivations. J. Therm. Biol., 30, 131–138.
- Longo V.D. and Mattson M.P. (2014) Fasting: Molecular Mechanisms and Clinical Application. a review. Cell Metabol., 19, 181-192.
- Martineaud J.P., Cisse F. and Samb A. (2000) Circadian variability of temperature in fasting subjects. Scripta Med. (Brno)., 73(1), 15–24.
- McCue M.D. (2010) Starvation physiology: reviewing the different strategies animals use to survive a common challenge. Comp. Biochem. Physiol. A Mol. Integr. Physiol., 156, 1–18.
- McKechnie AE. and Lovegrove B.G. (2002) Avian facultative hypothermic responses: a review. Condor., 104, 705–24.
- McKechnie A.E. and Mzilikazi N. (2011) Heterothermy in Afrotropical mammals and birds: a review. Integr. Comp. Biol., 51(3), 349–363.
- Minka N.S. and Ayo J.O. (2014) Influence of cold–dry (harmattan) season on colonic temperature and the development of pulmonary hypertension in broiler chickens, and the modulating effect of ascorbic acid. Open Access Anim. Physiol., 6, 1–11.
- Minka N.S. and Ayo J.O. (2016) Daily rhythms of colonic temperature and circulating blood enzymes, urea and calcium in Japanese quail (Coturnix coturnix japonica) under natural cold-dry (harmattan) and hot-dry conditions. Biol. Rhythm. Res., 48, 23-34.
- Mrosovsky N. (1999) Masking: history, definitions, and measurement. Chrobiol. Int., 16, 415-429
- Nagashima K., Tokizawa K., Marui S. and Uchida Y. (2018) Circadian Body Temperature Rhythm and the Interaction with Energy State in Homeostasis -An Integrated Vision. (IntechOpen. Japan). 10-28.
- Odekunle T.O. (2004) Rainfall and the length of the growing season in Nigeria. Int. J. Climatol., 24(4), 467-479.
- OECD. (2000) Guidance Document: Recognition, Assessment and Use of Clinical Signs as Humane Endpoints for Experimental Animals Used in Safety Evaluation OECD Environmental Health and Safety Publications Series on Testing and Assessment. No. 19. pp. 32-39.
- Oismenu C., Authier G. and Arochelle J. (1992) Physiology of prolonged fasting in greater snow geese (Chen caerulescens a tlantica). The Auk., 109(3), 511-521
- Oladele A.O. (2007) Harmattan haze and environmental health. Afr. J. Environ. Sci. Technol., 1, 1–3.
- Piccione G. and Caola G. (2002) Biological rhythm in livestock. J. Vet. Sci., 3, 145-157.
- Piccione G., Gianesella M., Morgante M. and Refinetti R. (2013) Daily rhythmicity of core and surface temperatures of sheep kept under thermoneutrality or in the cold. Res. Vet. Sci., 95, 261–265.
- Prusik M., Lewczuk B., Ziółkowska N. and Przybylska-Gornowicz B. (2015) Regulation of melatonin secretion in the pineal organ of the domestic duck – an in vitro study. Pol. J. Vet. Sci., 18(3), 635–644.
- Refinetti R., Cornélissen G. and Halberg F. (2007) Procedures for numerical analysis of circadian rhythms. Biol. Rhythm Res., 38, 275–325.
- Schleucher E. (2004). Torpor in birds: taxonomy, energetics, and ecology. Physiol. Biochem. Zool., 77, 942–949
- Tattersall G.J., Roussel D., Voituron Y. and Teulier L. (2016) Novel energy-saving strategies to multiple stressors in birds: the ultradian regulation of body temperature. Proc. R. Soc. B., 283, 20161551.
- The Humane Society of the United States. (2008) Welfare of Animals in the Duck Industry: Impacts on farm animals 23. An HSUS Report. Washington, DC. USA. https://animalstudyrepository.org/hsus-epsimpacts-on-animals/23
- Underwood H. and Edmonds K. (1995) The circadian rhythm of thermoregulation in Japanese quail. II. Multioscillator control. J. Biological. Rhythms., 10(3), 23.
- Welton N.J., Houston A.I., Ekman J. and McNamara J.M. (2002) A dynamic model of hypothermia as an adaptive response by small birds to winter conditions. Acta Biotheor., 50, 39–56.
- Yoda T., Crawshaw L., Yoshida K. and Su L. (2000) Effects of food deprivation on daily changes in body temperature and behavioral thermoregulation in rats. Am. J. Physiol., 278, R134-139.
- Zeman M., Buyse J., Herichova I. and Decuypere E. (2001) Melatonin Decreases Heat Production in Female Broiler Chickens. Acta Vet. Brno., 70, 15-18.


