Effects of Ni2+ toxicity on hill reaction and membrane functionality in maize
Автор: Ghasemi Fatemeh, Heidari Reza, Jameii Rashid, Purakbar Latifeh
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 4 т.8, 2012 года.
Бесплатный доступ
Soil contamination with heavy metals has become a worldwide problem, leading to losses in agricultural yield and hazardous health effects as they enter the food chain. Nickel as an essential trace element, affect a number of biochemical and physiological processes in plants in toxic levels. The most common symptoms are chlorosis, and inhibited photosynthesis and respiration. Zea mays seeds were germinated and cultured on nutrient solution with nickel concentrations of 50-200 μmol for a period of two weeks. Studied physiological makers included photosynthetic pigments content, the rate of Hill reaction, K+ efflux and carbohydrate leakage from the roots to the external solution and cell death as a Ni-induced membrane damage. By increasing Ni concentration up to 100 μmol, the content of chlorophyll a increased, but decreased at 200 μmol Ni. No significant changes in chlorophyll b and carotinoids content observed. The rate of Hill reaction as an ability of chlorophyll a in the reaction center of PSII680 to split water, decreased by increasing Ni concentration. Different concentrations of nickel increased the K+ efflux and sugar leakage from roots to the culture and the cell death of root tips. The present results suggested that the disruption of photosynthesis by Ni cannot be attributed to any single factor and appears to result from its combined effects on chloroplast structure, chlorophyll content and photosynthetic protein complexes and treatment with different levels of nickel may induce structural damage and alterations in membrane properties by generation of reactive oxygen species.
Chlorophyll content, hill reaction, k+ efflux, nickel toxicity, zea mays
Короткий адрес: https://sciup.org/14323698
IDR: 14323698
Текст научной статьи Effects of Ni2+ toxicity on hill reaction and membrane functionality in maize
The plasma membrane (PM) of root cells constitutes the first barrier for the entry of heavy metals but also a target of their toxic action (Llamas et al. 2008). Impairment of nutrient balance may result from metal-induced disorders in the functionality of cell membranes. Thus, Ni2+ affected the lipid composition of the plasma membrane (PM) and the PM-H+-ATPase activity in Oryza sativa shoots. Moreover, Gonnelli et al . (2001) reported that malondialdehyde (MDA) concentrations, a marker of lipid peroxidation, increased in shoots of plants from Ni2+-sensitive but not from tolerant populations of Silene . All these changes might disturb membrane functionality, and therefore the ion balance in the cytoplasm, particularly of K+, the most mobile ion across plant cell membranes.
The purpose of the present study is to contribute to a better understanding of the biochemical and physiological responses of plants subjected to nickel stress. Little information is available in the literature on the relationship between Ni-toxicity, Hill reaction and membrane permeability. Therefore, our work focuses in the study of the effects of different concentration of nickel on Hill reaction, photosynthetic pigment's content and plasma membrane as a target of Ni2+ effects.
MATERIALS AND METHODS
Maize ( Zea mays cv. 704) seeds after sterilization were germinated in Petri dishes (20 cm) containing distilled water at 27˚c under dark condition. Individual seedlings 3 days following germination, were transferred to 600-ml beakers containing 500 ml of aerated Hoagland’s solution with different concentration of nickel chloride (0, 50, 100 and 200 μmol) The experiments were arranged in a completely randomized design with three replicates and each replicate contained four seedlings. 15 days after treatment, fresh leaves of seedlings were harvested for determination of chlorophyll content and Hill reaction.
The content of chlorophyll a and b was determined by the method described by Lichtenthaler and Wellburn (1983). Chlorophyll was extracted in 100% acetone. Absorbance was measured at 663 and 645 nm. The chlorophyll and carotinoid contents were calculated from the Lichtenthaler and Wellburn (1983) formulae.
The Hill reaction was measured according to Bregman (1990). One gram of fresh leaves were homogenized using a chilled pestle and mortar in 3 ml phosphate buffer (pH 7) and then centrifuged at 12 000 rpm for 2 min. The pellet thoroughly was resuspended with a pasture pipet. The reaction mixture contained 0.5 ml of the chloroplast suspension, 2ml phosphate buffer and 0.2 ml 2,6-dichlorophenolindolphenol. Absorbance readings were taken at 1-min intervals of illumination for 15 minutes at 550 nm.
Potassium efflux from samples was measured according to Llamas et al. (2008) method. The roots of 3 day seedlings were thoroughly washed twice with cold 0.2 mmol CaSO 4 to eliminate the nutrient solution from the apoplast, blotted on filter paper and submerged in 15 ml of 0, 50, 100 and 200 μmol
Ni2+. They were then incubated for 2 days at 25 °C. K+ efflux from the roots to the external solution was measured by flame photometry.
The carbohydrate leakage from the roots was measured according to Costa and Spitz (1997). The reaction mixture contained 2ml of the external solution, 1 ml phenol 5% and 3 ml sulphuric acid 98%. After 60 min, absorbance was measured at 485 nm.
To determine the cell death, after treating the seedlings with 0, 50, 100 and 200 μmol Ni2+, 1 cm of three root tips of each replicates were put in Evans blue 0.025% for 30 min, then washed with water for 15 min and homogenized in 1 ml methanol 50% and SDS 1%. The extracts were then incubated for 15 min in a water bath at 50 °C and centrifuged for 15 min at 14000 rpm. Absorbance was measured at 600 nm (Baker and Mock, 1994).
The results of the particular determinations were statistically analyzed by ANOVA programme, Statistica 5.0. Data are reported as the mean ± SD. Three independent experiments for each condition were performed. Statistical significance was considered to be significant when the P value was less than 0.05.
RESULTS
According to figure 1, by increasing Ni concentration up to 100 μmol, the content of chl a increased, but decreased at 200 μmol Ni. No significant changes in chl b and carotinoids content were observed. Ni is now considered as an essential mineral nutrient (in this study up to 100 μmol, fig.1), but excess Ni in the medium alters various physiological processes, resulting in detrimental effects on plants and causing diverse toxicity symptoms(Chen et al. 2009).
Ouzounidou et al. (2006) reported that long- term exposure of wheat plants to 1 mmol Ni2+ reduces Fe contents and results in Fe and Mg deficiency symptoms. The chlorophyll content decrease in the presence of nickel could be caused both by intensified chlorophyllase action in the plants treated with the metal and sensitivity of other enzymes of porphyrins synthesis pathway as well as by synthesis inhibition of delta-aminolevulinic acid dehydratase – an enzyme participating in synthesis of assimilation dyes (Llamas et al. 2008). It can also result from decreased action of ironporphyrin enzymes – catalase and peroxydase, or from decreased availability of iron needed for chlorophyll synthesis (Pandey and Sharma, 2002). Nickel can displace manganese ion that is coordinated to the nitrogen (N) atoms of the tetrapyrolles of the chlorophyll molecule. This alteration in chlorophyll structure could lead to a loss of absorbing properties and hence the green coloration (Gajewska et al. 2007, Pandey and Sharma, 2002). It is worthy of note that this decline in chlorophyll content may account for the stunted growth. Therefore, yellowing of the leaves is an indication that the chlorophyll content is low. The influence of Ni on photosynthesis is pervasive, occurring both in isolated chloroplasts and whole plants (Molas, 2002, Chen et al. 2009).
reaction) were depleted following treatment with 1 mmol Ni (Boisvert et al. 2007). Taking these studies together, the disruption of photosynthesis by Ni cannot be attributed to any single factor and appears to result from its combined effects on chloroplast structure, chlorophyll content and photosynthetic protein complexes.
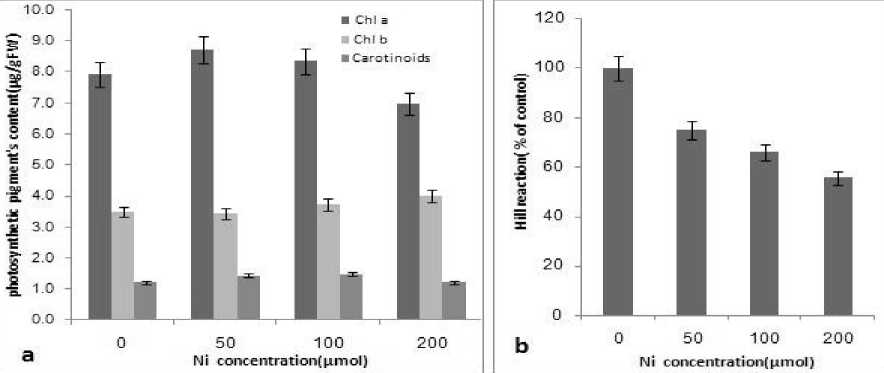
Figure 1: Mean weight of fishes per week reared at the different photoperiods (P<0.05).
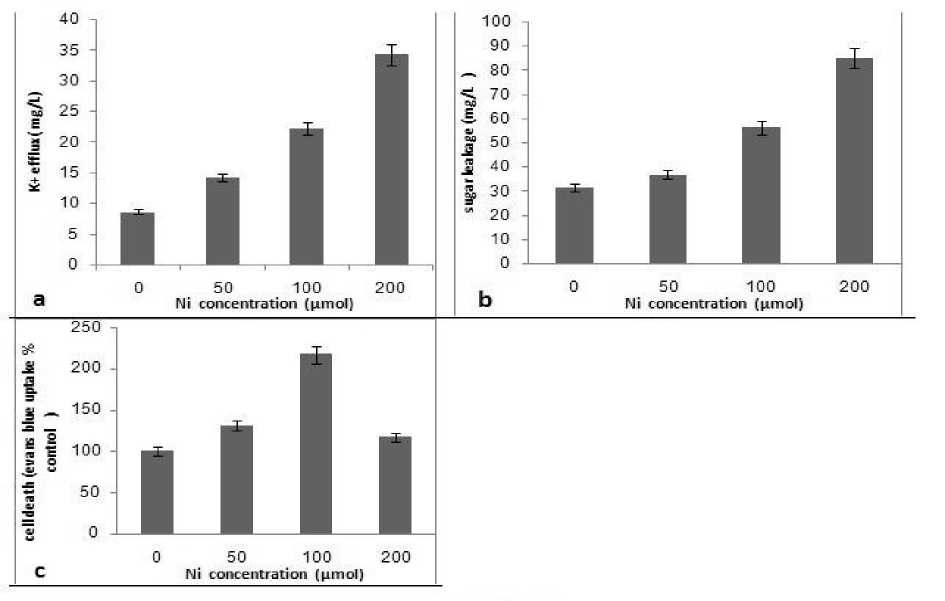
Figure 2: Effects of different concentrations of nickel on K+ efflux (a), sugar leakage (b) of the roots to the culture and root tip cells death (c) of Zea mays seedlings as root membrane functionality. Data points and error bars represent means ± SD.
The plasma membrane (PM) is the first site of contact with plant cells of heavy metals accumulated in the soil. It is not only a selective barrier which controls their uptake, but also a target of their toxic action. To study the effects of Ni toxicity on membrane functionality, the K+ efflux and sugar leakage of the roots to the culture and root tip cells death were measured as Ni-induced membrane damage. K+ efflux from the roots of Zea mays to the culture increased by increasing Ni concentration (fig. 2).
Ni has been shown to increase membrane permeability in rice and maize roots (Pavlovkin et al. 2006). Gabbrielli et al. (1999) showed that toxic concentrations of Ni2+ lowered K+ contents in shoots and roots of pea plants and since they did not cause an increase in K+ efflux, they suggested the effect could be due to a decrease in K+ uptake. They could be due to an inhibition of the PM-H+-ATPase or to a competition with other divalent cations, such as Ca2+, Mg2+ or Fe2+ in their transport across the membrane. In both cases, nutrient imbalance would result, and this would impair plant growth, as has been reported for various plant species (Ouzounidou et al. 2006). In this study, the cell death of root tips increased by increasing Ni concentration (fig. 2). Some authors reported that changes of the membrane proteins or lipidic composition caused by heavy metals induce structural damage and alterations in membrane properties (Llamas et al. 2008). Different concentrations of nickel had a significant effect on sugar leakage from roots of Zea mays seedlings to the culture (fig. 2). The observed results with respect to the high level of Ni may be due to its role on the structural damage and alterations in membrane properties (Llamas et al. 2008). Studies revealed that the resultant effect of nickel on plants arise from their ability to offset the balance between the production and quenching of reactive oxygen species as superoxide radical, singlet oxygen and peroxide is disturbed. The generation of HO-radicals may cause extensive damages of membranes by peroxidation of their constituent lipids (Eriyamremu and Lolodi, 2010). Oxidative stress can seriously disrupt normal metabolism through oxidative damage to lipids, protein and nucleic acids. This leads to change in selective permeability of bio-membranes and thereby membrane leakage and change in the activity of enzymes bound to membrane occurs (Boominathan and Doran 2002).
ACKNOWLEDGEMENTS
We gratefully acknowledge the contribution and enthusiasm of our coworkers in the present studies.
REFRENCES
Aravind, P. and Prasad, M.N.V. (2004) Zn protects chloroplasts and associated photochemical functions in cadmium exposed Ceratophyllum demersum L., a freshwater macrophyte. Plant Sci. , 166 : 1321 – 1327.
Baker, C.J. and Mock, N.M. (1994) An improved method for monitoring cell death in a cell suspension and leaf disc using Evans blue. The Plant Cell , 39 : 7-12.
Boisvert, S. (2007) Inhibition of the oxygen-evolving complex of photosystem II and depletion of extrinsic polypeptides by nickel. BioMetals, 20 : 879 – 889.
Boominathan, R. and Doran, P.M. (2002) Ni-induced oxidative stress in roots of the Ni hyperaccumulator Alyssum bertolonii . New Phytol. , 156 : 205–215.
Chen, C., Huang, D. and Liu, J. (2009) Functions and toxicity of nickel in plants: recent advances and future prospects. CLEAN - Soil, Air, Water, 37 : 304–313.
Costa, G. and Spitz E. (1997) Influence of cadmium on soluble carbohydrates, free amino acids, protein content of in vitro cultured Lupinus albus . Plant Sci. , 128 : 131.
Eriyamremu, G.E. and Lolodi, O. (2010) Alterations in lipid peroxidation and some antioxidant enzymes in germinating beans ( Vigna unguiculata ) and maize ( Zea mays ) exposed to nickel. Inter. J. Bot. , 6 : 170-175.
Gabbrielli, R., Pandolfini, T., Espen, L. and Palandri, M.R. (1999) Growth, peroxidase activity and cytological modifications in Pisum sativum seedlings exposed to Ni2+ toxicity. J. Plant Physiol. , 155 : 639–645.
Gajewska, E. and Skłodowska, M. (2007) Effect of nickel on ROS content and antioxidative enzyme activities in wheat leaves. Biometals , 20 : 27–36.
Gonnelli, C., Galardi, F. and Gabbrielli, R. (2001) Nickel and copper tolerance in three Tuscan populations of Silene paradoxa , Physiol. Plantarum , 113 : 507–514
Léon, V., Rabier, J., Notonier, R., Barthelémy, R., Moreau, X., Bouraïma-Madjèbi, S., Viano, J. and Pineau, R. (2005) Effects of three nickel salts on germinating seeds of Grevillea exul var. rubiginosa, an endemic serpentine proteaceae. Annu. Bot. , 95 : 609–618.
Lichtenthaler, H.K. and Wellburn, A. (1983)
Determination of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. , 603 : 591–592.
Llamas, A., Ullrich, C.I. and Sanz, A. (2008) Ni2+ toxicity in rice: Effect on membrane functionality and plant water content. Plant Physiol. Biochem. , 46 : 905-910.
Madhava Rao, K. V. and Sresty, T. V. (2000) Antioxidative parameters in the seedlings of pigeonpea ( Cajanus cajan (L.) Millspaugh ) in response to Zn and Ni stresses. Plant Sci. , 157 : 113 – 128.
Molas, J. (2002) Changes of chloroplast ultrastructure and total chlorophyll concentration in cabbage leaves caused by excess of organic Ni (II) complexes. Environ. Exp. Bot. , 47 : 115.
Ouzounidou, G., Moustakas, M., Symeonidis, L. and Karataglis S. (2006) Response of wheat seedlings to Ni stress: effects of supplemental calcium. Arch. Environ. Contam. Toxicol., 50 : 346–352.
Pandey, N. and Sharma, C. (2002) Effect of heavy metals Co2+, Ni2+ and Cd2+ on growth and metabolism of cabbage. Plant Sci., 163 : 753– 758.
Pavlovkin, J., Luxová, M., Mistríkova, I. and Mistrík, I. (2006) Short and long-term effects of cadmium on transmembrane electric potential (Em) in maize roots. Biol. Plant., 61 : 109–114.
Seregin, I.V. and Kozhevnikova, A.D. (2006) Physiological role of nickel and its toxic effects on higher plants. Russ. J. Plant Physiol. , 53 : 257–277.
Список литературы Effects of Ni2+ toxicity on hill reaction and membrane functionality in maize
- Aravind, P. and Prasad, M.N.V. (2004) Zn protects chloroplasts and associated photochemical functions in cadmium exposed Ceratophyllum demersum L., a freshwater macrophyte. Plant Sci., 166: 1321 -1327.
- Baker, C.J. and Mock, N.M. (1994) An improved method for monitoring cell death in a cell suspension and leaf disc using Evans blue. The Plant Cell, 39: 7-12.
- Boisvert, S. (2007) Inhibition of the oxygen-evolving complex of photosystem II and depletion of extrinsic polypeptides by nickel. BioMetals, 20: 879 -889.
- Boominathan, R. and Doran, P.M. (2002) Ni-induced oxidative stress in roots of the Ni hyperaccumulator Alyssum bertolonii. New Phytol., 156: 205-215.
- Bregman, A. (1990) Laboratory Investigations in Cell and Molecular Biology. Third Edition, John Wiley & Sons, New York.
- Chen, C., Huang, D. and Liu, J. (2009) Functions and toxicity of nickel in plants: recent advances and future prospects. CLEAN -Soil, Air, Water, 37: 304-313.
- Costa, G. and Spitz E. (1997) Influence of cadmium on soluble carbohydrates, free amino acids, protein content of in vitro cultured Lupinus albus. Plant Sci., 128: 131.
- Eriyamremu, G.E. and Lolodi, O. (2010) Alterations in lipid peroxidation and some antioxidant enzymes in germinating beans (Vigna unguiculata) and maize (Zea mays) exposed to nickel. Inter. J. Bot., 6: 170-175.
- Gabbrielli, R., Pandolfini, T., Espen, L. and Palandri, M.R. (1999) Growth, peroxidase activity and cytological modifications in Pisum sativum seedlings exposed to Ni2+ toxicity. J. Plant Physiol., 155: 639-645.
- Gajewska, E. and Skłodowska, M. (2007) Effect of nickel on ROS content and antioxidative enzyme activities in wheat leaves. Biometals, 20: 27-36.
- Gonnelli, C., Galardi, F. and Gabbrielli, R. (2001) Nickel and copper tolerance in three Tuscan populations of Silene paradoxa, Physiol. Plantarum, 113: 507-514
- Léon, V., Rabier, J., Notonier, R., Barthelémy, R., Moreau, X., Bouraïma-Madjèbi, S., Viano, J. and Pineau, R. (2005) Effects of three nickel salts on germinating seeds of Grevillea exul var. rubiginosa, an endemic serpentine proteaceae. Annu. Bot., 95: 609-618.
- Lichtenthaler, H.K. and Wellburn, A. (1983) Determination of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans., 603: 591-592.
- Llamas, A., Ullrich, C.I. and Sanz, A. (2008) Ni2+ toxicity in rice: Effect on membrane functionality and plant water content. Plant Physiol. Biochem., 46: 905-910.
- Madhava Rao, K. V. and Sresty, T. V. (2000) Antioxidative parameters in the seedlings of pigeonpea (Cajanus cajan (L.) Millspaugh) in response to Zn and Ni stresses. Plant Sci., 157: 113 -128.
- Molas, J. (2002) Changes of chloroplast ultrastructure and total chlorophyll concentration in cabbage leaves caused by excess of organic Ni (II) complexes. Environ. Exp. Bot., 47: 115.
- Ouzounidou, G., Moustakas, M., Symeonidis, L. and Karataglis S. (2006) Response of wheat seedlings to Ni stress: effects of supplemental calcium. Arch. Environ. Contam. Toxicol., 50: 346-352.
- Pandey, N. and Sharma, C. (2002) Effect of heavy metals Co2+, Ni2+ and Cd2+ on growth and metabolism of cabbage. Plant Sci., 163: 753-758.
- Pavlovkin, J., Luxová, M., Mistríkova, I. and Mistrík, I. (2006) Short and long-term effects of cadmium on transmembrane electric potential (Em) in maize roots. Biol. Plant., 61: 109-114.
- Seregin, I.V. and Kozhevnikova, A.D. (2006) Physiological role of nickel and its toxic effects on higher plants. Russ. J. Plant Physiol., 53: 257-277.


