Effects of water deficit and chitosan spraying on osmotic adjustment and soluble protein of cultivars castor bean (Ricinus communis L.)
Автор: Karimi Sara, Abbaspour Hossein, Sinaki Jafar Masoud, Makarian Hassan
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 3 т.8, 2012 года.
Бесплатный доступ
The present study was aimed investigating the effect of water deficit and chitosan spraying on osmotic adjustment and soluble protein of cultivars castor bean under field condition. experiment was carried out as a split factorial based on randomized complete block design with three replications. The results showed that water deficit caused increase a significant (P
Castor bean, chitosan, proline, soluble protein, total soluble sugars
Короткий адрес: https://sciup.org/14323659
IDR: 14323659
Текст научной статьи Effects of water deficit and chitosan spraying on osmotic adjustment and soluble protein of cultivars castor bean (Ricinus communis L.)
Castor bean (Ricinus communis L.) is an important oilseed crop which produces an oil rich in ricinoleic acid, commonly over 80%This property makes the castor bean a promising candidate for bio fuel production as well as other industrial applications (Li et al., 2010). Among the environmental stresses, drought stress is one of the most adverse factors of plant growth and productivity, More than 70% of Total area of Iran is arid and semi arid (Rafiee, 2011). A decrease in osmotic potential in response to water stress is a well-known mechanism by which many plants can cope with drought conditions, Most organisms increase the cellular concentration of osmotically active compounds, termed "compatible solutes", under desiccation by drought or lowering of osmotic potential Proline and soluble sugar are important solutes for adaptation to low water potential (Saglam et al., 2010). proline is one amongst the most important cytosolutes and accumulates in plants during the adaptation to various types of environmental stress, such as drought, salinity, high temperature, nutrient deficiency, and exposure to heavy metals and high acidity (Nazarli et al., 2011). Free proline and sugar contents significantly increased in Vigna radiata nodules under drought, but nodules had more proline than leaves (Ashraf and Iram, 2005). Osmotic adjustment has been reported also in chickpea under water deficit conditions (Najaphy et al., 2010).
Proteins are compounds of fundamental importance for all functions in the cell, It is well known that alteration of gene expression is always involved in preparing plants for an existence under stress. Protein variation is an essential part of plant response to environmental stress as well as for adaptation to environmental conditions, Under conditions of water deficit (dehydration) numerous processes are modified or impaired, Water stress affects the protein levels of plants but the results of different authors are contradictory. Some authors show decreased protein levels under water stress. (Bakalova et al., 2008).
Chitosan, the deacetylated derivative of chitin, is one of the abundant, renewable, nontoxic and biodegradable carbohydrate polymers, and available largely in the exoskeletons of shellfish and insects, chitosan has been widely applied as a functional biopolymer in food and pharmaceutics (Hanafi, 2012; Cho et al., 2010; Dong et al., 2004; Dai et al., 2009). Chitosan chemical structure, β-1, 4-linked polymer of D-glucosamine, chitosan does not get broken down or digested by human gastrointestinal enzymes. It is the most abundant natural polymer after cellulose (Lamiaa and Barakat, 2011). Chitosan can be made into gelatin, orb, fiber and membrane shapes for various uses, Moreover, because chitosan can be easily obtained and confirms to tissue engineering application requirements; can be implanted into human body and causes no harm; it is a notably suitable material for use in tissue engineering (Hsieh et al., 2007). Chitosan seems to act as an stress tolerance inductor when directly applied to plant tissue, unchaining a hypersensible reaction and lignification, inducing lipid peroxidation, production of defense against pathogens (Ortiz et al., 2007).
MATERIALS AND METHODS
Field studies were conducted during the spring of 2010, Damghan Branch , Iran. The experiment was performed in a split-factorial based on randomized complete block design with three replication. Water treatment, including three levels: control, cut of Irrigation in beginning flowering and seedling stage, caltivars used (Commerical, Ahvaz local, Mashhad local), chitosan spraying in 1 level: (control, Spraying in 5 g/l concentration). Were allocated as main and sub-plots, respectively.
Free proline
Proline was determined following Bates et al., 1973. Fresh plant material (1-0.5 g) was homogenized in 10 ml of 3% sulfosalicylic acid and the homogenate filtered. The filtrate (2ml) was treated with 2ml acid ninhydrin and 2ml of glacial acetic acid, then with 4ml of toluene, Absorbance of the colored solutions was read at 520 nm.
Total soluble sugars
The amino acids were determined in 50 mg of leaf dry matter powder incubated in 5 mL of sterile distilled water at 100 °C for 30 min. After being homogenized, it was centrifuged at 2000 ×g for 5 min at 20 °C and the supernatant was removed. The quantification of the total soluble amino acids was executed at 570 nm according to Peoples et al., 1989, and L-asparagine + L-glutamine (Sigma Chemicals) was used as a standard.
Total soluble proteins
The determination of the total soluble proteins was performed in 100 mg of leaf powder incubated in 5 mL of extraction buffer (Tris-HCl at 25 mM and pH 7.6). The mixture was kept in agitation for 2 h, after wards centrifuged at 2000 g for 10 min. at 20 °C and subsequently the supernatant was removed. The quantification of the total soluble proteins was carried out at 595 nm according to Bradford (1976) with albumin bovine (Sigma Chemicals) used as standard.
Statistical analysis
Data were subjected to analysis of variance (ANOVA), and means were compared using Duncan’s range test at P = 0.05. All calculations were performed in Statistical analysis Version SAS 9.1 (2007) (Mistake, 2009) software for Windows program and Excel software was used for drawing diagrams.
RESULTS
Soluble protein
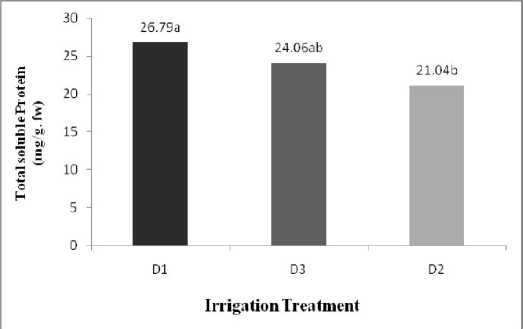
The Result from the ANOVA statistical analysis indicated that Water deficit caused effect significant (P<0.05) in Protein content and the other between treatments in protein content no had a significant ( Table 1 ). The mean comparison shows that maximum amount Protein related to (D1: complete Irrigation, 26.79%) and the minimum amount Protein obtain from (D2: Water deficit in beginning of Flowering stage, 21.04%) ( Table 2 ). Water deficit caused decrease in protein content ( Figure 1 ).
Total Soluble Sugars
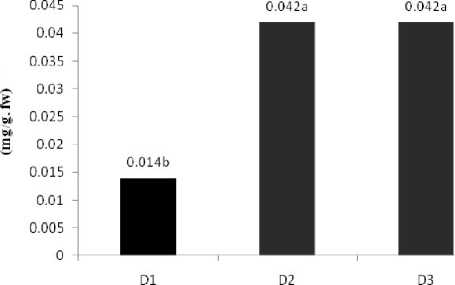
The Result Shown that Water deficit caused increase a significant (P<0.05) in total soluble sugars, also a had cultivars between different a significant (P<0.01) and a had Factorial effect different Between(Water deficit *Cultivar) and (Water deficit *Cultivar*Solution) a significant (P<0.05).( Table1 ).
Table 2 shown that no had different a significant between water deficit levels but rate of Control treatment highest amount. The most amount of sugars obtain of water deficit Levels (D2:Water deficit in beginning of flowering stage, D3: Water deficit in beginning of seedling stage, 0.042%) and minimum amount related to treatment control(D1: complete Irrigation, 0.014%) ( Figure 1 ). Also the mean comparsion shown that a had cultivars between a significant, the most amount related to cultivar (V2: Ahvaz local, 0.047%) and the lost amount related to cultivar (V1: commercial local, 0.017%). ( Table 2 ). Figure 2 shown the most amount carbohydrate related to (D3: Water deficit in beginning of seedling stage, V2: Ahvaz local cultivar, 0.063%) and the lost amount obtain from
(D1: complete Irrigation, V1: commercial cultivar, 0.001%).
Proline content
The results from the ANOVA statistical analysis indicated water deficit caused effect significant (P<0.05) and no had significant other treatments.
( Table 1 ). The mean comparsion indicated between treatment different Irrigation had a significant. maximum proline content related to (D3: water deficit in beginning seedling stage) and minimum proline content related to (D1: complete Irrigation). ( Table 2 ), ( Figure 1 ).
Irrigation Treatment
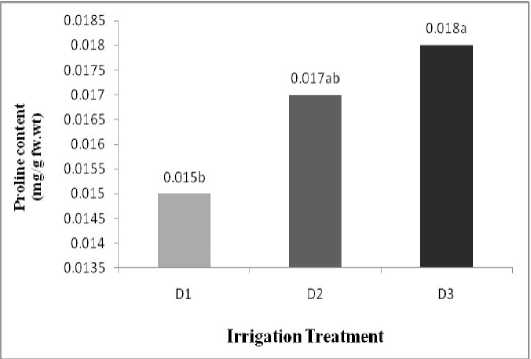
Figure 1: Effect water stress on soluble Protein, Total Soluble carbohydrate, Proline content .Letters on
bars indicate results of Duncan’s multiple range test different letters on the histograms indicate that the means differ significantly (P <0.05).
D1: complete Irrigation, D2:water deficit in beginning seedling stage, D3: water deficit in beginning flowering stage
T able 1: Analysis of variance (Mean Square) water Deficit, cultivars and spraying chitosan on studied traits
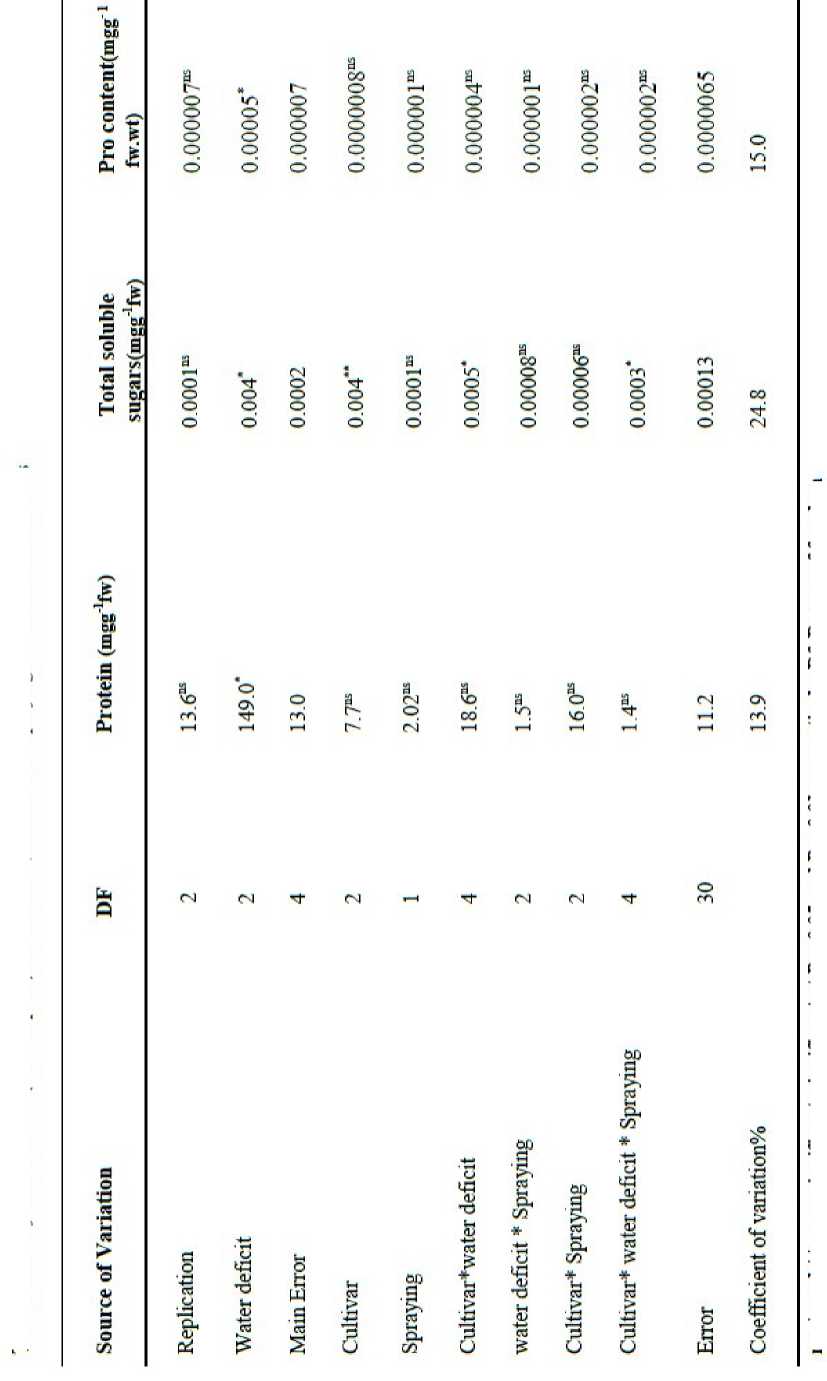
ns, *, and ** , non-significant, significant at P < 0.05 and P < 0.01, respectively. Df. Degree of freedom
Table 2: Effect of various treatments Irrigation, cultivar, Spraying on studied traits
|
Treatments |
Protein (mgg-1fw) |
Soluble Carbohydrate(mgg-1fw) |
Pro (mgg-1fw.wt) |
|
Irrigation |
|||
|
D 1 |
26.79a |
0.014b |
0.015b |
|
D 2 |
21.04b |
0.042a |
0.017ab |
|
D 3 |
24.06ab |
0.042a |
0.018a |
|
Cultivars |
|||
|
V 1 |
23.4a |
0.017c |
0.017a |
|
V 2 |
23.7a |
0.047a |
0.016a |
|
V 3 |
24.7a |
0.034b |
0.017a |
|
Solution |
|||
|
M 1 |
23.7a |
0.031a |
0.016a |
|
M 2 |
24.1a |
0.034a |
0.017a |
|
Factorial Effect |
|||
|
D 1 V 1 |
25.9abc |
0.001g |
0.020a |
|
D 1 V 2 |
28.53a |
0.028de |
0.011a |
|
D 1 V 3 |
25.95abc |
0.014ef |
0.016a |
|
D 2 V 1 |
20.81d |
0.035cd |
0.02a |
|
D 2 V 2 |
20.54d |
0.050b |
0.016a |
|
D 2 V 3 |
21.78 cd |
0.040bc |
0.02a |
|
D 3 V 1 |
23.74bcd |
0.014f |
0.018a |
|
D 3 V 2 |
22.04bcd |
0.063a |
0.021a |
|
D 3 V 3 |
26.40ab |
0.047bc |
0.018a |
Data represent the mean values of three replicates. Within a column, mean values followed by different letters are statistically different based on Duncan’s range test at P = 0.05.
DISCUSSION
Proline content
A common response to water deficit in plants is the accumulation of osmo protectants such as proline (Moradshahi et al., 2004). Proline accumulation is responsible for the hydration of biopolymers surviving as areadily utilizable energy source and serving as a nitrogen source compound during periods of inhibited growth (Kala and Godara, 2011).
A marked increase in proline content in the leaves of could be an indicator of its high drought tolerance (Ashraf and Iram, 2005). Proline accumulation is believed to play adaptive roles in plant stress tolerance (Mafakheri et al., 2011). Also, Din et al., 2011 found that metabolic factors such as free proline contents in leaves increased significantly under sever drought stress. Thus, it appears that increase in proline contents during drought stress induction is an adaptive mechanism in castor oil.
Total Soluble Sugar
Soluble sugars is an important constituent and source of energy for all living organisms, plants manufacture this organic substance during photsyntheis and breakdown during respiration (Seyyednejad and Koochak, 2011). Under water stress condition the breakdown of polysaccharides caused an accumulation of soluble sugars which helpmaintenance of turgor (Nazarly et al., 2011). Under drought conditions, the accumulation of soluble sugars seemed to be associated with drought tolerance in many plant species, soluble sugars also contributed to improving drought tolerance of peas, sugar beets and black poplars (Liu et al., 2011). The accumulation soluble sugars the cell under stress by balancing the osmotic strength of the cytosol with that of the vacuole and the external environment (Abdalla, 2011). In order to tolerate drought stress, plants will accumulate high
167 concentration of low molecular-mass organic solutes such as soluble sugars or other amino acids to regulate the osmotic potential of cells aming at improving absorbtion of water under drought stress (Abbaspour et al., 2011).
Total Soluble Protein
It seems that decrease in soluble protein during drought stress was due to a severe decrease in photosynthesis. Photosynthesis decreased in Drought stress and Material for protein synthesis werent provided, therefore, protein synthesis dramatically reduced or even stopped (Mohammadkhani and Heidari, 2007). The progressive reduction of total soluble proteins during water deficiency in the plants was induced by proteolysis, with the liberated amino acids used during the plant osmotic adjustment, This fact indicates a slow recuperation of this parameter probably because the proteins depend on other nitrogen compounds for synthesis (Costa and LoBato, 2009). our result are in agreement with those of (lqbal and Bano, 2009; Bayramov et al., 2010).
CONCLUSION
Progressive water deficit stress increased concentration of proline and soluble sugars in castor oil leaves. The accumulation of the osmolytes can help the castor bean plant to maintain the cell turgor and the structural integrity of membranes. suggested that lower accumulation of osmolyte function in protecting macromolecules either by protecting the tertiary structure of protein or by scavenging ROS (reactive oxygen species) produced in response to drought, the other soluble sugars content and proline improve stress tolerance by protecting and stabilizing membranesand enzymes during stress conditions. In generally, Osmotic adjustment is a mechanism to maintain water relations and sustains photosynthesis by maintaining leaf water content at reduced water potentials. Damghan regions ingredient arid and semi arid in Iran, also castor bean herb is drought tolerant, the experimental our, cultivars between no had a significant different of proline and protein content. But, cultivars between had a significant different of soluble sugars, the result show that cultivar Ahvaz local the most amount of soluble sugars. therefore suggested that Ahvaz Local cultivar in drought stress condition rate of other cultivar Toleranter, we can be with attention Damghan Climate condition, there Cultivate Ahvaz local cultivar.
Список литературы Effects of water deficit and chitosan spraying on osmotic adjustment and soluble protein of cultivars castor bean (Ricinus communis L.)
- Abbaspour, H., Saeidisar, S. and Afshari, H. (2011) Improving drought tolerance of Pistacia vera L. seedlings by arbuscular mycorrhiza under greenhouse conditions. Journal of Medicinal Plants Research, 5(32), 7065-7072.
- Abdalla, M.M. (2011) Beneficial effects of diatomite on the growth, the biochemical contents and polymorphic DNA in Lupinus albus plants grown under water stress. Agriculture and Biology Journal of North America, 2(2), 207-220.
- Ashraf, M., Iram, A. (2005) Drought stress induced changes in some organic substances in nodules and other plant parts of two potential legumes differing in salt tolerance. Flora, 200, 535-546.
- Bakalova, S., Nedeva, D. and Mckee, J. Protein profiles in wheat seedlings subjected to dehydration stress, APPLIED Ecology and Environmental Research, 6(2), 37-48.
- Bates, L.S., Waldren, R.P., Teare, I.D. (1973) Rapid determination of free proline for water stress studies, Plant soil, 39, 205-207.
- Bayramov, M.S., Babayen, G.H., Khaligzade, N.M., Guliyev, M.N. and Raines, A.C. (2010) Effect of Water Stress on Protein Content of Some Calvin Cycle Enzymes in Different Wheat Genotypes. Proceedings of ANAS (Biological Sciences), 65(5-6), 106-111.
- Bradford, M.M. (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem, 72, 248-254.
- Cho, Y., Shi, R. and Borgens, B.R. (2010) Chitosan produces potent neuroprotection and physiological recovery following traumatic spinal cord injury, The Journal of Experimental Biology, 213, 1513-1520.
- Costa, D.L.C.R., Lobato, S.D.K.A., Silvera, D.G.A.J. and Laughinghousevi, D.H. (2011) ABA-mediated proline synthesis in cowpea leaves exposed to water deficiency and rehydration, Turk. J. Agric. For., 35, 309-317.
- Dai, M., Zheng, X., Xu, X., Kong, Y.X., Guo, G., Luo, F., Zhao, X., Wei, Q.Y. and Qin, Z. (2009) Chitosan-Alginate Sponge: Preparation and Application in Curcumin Delivery for Dermal Wound Healing in Rat. Journal of Biomedicine and Biotechnology, Article ID 595126, 8 pages.
- Din, J., Khan, U., Ali, I., and Gurmani, R.A. (2011) Physiological and agronomic response of Canola varieties to drought stress, The Journal of Animal & Plant Sciences, 21(1), 78-82.
- Dong, Y., Ruan, Y., Wang, H., Zhao, Y. and Bi, D. (2004) Studies on Glass Transition Temperature of Chitosan with Four Techniques, Journal of Applied Polymer Science, 93, 1553-1558.
- Hanafi, N. (2012) Role of Chitosan Nanoparticles in Targeting Ehrlich Tumor Cells Transplanted in Albino Mice, International Journal of Research in Biological Sciences, 2(1), 6-17.
- Hsieh, C.W., Chang, P.C. and Lin, M.S. (2007) Morphology and characterization of 3D micro-porous structured chitosan scaffolds for tissue engineering, Colloids and Surfaces B: Biointerfaces, 57, 250-255.
- Kala, S. and Godara, A.K. (2011) Effect of moisture stress on leaf total proteins, proline and free amino acid content in commercial cultivars of Ziziphus mauritiana. Journal of Scientific Research, 55, 65-69
- Lamiaa, A., Barakat, A. (2011) Hypolipidemic and Antiatherogenic Effects of Dietary Chitosan and Wheatbran in High Fat-High Cholesterol Fed Rats. Australian Journal of Basic and Applied Sciences, 5(10), 30-37.
- Li, G., Wan, Sh., Zhou, J., Yang, Z. and Qin, P. (2010) Leaf chlorophyll fluorescence, hyperspectral reflectance, pigments content, malondialdehyde and proline accumulation responses of castor bean (Ricinus communis L.) seedlings to salt stress levels, Industrial Crops and Products, 31, 13-19.
- Liu, C., Liu, Y., Guo, K., Fan, D., Li, G., Zheng, Y., Yu, L. and Yang, R. (2011) Effect of drought on pigments, osmotic adjustment and antioxidant enzymes in six woody plant species in karst habitats of southwestern China, Environmental and Experimental Botany, 71, 174-183.
- Lqbal, S. and Bano, A. (2009) Water stress induced changes in antioxidant enzymes, membrane stability and seed protein profile of different wheat accessions, African Journal of Biotechnology, 8(23), 6576-6587.
- Mafakheri, A., Siosemardeh, A., Bahramnejad, B., Straik, P.C. and Sohrabi, E. (2010) Effect of drought stress on yield, proline and chlorophyll contents in three chickpea cultivars, AJCS, 4(8), 580-585.
- Mohammadkhani, N. and Heidari, R. (2008) Effects of Drought Stress on Soluble Proteins in two Maize Varieties, Turk. J. Biol., 32, 23-30.
- Moradshahi, A., Eskandari, S.B. and Kholdebarin, B. (2004) Some Physiological Responses of Canola (Brassica napus L.) to Water Deficit Stress Under Laboratory Conditions, Iranian Journal of Science & Technology, Transaction A, 28(A1) 43-50
- Najaphy, A., Khamssi, N.N., Mostafaie, A. and Mirzaee, H. (2010) Effect of progressive water deficit stress on proline accumulation and protein profiles of leaves in chickpea, African Journal of Biotechnology, 9(42), 7033-7036.
- Nazarli, A., Faraji, F. and Zardashti, M.R. (2011) Effect of drought stress and polymer on osmotic adjustment and photosynthetic pigments of sunflower Cercetări Agronomice în Moldova, 44(1) 35-42
- Ortiz, O.H., Mendoza, B.A., Villarreal, M.R., Rodriguez, R.H. and Romenus, A.D.K. (2007) Enzymatic Activity in Tomato Fruits as a Response to Chemical Elicitors. J. Mex. Chem. Soc., 51(3), 141-144.
- Peoples, M.B., Faizah, A.W., Reakasem, B.E. and Herridge, D.F. (1989) Methods for evaluating nitrogen fixation by nodulated legumes in the fi eld. Australian Centre for International Agricultural Research, Canberra.
- Rafiee, M. (2012) Effect of Every Other Furrow Irrigation and Planting Density on Physiological Traits in Corn (Zea mays L.), World Applied Sciences Journal, 17(2), 189-193.
- Saglam, A., Terzi, R., Nar, H., Saruhan, N., Ayaz, A.F. and Kadioglu, A. (2010) Inorganic and Organic solutes In apoplastic and symplastic spaces contribute to osmotic Adjustment during leaf rolling in Ctenanthe setosa. Acta Biologica Cracoviensia Series Botanica, 52(1), 37-44.
- Seyyednejad, M.S. and Koochak, H. (2011) A Study on Air Pollution effects on Eucalyptus camaldulensis. International Conference on Environmental, Biomedical and Biotechnology, IPCBEE vol.16, IACSIT Press, Singapoore.


