Effects of Pseudomonas putida and Vitazyme® on growth and development of the potato tuber moth
Автор: Idris I., Adam A., Hashem A., Saour G.
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 4 т.19, 2023 года.
Бесплатный доступ
The ability of Pseudomonas putida strain BTP1 and the biostimulant (Vitazyme®) to protect potato plants from the potato tuber moth Phthorimaea operculella (PTM) (Zeller) was investigated. Significant negative effects on survival, pupal weight, and fertility of the insect were observed between treatments and control. The results revealed that the BTP1-treated foliage had significantly the highest negative impact on PTM development and reproduction compared to other treatments. The combination of BTP1 and Vitazym® did not result in a synergistic detrimental effect on potato tuber moth reproduction. However, the biostimulant and BTP1 treatments showed the largest negative effects on PTM reproduction due to the density of hairs and trichomes on the treated foliage. Application of BTP1 and Vitazyme® could be a potential tool to reduce the use of insecticides and enhance integrated pest management against potato tuber moth.
Biostimulant, leaf hairs, plant resistance, potato tuber moth, pseudomonas putida
Короткий адрес: https://sciup.org/143180991
IDR: 143180991
Текст научной статьи Effects of Pseudomonas putida and Vitazyme® on growth and development of the potato tuber moth
The potato tuber moth (PTM) Phthorimaea operculella Zeller (Lepidoptera: Gelechiidae) is a serious insect pest that damages potato crop both in the field and during storage; it is currently present in over 90 countries in Mediterranean basin, tropical and subtropical areas due to climate change (Cho et al., 2021). The larvae feed on the leaves, stems, and petioles of potatoes, making tunnels through the tubers (Clough et al., 2010; Zhang et al., 2021). Chemical insecticides have been used frequently and dominantly to control P. operculella. However, insecticides are still a major health and environmental concern. Additionally, PTM is becoming increasingly resistant to the use of these insecticides. An organic biostimulant developed through biotechnology can improve plants growth rates, nutrient efficiency, or impart tolerance to biotic and abiotic stressors, without having nutritional or insecticidal properties (Dara, 2021; Pereira et al. 2021). There are many compositions of biostimulants, but the main components are humic substances and marine algae extracts (carriers), vitamins (ascorbate, vitamins B, and a-tocopherol), C-hydrolysate, mycorrhizal fungi, and other compounds whose proportions vary from manufactures. Although originally developed for tissue culture applications, they have also shown to be effective in enhancing plant growth by increasing nutrient uptake as well as root development without heavy reliance on fertilizers (Richardson et al., 2004). The increased efficiency of nutrient uptake improves the health and vigor of the plant and increases production of secondary metabolites such as polyphenols (Sivaramakrishnan et al., 1996). Previous studies have demonstrated that mortality of neonate larvae of P. operculella on biostimulant-treated foliage differed significantly than those on untreated foliage (Saour, 2010). On the other hand, a very important additional factor for crop protection is the induction of systemic resistance (ISR) by non-pathogenic rhizobacteria (Racke and Sikora, 1992; Zehnder et al., 1997). According to several studies, plant growth-promoting rhizobacteria (PGPR) strains cause systemic resistance to several insect pests, (Zehnder et al., 1997; Adam et al., 2016; Delgado-Ramírez, 2021). In previous studies, Pseudomonas putida BTP1 influences the development and reproduction of grapevine phylloxera Daktulosphaira vitifoliae (Hemiptera: Phylloxeridae), in addition to potato tuber moth P. operculella in potato plants were demonstrated (Adam et al., 2013, Adam et al., 2016). Therefore, this study aims to determine whether organic biostimulants and P. putida strain BTP1 have negative impacts on P. operculella growth and development under field conditions.
MATERIALS AND METHODS
Insects
The insects used in this experiment were obtained from a laboratory stock culture, which was replenished with field collections of PTM individuals each year. As described by Saour and Makee (1997), larvae were reared in plastic containers (40 x 25 x 10 cm) on wax-coated potato tubers.
Bacterial strain and biostimulant (Vitazyme®) preparation
A strain of Psudomonas putida , BTP1, was originally selected due to its specific properties regarding pyoverdine-mediated iron transport (Jacques et al ., 1995; Ongena et al ., 2002). As described previously by Ongena et al ., 2002 it was maintained and prepared for use in ISR assays. For the bioassays, BTP1 strains were grown on a rotary shaker at 28°C for 24 h in Erlenmeyer flasks (250 ml) containing 100 ml of Casamino Acids medium (CAA). The cells were centrifuged at 16500 g for 15 minutes at 4°C and then rinsed in sterile NaCl (5g l –1). In order to obtain a bacterial suspension of 108 CFU ml-1, the final pellet was resuspended in sufficient sterile distilled water.
Potato tubers were washed in sterile water, dipped separately in a suspension of P. putida strain BTP1 for 30 min, and air-dried, while control tubers were treated with sterile water.
Vitall Earth Resources (Gladewater, Texas, USA) the organic biostimulant (Vitazyme®) were used for this experiment. Among the biological stimulants included in Vitazyme® are sea algae (kelp) and fish soluble solids, natural humic acids, natural chelates, compost percolates, sequestrates, digested multifermented cereal grain extracts, vitamins, and growth regulators. The organic biostimulant-treated rows were sprayed to runoff, with a
0.75% Vitazyme solution diluted in water, directly on the seed pieces at planting. Thirty days after planting, a second application (0.75% Vitazyme solution/row) was performed to the leaves and soil.
Potato planting and design treatments
Seed tubers (commercial Draja cultivar) were planted at a depth of 8-10 cm with a spacing of 25 cm between plants and 70 cm between rows. In this trial, 500 m2 of land was cultivated arranged in eight rows (25 m long) with two rows for each of the treatments (BTP1, Vitazym®, BTP1 with BTP1) and two rows for control, separated by an alley of 1.5 meters to prevent row interspersion. The rows contained 100 potatoes and were fertilized appropriately (120 kg K 2 O, 120 kg P 2 O 5 , 120 kg N / h). Weekly irrigation was provided.
Approximately 800 leaves were excised from potato plants (six to seven weeks old) from the third, fully expanded leaf of similar size. To remove all impurities, the leaves were thoroughly washed with distilled water and shook to remove excess water. Each leaflet was placed individually on filter paper (Grade 1, Whatman, England), moistened with distilled water, and placed in a plastic Petri dish with an ID of 85 mm. As part of each treatment, 200 larvae (aged ≤ 24 h) were placed in 20 plastic boxes (18 x 12 x 8 cm), fed 200 leaves until they reached the pupa stage. Four days after placement, the larvae were gently prodded with a camel-hair brush; if no reaction was evident, the neonate was declared dead. Survivor larvae were carefully placed in transparent plastic boxes (4 x 3 x 2 cm), and fresh leaves were presented every four days, resealed with parafilm to prevent larvae escape.
After 7 days, the survival rate of larvae was calculated based on the number of larvae (aged ≤ 24 hours). Every tow-day-old pupa (31 pupae per treatment) was weighed and placed in a plastic tube. Based on the survival larvae rate, the pupae and adult moths survival rates were estimated, and the mortality rate was calculated. Newly emerged females (0-18 hours) (n = 25) were paired with 1-day-old normal males in 350 ml transparent plastic boxes with a filter paper oviposition site and a 10% sucrose solution as food sources. In the experiment, males and females were kept together until death. Eggs were removed daily, counted, and left to hatch for determining fertility (percentage egg hatch). Light and relative humidity were held at 25°C and 70 %, respectively.
Forty-five days after planting, the number of hairs and trichomes per unit leaf area (1 cm2) on the adaxial leaflet surface for and treatments and control were counted using a binocular microscope at 15x magnification (Kyowa Optical, Japan). Leaflets (n= 40 leaflet/plant) from the third-last fully expanded leaf were randomly picked from potato plants and subjected to count procedure. For each treatment, the experiment was repeated three times.
Statistical analysis
Statistical significance is defined as P < 0.05. Stat View statistics software (version 5.0; SAS Institute, Cary, North Carolina, USA) (Landau et al ., 1999) was utilized to perform all statistics analyses. ANOVA-Tukey HSD test was used to determine the statistical significance of the mean of fertility females, number of hairs trichomes per unit leaf area (1 cm2), and percentage difference between mortality and pupae weight. Schneider-Orelli (Kroschel and Koch, 1996) formula used to calculate PTM mortality parameters was introduced as follows: % Effectiveness = % Mortality - % Mortality in control/100 - Mortality in control, and
% Decrease = ((Control – Treatment) / Control) *100
RESULTS
Table 1 shows that P. operculella larval, pupal and adult mortality rates were highest on P. putida BTP1-treated potato leaves compared to other treatments (df= 4, f = 1064.177; P < 0.0001; df= 4, f = 60.162; P < 0.0001; df= 4, f = 132.195; P < 0.0001). Despite the fact that BTP1, Vitazym® and BTP1+ Vitazym® treatments significantly differ from control and fertilizer treatments, there was a synergistic effect observed in adult s mortality when the BTP1 and Vitazym® treatments were combined.
The pupal weight and female fertility differed significantly between treatments and control across all experiments (df= 4, f = 155.166; P < 0.0001; df= 4, f = 198.158; P < 0.0001) (Table 2). Results showed that BTP1 treatment negatively affected mean pupal weight and female fertility. The mean pupal weight and female fertility decreased by 21% and 19% compared to the control (8.98, 7.08 mg and 99.24, 79.56 eggs, for control and BTP1 treatments, respectively) (Table 2).
Our results show that on the adaxial leaflet surface of the potato s plants treated with BTP1, Vitazym® and BTP1+ Vitazym®, the number of hairs and trichomes density/cm2 on potato leaflet increased and differed significantly from that of control and fertilizer. The highest increase was observed in BTP1 treatments (df= 4, f = 202.704; P < 0.0001) (Fig. 1).
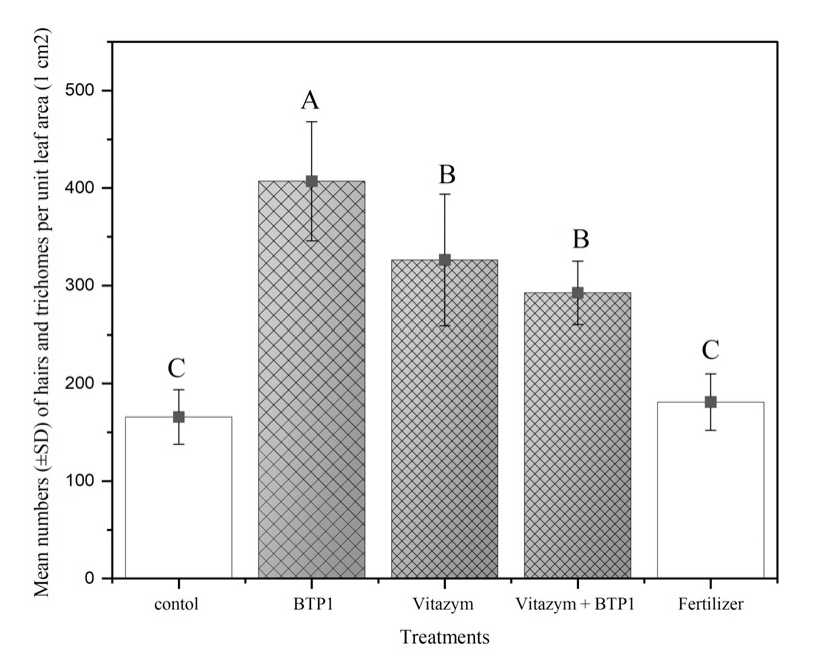
Figure. 1. Mean number (±SD) of hairs and trichomes per unit leaf area (1 cm2) on the adaxial leaflet surface of plant potato sprayed with an organic bio- stimulant (Vitazym®) and P. putida BTP1under field conditions (Means followed by different capital letters are significantly different at P < 0.05 (Tukey HSD test))
Table 1: Mean percentages (±SD) of larval, nymphs, and adults mortality of potato tuber moth fed on foliage of potato plants sprayed with an organic biostimulant (Vitazym®) and P. putida BTP1 under field conditions
|
Mortality % |
control |
BTP1 |
Vitazym® |
Vitazym® + BTP1 |
Fertilizer |
F- value |
P< |
|
Larvae |
19.3±2.7 E |
54.5 ± 1.7 A |
33.5± 2.2 C |
49.9 ± 1.6 B |
23.35 ± 2.23 D |
1064.74 |
0.0001 |
|
Nymph |
30.8±2.9 B |
43.7 ± 3.7 A |
40.5± 3.6 A |
41.7 ± 2.3 A |
33.00 ± 2.7 B |
60.162 |
0.0001 |
|
Adults |
49.3±1.2 D |
63.3 ± 2.4 A |
60.2± 2.6 B |
65.3 ± 2.4 A |
56.5 ± 3.1 C |
132.195 |
0.0001 |
Effectiveness %
|
Mortality % |
BTP1 |
Vitazym® |
Vitazym® + BTP1 |
Fertilizer |
|
Larvae |
35 |
14 |
30 |
4 |
|
Nymph |
13 |
9 |
11 |
2 |
|
Adults |
14 |
10 |
16 |
7 |
Means within rows marked with the same letter are not significantly different at P < 0.05 (Tukey HSD test).
Table 2: Means (±SD) of weight nymph and female fertility of potato tuber moth fed on foliage of potato plants sprayed with an organic bio- stimulant (Vitazym®) and P. putida BTP1 under field conditions
|
Means |
Control |
BTP1 |
Vitazym® |
Vitazym® + BTP1 |
Fertilizer |
F- value |
P< |
|
Nymph weight |
8.98±0.33 A |
7.08±0.32 E |
8.38± 0.23 D |
8.58 ± 0.26 C |
8.7 ± 0.34 B |
155.166 |
0.0001 |
|
Fertility per female |
99.24±3.4 A |
79.56 ± 3.7 E |
84.68± 3.6D |
93.25 ± 4.3 C |
97.23± 3.7 B |
198.958 |
0.0001 |
Decrease %
|
Means |
BTP1 |
Vitazym® |
Vitazym® + BTP1 |
Fertilizer |
|
Nymph weight |
21.16 |
6.68 |
4.45 |
3.12 |
|
Fertility per female |
19.83 |
14.67 |
6.04 |
2.03 |
Means within rows marked with the same letter are not significantly different at P < 0.05 (Tukey HSD test).
DISCUSSION
Plants treated with PGPR may have a decreased level of necessary nutrients, or they may have compounds that inhibit growth (Reese and Field, 1986; Bong and Sikorowski, 1991; Yaman et al .,1999). Our results are consistent with previous studies on whiteflies of tomato Lycopersicon esculantum plants, rice leaf rollers Cnaphalocrocis medinalis in rice, and American bollworms Helicoverpa armigera in cotton (Valenzuela-Soto et al ., 2010; Commare, 2002; Vijayasamundeeswari et al ., 2009).
Although P . putida strain BTP1 and the biostimulant (Vitazym®) have different mechanisms of action, they both affect potato tuber moth development (Saour, 2010; Adam et al ., 2016). Previously, organic biostimulants were reported to improve plant health and vigor by increasing the number, thickness, and diameter of xylem cells (Berlyn and Sivaramakrishnan, 1996). Mostafa et al ., (2021) found that bio-based stimulator compounds (BSTC) significantly reduced the infestation of Liriomyza trifolii on plants by closing the tunnel end, resulting in the larvae s suffocation and death.
PTM neonate larvae mortality confined to biostimulant-treated potato foliage may be due to thicker cell walls (leaf toughness), stronger vascular tissues, and hardness (due to localized amorphous silica), all of which affect the wear and tear of larvae mandibles (Zalucki et al., 2002; Idris et al., 2023). In L2, L3 and L4 larval stages, the treatment of potato tubers with BTP1 causes secondary metabolic changes in treated plant cells which elicit the production of defense compounds, and the accumulation of some toxic phenolic compounds in resistant plant cells leads to an increase in the death rate (Lattanzio et al., 2000; Zehnder et al., 2001; Arimura et al., 2005; Joe and Muthukumaran, 2008).
Several studies have demonstrated that foliar glandular trichomes play a significant role in protecting Solanum species from insect pests through behavioral or physical barriers (Zalucki et al., 2002; Horgan et al., 2007; Peiffer et al., 2009). In one study, removing glandular trichomes from wild potato foliage, Solanum berthaultii Hawkes, resulted in increased larval mobility, more leaf feeding, shorter larval development and larger pupae (Malakar and Tingey, 2000). In addition, factors such as light intensity, temperature, day length, and application of chemical elicitors have been reported to affect the amount of trichomes per unit area in Lycopersicon, Solanum, Ulmus, and Madia species (Duffey, 1986; Simmons et al.,2003; Boughton et al., 2005; Bosu and Wagner, 2014; Gonzáles et al., 2008). Saour (2010) reported that the higher density of hairs and trichomes on the foliage of biostimulant-treated plants is responsible for the pseudoresistance phenomenon. However, P. putida strain BTP1 is able to promote induced systemic resistance (ISR) in a wide spectrum of pathosystems, including potato plants (Adam et al., 2016). Consequently, biostimulant and BTP1 treatments had the largest negative effects on PTM, as their foliage contained more hairs and trichomes. Finally, our results were obtained from excised leaves experiments and further experiments in planta leaves are needed.
CONCLUSION
In this study, P. operculella reproduction on potato leaves treated with four treatments under field conditions was investigated. By applying Vitazym® and BTP1 separately, PTM development decreased, while plant growth and nutrient efficiency were improved. BTP1 induces resistance in potato plants against PTM more effectively than the biostimulant (Vitazym®) and the combination between BTP1 and Vitazym® did not result in a synergistic effect on PTM reproduction.
ACKNOWLEDGEMENT
The authors acknowledge Professor I. Othman, the General Director of Syrian Atomic Energy Commission, Professor N. Mirali head of molecular biology and biotechnology department for their encouragement and support.
CONFLICT OF INTERESTS
The authors declare that they have no potential conflicts of interest.
Список литературы Effects of Pseudomonas putida and Vitazyme® on growth and development of the potato tuber moth
- Adam, A., Idris, I. and Ayyoubi, Z. (2013). In vitro Pseudomonas putida BTP1-induced systemic resistance in grapevine rootstocks against phylloxera (Daktulosphaira vitifoliae). Adv. Hortic. Sci. 27:137142.
- Adam, A., Idris, I., Khalil, N. and Houssian, K. (2016) Arimura, G. I., Kost C. and Boland, W. (2005) Herbivore-induced, indirect plant defences. Biochim. Biophys. Acta, Mol. 1734: 91-111.
- Berlyn, G. P., and Sivaramakrishnan, S. (1996) The use of organic biostimulants to reduce fertilizer use, increase stress resistance, and promote growth, p 106-112 in TD Landis and DB South. National
- Proceedings, Forest and Conservation Nursery Associations. Gen. Tech. Rep. PNW-GTR389, Portland, OR: Department of Agriculture, Forest Service, Pacific Northwest Research Station. National Proceedings: Forest and Conservation Nursery Associations.
- Bong, C. F. J. and Sikorowskip, P. (1991) Effects of cytoplasmic polyhedrosis virus and bacterial contamination on growth and development of the corn earworm, Helicoverpa zea (Lepidoptera: Noctuidae). J. Invertebr. Pathol. 57: 406-412.
- Bosu, P.P. AND Wagner, M.R. (2014). Effects of induced water stress on leaf trichome density and foliar nutrients of three elm (Ulmus) species: implications for resistance to the elm leaf beetle. Environ. Entomol. 36: 595-601.
- Boughton, A. J., Hoover, K. and Felton, G. W. (2005). Methyl jasmonate application induces increased densities of glandular trichomes on tomato, Lycopersicon esculentum. J. Chem. Ecol. 31: 22112216.
- Cho, S. R., Kim, M., Shin, E., Kim, H. K., Koo, H. N. and Kim, G. H. (2021). X-ray Irradiation-Induced Abnormal Development and DNA Damage in Phthorimaea operculella (Lepidoptera: Gelechiidae). Appl. Sci. 11: 5068. https://doi.org/10.3390/app11115068.
- Clough, G. H., Rondon, S. I., Debano, S. J., David, N. and Hamm, P. B. (2010). Reducing tuber damage by potato tuberworm (Lepidoptera: Gelechiidae) with cultural practices and insecticides. J. Econ. Entomol. 103 1306-1311.
- Commare, R. R., Nandakumar, R., Kandan, A., Suresh, S., Bharathi, M., Raguchander, T. and Samiyappan, R. (20020. Pseudomonas fluorescens based bioformulation for the management of sheath blight disease and leaffolder insect in rice. Crop.Prot. 21: 671-677.
- Dara, S. K. (2021). Advances in biostimulants as an integrated pest management tool in horticulture. Published by Burleigh Dodds Science Publishing Limited. http://dx.doi.org/10.19103/AS.2021.0095.03.
- Delgado-ramirez, C. S., Hernandez-martinez, R. and Induced resistance in potato plants by a nonpathogenic Pseudomonas putida BTP1 against potato tuber moth (Phthorimaea operculella Zeller). Adv. Hortic. Sci. 30: 47-52.
- Sepulveda, E. (2021). Rhizobacteria associated with a native Solanaceae promote plant growth and decrease the effects of Fusarium oxysporum in tomato. Agron. 11: 579.
- Duffey, S. S. (1986). Plant glandular trichomes: their partial role in defence against insects. Insects and the plant surface. 151-172.
- Gonzales, W. L., Negritto, M. A., Suarez, L. H. and Gianoli, E. (2008). Induction of glandular and non-glandular trichomes by damage in leaves of Madia sativa under contrasting water regimes. Acta Oecol. 33: 128-132.
- Idris, I., Naddaf, M., Harmalani, H., Alshater, R., Alsafadi, R., (2023). Efficacy of Olive Stones and Corncobs Crystalline Silica Nanoparticles (SiO2, NPs) Treatments on Potato Tuber Moths (Phthorimaea operculella). Silicon. https://doi.org/10.1007/s12633-022-02286-2.
- Jacques, P., Ongena, M. A. R. C., Gwose, I., Seinsche, D., Schröder, H., Delfosse, P., Thonart, P., Taraz, K. and Budzikiewicz, H. (1995). Structure and characterization of isopyoverdin from Pseudomonas putida BTP1 and its relation to the biogenetic pathway leading to pyoverdins. Zeitschrift für Naturforschung C. 50: 622-629.
- Joe, M. M. and Muthukumaran, N. (2008). Role of certain tomato against the leaf caterpillar Spodoptera litura Fab. Notulae Botanicae Horti Agrobotanici Cluj-Napoca., 36:71-75.
- Kroschel, J., Koch, W. and (1996). Studies on the use of chemicals, botanicals and Bacillus thuringiensis in the management of the potato tuber moth in potato stores. Crop. Prot. 15: 197-203.
- StatView for windows, version 5.0. Statistical Methods in Medical Research. 8: 337-341.
- Linsalata, V. (2000). Role of endogenous flavonoids in resistance mechanism of Vigna to aphids. J. Agric. Food Chem. 48: 5316-5320.
- Malakar, R. and Tingey, W. M. (2000). Glandular trichomes of Solanum berthaultii and its hybrids with potato deter oviposition and impair growth of potato tuber moth. Entomol. Exp. Appl.94: 249-257.
- Mostafa, A. A., El-rahman, S. N. A., Shehata, S., Abdallah, N. A. and Omar, H. S. (2021). Assessing the effects of a novel biostimulant to enhance leafminer resistance and plant growth on common bean. Sci. Rep. 11: 1-14.
- Ongena, M., Giger, A., Jacques, P., Dommes, J. and Thonart, P. (2002). Study of bacterial determinants involved in the induction of systemic resistance in bean by Pseudomonas putida BTP1. Eur. J. Plant Pathol. 108: 187-196.
- Peiffer, M., Tooker, J. F., Luthe, D.S. and Felton, G.W. (2009). Plants on early alert: glandular trichomes as sensors for insect herbivores. New. Phytol. 184: 644656.
- Pereira, R. V., Filgueiras, C, C., Doria, J., Penaflor, M. F. G. V. and Willett, D. S. (2021). The effects of biostimulants on induced plant defense. Front. Agron. Vol 3/Article 630596. https.//doi.org/10.3389/fagro.2021630596.
- Racke, J. and Sikora, R.A., (1992). Isolation, formulation and antagonistic activity of rhizobacteria toward the potato cyst nematode Globodera pallida. Soil Biol. Biochem. 24: 521-526.
- Richardson, A. D., Aikens, M., Berlyn, G. P. and Marshall, P. (2004). Drought stress and paper birch (Betula papyrifera) seedlings: effects of an organic biostimulant on plant health and stress tolerance, and detection of stress effects with instrument-based, noninvasive methods. Arboric. Urban. For. 30(1) 52.
- Saour, G. (2010). Organic biostimulant application induces increased densities of leaf hairs and trichomes on potato: implication for susceptibility to potato tuber moth Phthorimaea operculella (Zeller) (Lepidoptera: Gelechiidae). Adv. Hortic. Sci.24:104-108.
- Martin, P. M. (2003). Trichomes of Lycopersicon spp. and their effect on Myzus persicae (Sulzer) (Hemiptera: Aphididae). Aust. J. Entomol. 42: 373378.
- Sivaramakrishnan, S., Berlyn, G. P., Montgomery, M. E. and Ashton, P. M. S. (1996). White oaks, gypsy moths and organic biostimulants: the effect of defoliation and nutrients on plant anatomy and physiology. Bull. Ecol. Soc. Am. 77: 11.
- Valenzuela-soto, J. H., Estrada-hernandez, M. G., Ibarra-laclette, E., Delano-frier, J. P. (2010). Inoculation of tomato plants (Solanum lycopersicum) with growth-promoting Bacillus subtilis retards whitefly Bemisia tabaci development. Planta, 231: 397-410.
- Vijayasamundeeswari, A., Ladhalakshmi, D., Sankaralingam, A. and Samiyappan, R. (2009). Plant growth promoting rhizobacteria of cotton affecting the developmental stages of Helicoverpa armigera. J. Plant Prot. Res. 49(3). DOI: 10.2478/v10045-009-0036-y.
- Yaman, M., Demirbag, Z., and Belduz, A. O. (1999). Investigations on the bacterial flora as a potential biocontrol agent of chestnut weevil, Curculio elephas (Coleoptera: Curculionidae) in Turkey. Biol. 54 : 679683.
- Zalucki, M. P., Clarke, A. R. and Malcolm, S. B. (2002). Ecology and behavior of first instar larval Lepidoptera. Annu. Rev. Entomol. 47: 361-393.
- Zehnder, G., Kloepper, J., Yao, C. and Wei, G. (1997). Induction of systemic resistance in cucumber against cucumber beetles (Coleoptera: Chrysomelidae) by plant growth-promoting rhizobacteria. J. Econ. Entomol. 90: 391-396.
- Zehnder, G. W., Murphy, J. F., Sikora, E. J., Kloepper, J.W. (2001). Application of rhizobacteria for induced resistance. Eur. J. Plant Pathol. 107: 39-50.
- Zhang, M., Yan, J., Ali, A. and GAO, Y., (2021). Different performance of Phthorimaea operculella Zeller (Lepidoptera: Gelechiidae) among four potato tuber varieties under laboratory condition. Insects. 12:580. DOI: 10.3390/insects12070580.


