Elucidating the mechanism of anti-apoptotic activity of а-crystallin and its therapeutic potential
Автор: Chakraborty Aparajita, De Priyanka, Saha Sudipa
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 1 т.21, 2025 года.
Бесплатный доступ
Α- Crystallins are the structural proteins of the eye lens which possess anti-apoptotic activity. Both αA- and αB- crystallins are distinct antiapoptotic regulators which can interact with Bax and Bcl-XS, proapoptotic members of the Bcl-2 family in order to sequester their translocation into the mitochondria. Thus they may interfere with the mitochondrial apoptotic pathway which triggers Bax pro-apoptotic activity and the downstream activation of effector caspases such as Caspase-9 and Caspase-3. The differential regulation of α- crystallins has been observed in several ocular diseases such as age-related macular degeneration and many others. Crystallins interact with pro-apoptotic Bax and displayed cytoprotection against Bax-triggered apoptosis. αA-crystallin was found to inhibit chemical-induced apoptosis by inhibiting the activation of caspase-3 and caspase-9. Its antiapoptotic activity was found to be directly related to its chaperone activity. On the other hand, αB- crystallin associated with IKK-β activates its kinase activity which in turn, leads to the activation of NF- ĸB; this activation protects myoblasts from tumor necrosis factor-α (TNF-α) - induced cytotoxicity by enhancing the expression of Bcl-2, an anti-apoptotic protein. The anti- apoptotic mechanisms may be exploited for therapeutic purposes in near future.
Α- crystallin, antiapoptotic mechanism, pro-apoptotic members, bax, bcl-2, caspase-3, caspase-9, retinal degenerations, ikkβ, nf- ĸb, therapeutic purposes
Короткий адрес: https://sciup.org/143183776
IDR: 143183776
Текст обзорной статьи Elucidating the mechanism of anti-apoptotic activity of а-crystallin and its therapeutic potential
α - Crystallin belongs to the small heat shock protein (sHSP) family which are antiapoptotic proteins as well (de Jong et al. , 1993). It has been reported in previous studies that cells overexpressing αA- or αB- crystallin are more resistant to osmotic, thermal and oxidative stress (Dasgupta et al. , 1992, Aoyama et al. , 1993, Andley, 2007). At the same time, α- Crystallin is known to prevent apoptosis induced by a variety of agents which include stautosporine, UVA light, etoposide or TNF-α (Mehlen et al. , 1996, Mao et al. , 2004, Andley, 2008). There are various mechanisms by which α-crystallin may function as an antiapoptotic protein. For instance, it may interact with the proapoptotic molecules p53, Bax and Bcl X(S) and thus prevent their translocation to mitochondria during apoptosis ( iu et al. , 2007). During the process of lens fiber cell differentiation in the lens, αA- crystallin suppresses caspase activity which leads to the retention of lens fiber cell integrity following mitochondrial degradation and other organelles (Morozov and Wawrousek, 2006, iu et al. , 2007). On the other hand, αB- crystallin is able to suppress the autocatalytic maturation of procaspase-3 and inhibit cytochrome c release during mitochondria (Kamradt et al. , 2002). Thus the ability of both crystallin subunits to regulate intracellular apoptotic signals is a remarkable feature which may be studied in greater details in future research.
Differences in gene regulation of α- crystallins have been observed in several ocular diseases such as age-related macular degeneration, stress-induced or inherited retinal degenerations. Their altered expression in pathological conditions reflects a possible role in cellular defensive response (Hamann et al. , 2013). An upregulation of αB- crystallins protects against cell death under stress conditions (Nagaraj et al. , 2016). Thus the ability of crystallins to protect cells against the undesirable consequences of cellular stress and protein denaturation highlights their therapeutic role in blocking protein aggregation and apoptosis.
Role of αA- crystallins in apoptosis
α- crystallins are known to possess antiapoptotic function by the regulation of intracellular apoptotic signals. Both αA and αB subunits are known to interfere with the mitochondrial apoptotic pathway by triggering the Bax pro-apoptotic activity and the downstream activation of effector caspases (Hamann et al., 2013). While αB- crystallin is involved in the abrogation of apoptosis via repression of Raf/MEK/ERK signal, αA-crystallin activates the Akt surviving pathway to inhibit apoptosis ( iu et al., 2004, Hamann et al., 2013). It has been demonstrated that αA- crystallin is able to inhibit the activation of two caspase proteins, caspase-3 and caspase-9 as well as prevent the chemically-induced apoptosis and the apoptosis induced by the overexpression of pro-apoptotic Bim and Bax (Mao et al., 2004, Pasupuleti et al., 2010).
Another level of prevention of apoptosis (Bax-independent or dependent) is performed by αA-crystallin. PI3K is responsible for the stimulation of the PI-dependent kinase through PIP 3 which leads to Akt phosphorylation and thus the activity of Akt. The PI3K activity was elevated in R21 mutated cells as compared to Wt cells but how its activity was enhanced by αA-crystallin is yet to be deciphered but data suggested a possibility of PI3K phosphorylation might be a possible cause (Pasupuleti et al. , 2010). It had been observed that whenever PI3K was inhibited either by Y294002 or any dominant-negative mutation then the anti-apoptotic function of αA- crystallin was completely abolished ( iu et al. , 2004, Pashupuleti et al. , 2010). Therefore a certain observation led to the inference of the fact that AKt/PI3K pathway activation was responsible for αA-crystallin’s antiapoptotic function (Fig 1).
Originally αA- crystallin was described as an endogenous neuroprotective factor in retinal neurons which was exhibited in overexpression-related studies in hypoxic stress or optic neuropathies (MacRae, 2000, Nath et al., 2022). Previous studies had revealed that the exogenous administration of αA- crystallin resulted in a protection and rescue of neurons from degeneration associated with metabolic or hypoxic stress (MacRae, 2000, Ying et al., 2014, Nath et al., 2022). It was associated with significantly decreased levels of GFAP in both the retina and the crush site following the third day of optic nerve crush injury and the induction of astrocyte architecture remodelling at the crush site (Piri et al., 2016). Such data demonstrated the protective potential of αA- crystallin (functionally enhanced) recombinant proteins against neurodegeneration.
Role of αB- crystallins in the apoptotic pathway
αB- crystallin, a member of the small heat shock protein family is known for its role in biological functions which includes response to heat shock, differentiation and apoptosis (Adhikari et al., 2011). During differentiation, myoblasts, the precursor cells in muscle regeneration express increased levels of αB- crystallin and TNF-α even though the connecting link between these proteins in cell signalling is not clearly understood. Use of different approaches to induce the expression of αB- crystallin or its functionally compromised mutant R120GαB-crystallin had revealed that an increased expression of wild type αB- crystallin enhances NF-ĸB activity (Ito et al., 2002, aunay et al., 2006, Adhikari et al., 2011). Upon treatment with TNF-α, αB- crystallin associates with IKKβ and increases its kinase activity which in turn leads to phosphorylation and subsequent degradation of IκB-α, which is a negative regulator of NF-κB activity (Dodd et al., 2009, Adhikari et al., 2011). The activation of NF-κB by αB- crystallin enhances the expression levels of Bcl 2 which is an anti apoptotic protein and protects the cells from TNF-α induced cytotoxicity (Fig 1) (Kannan et al., 2012). There are other reported mechanisms by which αB- crystallin prevents apoptosis in cells. An important one is the prevention of caspase-3 maturation and activity and therefore the apoptotic inhibition (Kamradt et al., 2002). A careful scrutiny of mechanisms revealed that αB-crystallin prevented cell death by inhibiting pro-apoptotic molecules such as caspase-9 which is responsible for caspase-3 activation (Kamradt et al., 2002, Adhikari et al., 2011). At the same time, the crystallin protected mitochondrial integrity by preventing the translocation of Bax and Bcl Xs to the mitochondria. A recent study performed had revealed αB- crystallin is able to prevent apoptosis via several mechanisms such as the inhibition of RAS-initiated RAF/MEK/ERK signaling pathway or downstream which blocks the BAX and Bcl-2 translocation from the cytoplasm to the mitochondria (Antinioni et al., 2020, Dimauro and Caporossi, 2022). It interacts with p53 to retain it in the cytoplasm and by the probable mechanism of caspase-3 activation (Fittipaldi et al., 2015). An increased level of phosphorylation of αB crystallin determine its translocation to the myofilaments where it binds various proteins such as titin, desmin,vimentin,nebulette and the inactive precursor of caspase-3 thereby leading to the stabilisation of myofilament and thus the inhibition of apoptosis (Webster, 2003, Dimauro and Caporossi, 2022).
An overview of different chaperones and their role as antiapoptotic agents has been summarised in Table 1.
Table 1. Some molecular Chaperones and their role as anti apoptotic agent
|
Name of Chaperone |
Role in antiapoptosis |
Disease regulated |
Reference |
|
Hsp 90 |
Block cell death upon association with key apoptotic factors |
Cancer |
Xia et al. , 2014,Wang et al. , 2014 |
|
αA- crystallin |
Interaction with caspase-3 and caspase-9 and inhibiting their activation |
Retinal neurodegenerative disorders |
iu et al. , 2004, Pasupuleti et al. , 2010, Piri et al. , 2016, Chakraborty et al. , 2023 |
|
αB- crystallin |
Activation of NF-κB increase levels of Bcl-2, an antiapoptotic protein; Interaction with caspase proteins |
Bacterial endolphthalmities |
Kamradt et al. , 2002, Webster, 2003, Fittipaldi et al. , 2015 |
|
Hsp 70 |
Cytoprotective action; inhibition of TNF-α related apoptosis |
Cancer |
Garrido et al. , 2003,Wang et al. , 2014 |
|
Hsp 27 |
Inactivation of caspase-3 and cytochrome c released from mitochondria |
Cancer |
Acunzo et al. , 2012,Wang et al. , 2014 |
|
Hsp 60 |
Possess Anti apoptotic threshold in tumor cells; cytoprotective action |
Cancer |
Dumont et al. , 2007, Ghosh et al. , 2007 |
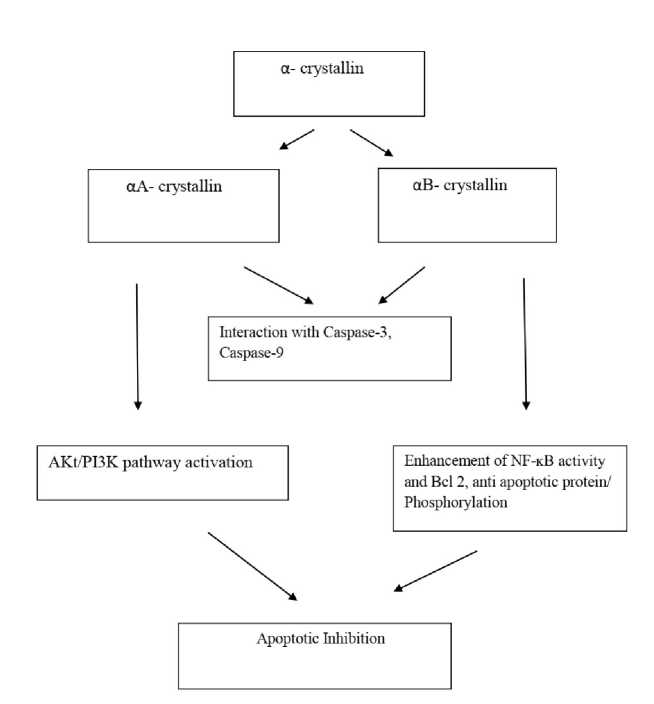
Figure 1 : Subunits of α-crystallin are apoptotic inhibitors. Both share a common mechanism of interaction with caspase factors.
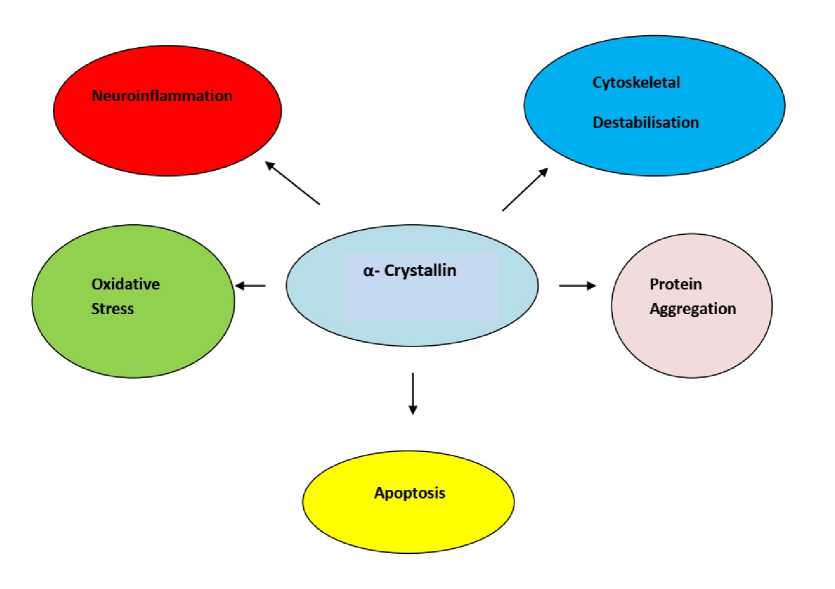
Figure 2: α- crystallin inhibits several processes responsible for causing disease and thus exploited for therapeutic benefits.
Therapeutic potential of α-crystallins as anti apoptotic agents
The ability of α- crystallin to inhibit apoptosis has been exploited for its therapeutic use. For instance, intravitreally injected α- crystallin was found to promote axonal regeneration after optic nerve crush in rats (Fig 2) The direct delivery of α- crystallin to retinal ganglion cells resulted in the upregulation of αA- crystallin in the retina via Toll-like receptor but the absence of αA-led to retinal degeneration (Wang et al. , 2014, Nagaraj et al. , 2016) The absence of αB- crystallin on the other hand, enhanced retinal apoptosis during bacterial endolphthalmities (Whiston et al. , 2008, Chakraborty et al. , 2023). Pasupuleti et al. , 2010 had demonstrated in his previous study about the possible linkage of α-crystallin’s chaperone activity and its antiapoptotic activity. It has other beneficial effects such as the ability to bind to copper and quench reactive oxygen species (ROS) formation. The phosphorylation of αA- or αB-crystallins (serine or threonine residues) had been shown to possess a possible role in the anti-apoptotic activity of the respective crystallins (Sharma and Santhoshkumar, 2009, Phadte et al. , 2021) It was found that cardiomyocytes expressing an alanine substituted triple mutant of αB- crystallin (S19A/S45A/S59A) expressed an increased susceptibility to sorbitol and hypoxia induced apoptosis in comparison to wild type cells (Morrison et al. , 2003, Phadte et al. , 2021) When cultured rat astrocytes were treated with a p38 protein kinase inhibitor SB203580 or the ERK1/2 inhibitor PD98059, two kinases responsible for phosphorylation of αB-crystallin, increased their susceptibility to ceramide and staurosporine induced apoptosis which revealed the role of αB- crystallin phosphorylation in the crystallin’s anti apoptotic activity ( i et al. , 2005, Ruebsam et al. , 2018, Phadte et al. , 2021). Though evidences for role of αA- crystallin were scarce but a recent study determined the key role of phosphorylation in the cytoprotective function of αA- crystallin. Cell culture experiments which were done showed that the expression of the αA-crystallin phosphomimetic T148D resulted in an increased survival rate of R28 cells subjected to metabolic stress (Takemoto et al. , 1996,
Schaeffer et al. , 2003, Ruebsam et al. , 2018) An in vitro assessment of the chaperone activity of the αA-crystallin phosphomimetic T148D revealed a two-fold increase in its activity as compared to the wild-type protein (Nahomi et al. , 2013, Phadte et al. , 2021) These results revealed the possible role of retinal αA- crystallin on its neuroprotective protection. The data overall suggested the possible therapeutic roles of α- crystallins as apoptotic inhibitors.
Concluding remarks
α- Crystallins are better known as molecular chaperones and for their anti apoptotic activities. The interaction of crystallins with pro apoptotic molecules such as p53, Bax and Bcl X(S) and preventing their translocation into mitochondria unleash their tendency to act as apoptotic inhibitors. Both subunits of α- Crystallin possess distinguishable roles as antiapoptotic agents. αA- Crystallin is able to inhibit the activation of two Caspase proteins, Caspase-3 and Caspase-9 while at the same time it may enhance the levels of PI3K activity which in turn is responsible for the anti apoptotic function of αA- crystallin.
αB- Crystallin on the other hand, in found to inhibit apoptosis via the activation of NF-κB, which increases the expression of Bcl 2, an anti apoptotic protein which protects the cells from TNF-induced cytotoxicity. A similar mechanism by which both subunits of α-crystallin can inhibit apoptosis is by interacting with caspase proteins i.e. caspase-9 which is responsible for the activation of Caspase-9. Phosphorylation of αB-Crystallin is responsible for interacting with a variety of proteins such as titin, desmin, vimentin which leads to the stabilisation of myofilaments and thus apoptotic inhibition.
Both subunits of α- Crystallins have been exploited for their role as anti apoptotic agents for therapeutic purposes. The anti apoptotic activity of crystallins seem to hold a possible connection with its chaperone activity. Phosphorylation seems to hold a possible role here because a previous study using phosphomimetic proteins revealed a two fold increase in the chaperone activity of crystallins. The roles of both subunits of α-crystallin can be deciphered owing to the fact that their absence results in an increased retinal degeneration or apoptosis.
The anti apoptotic activity of α-Crystallins thus hold a vital role as disease-inhibting but some studies highlighted its disease causing property as well. These two contradictory functions of the protein must be considered carefully for therapeutic considerations.
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
Список литературы Elucidating the mechanism of anti-apoptotic activity of а-crystallin and its therapeutic potential
- Acunzo, J., Katsogiannou, M., & Rocchi, P. (2012). Small heat shock proteins HSP27 (HspB1), αBcrystallin (HspB5) and HSP22 (HspB8) as regulators of cell death. The international journal of biochemistry & cell biology, 44(10), 1622-1631. https://doi.org/10.1016/j.biocel.2012.04.002
- Adhikari, A. S., Singh, B. N., Rao, K. S., & Rao, C. M. (2011). αB-crystallin, a small heat shock protein, modulates NF-κB activity in a phosphorylationdependent manner and protects muscle myoblasts from TNF-α induced cytotoxicity. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research, 1813(8), 1532-1542. https://doi.org/10.1016/j.bbamcr.2011.04.009
- Antonioni, A., Dimauro, I., Fantini, C., Barone, R., Macaluso, F., Di Felice, V., & Caporossi, D. (2020). αB-crystallin response to a pro-oxidant noncytotoxic environment in murine cardiac cells: An “in vitro” and “in vivo” study. Free Radical Biology and Medicine, 152, 301-312. https://doi.org/10.1016/j.freeradbiomed.2020.03.01
- Aoyama, A., Steiger, R. H., Fröhli, E., Schäper, R., Deimling, A., Wiestler, O. D., & Klemenz, R. (1993). Expression of αB‐crystallin in human brain tumors. International journal of cancer, 55(5), 760-764. https://doi.org/10.1002/ijc.2910550511
- Andley, U. P. (2007). Crystallins in the eye: function and pathology. Progress in retinal and eye research, 26(1), 78-98. https://doi.org/10.1016/j.preteyeres.2006.10.003
- Andley, U. P. (2008). The lens epithelium: Focus on the expression and function of the α-crystallin chaperones. The international journal of biochemistry & cell biology, 40(3), 317-323. https://doi.org/10.1016/j.preteyeres.2006.10.003
- Chakraborty, A., Nandy, S., Saha, S., & De, P. (2023). An Insight on α-crystallin Interactions with Various Proteins in Systemic Disorders. Journal of Stress Physiology & Biochemistry, 19(3), 35-46.
- Dasgupta, S., Hohman, T. C., & Carper, D. (1992). Hypertonic stress induces αB-crystallin expression. Experimental eye research, 54(3), 461-470. https://doi.org/10.1016/0014-4835(92)90058-Z
- Dimauro, I., & Caporossi, D. (2022). Alpha B-crystallin in muscle disease prevention: the role of physical activity. Molecules, 27(3), 1147. https://doi.org/10.3390/molecules27031147
- Dodd, S. L., Hain, B., Senf, S. M., & Judge, A. R. (2009). Hsp27 inhibits IKKβ-induced NF-κB activity and skeletal muscle atrophy. The FASEB Journal, 23(10), 3415. https://doi.org/10.1096%2Ffj.08-124602
- Dumont, D., Noben, J. P., Moreels, M., Vanderlocht, J., Hellings, N., Vandenabeele, F., ... & Robben, J. (2007). Characterization of mature rat oligodendrocytes: a proteomic approach. Journal of neurochemistry, 102(2), 562-576. https://doi.org/10.1111/j.1471-4159.2007.04575.x
- Fittipaldi S, Mercatelli N, Dimauro I, Jackson MJ, Paronetto MP, Caporossi D. Alpha B-crystallin induction in skeletal muscle cells under redox imbalance is mediated by a JNK-dependent regulatory mechanism. Free Radic Biol Med. 2015 Sep;86:331-42. https://doi.org/10.1016/j.freeradbiomed.2015.05.03
- Garrido, C., Schmitt, E., Candé, C., Vahsen, N., Parcellier, A., & Kroemer, G. (2003). HSP27 and HSP70: potentially oncogenic apoptosis inhibitors. Cell cycle, 2(6), 578-583. https://doi.org/10.4161/cc.2.6.521
- Ghosh, J. G., Shenoy, A. K., & Clark, J. I. (2007). Interactions between important regulatory proteins and human αB crystallin. Biochemistry, 46(21), 6308-6317. https://doi.org/10.1021/bi700149h
- Hamann, S., Métrailler, S., Schorderet, D. F., & Cottet, S. (2013). Analysis of the cytoprotective role of α- crystallins in cell survival and implication of the αAcrystallin C-terminal extension domain in preventing Bax-induced apoptosis. PloS one, 8(2), e55372. https://doi.org/10.1371/journal.pone.0055372
- Ito, H., Kamei, K., Iwamoto, I., Inaguma, Y., Garcia-Mata, R., Sztul, E., & Kato, K. (2002). Inhibition of proteasomes induces accumulation, phosphorylation, and recruitment of HSP27 and αβ-crystallin to aggresomes. The journal of biochemistry, 131(4), 593-603. https://doi.org/10.1093/oxfordjournals.jbchem.a003
- Kamradt, M. C., Chen, F., Sam, S., & Cryns, V. L. (2002). The small heat shock protein αB-crystallin negatively regulates apoptosis during myogenic differentiation by inhibiting caspase-3 activation. Journal of Biological Chemistry, 277(41), 38731-38736. https://doi.org/10.1074/jbc.M201770200
- Kannan, R., Sreekumar, P. G., & Hinton, D. R. (2012). Novel roles for α-crystallins in retinal function and disease. Progress in retinal and eye research, 31(6), 576–604. https://doi.org/10.1016/j.preteyeres.2012.06.001
- Launay, N., Goudeau, B., Kato, K., Vicart, P., & Lilienbaum, A. (2006). Cell signaling pathways to αB-crystallin following stresses of the cytoskeleton. Experimental cell research, 312(18), 3570-3584. https://doi.org/10.1016/j.yexcr.2006.07.025
- Li, D. W. C., Liu, J. P., Mao, Y. W., Xiang, H., Wang, J., Ma, W. Y., ... & Reed, J. C. (2005). Calciumactivated RAF/MEK/ERK signaling pathway mediates p53-dependent apoptosis and is abrogated by αB-crystallin through inhibition of RAS activation. Molecular biology of the cell, 16(9), 4437-4453. https://doi.org/10.1091/mbc.e05-01-0010
- Liu, S., Li, J., Tao, Y., & Xiao, X. (2007). Small heat shock protein αB-crystallin binds to p53 to sequester its translocation to mitochondria during hydrogen peroxide-induced apoptosis. Biochemical and biophysical research communications, 354(1), 109-114. https://doi.org/10.1016/j.bbrc.2006.12.152 MacRae, T. H. (2000). Structure and function of small heat shock/α-crystallin proteins: established concepts and emerging ideas. Cellular and Molecular Life Sciences CMLS, 57, 899-913. https://doi.org/10.1007/PL00000733
- Mao, Y. W., Liu, J. P., Xiang, H., & Li, D. W. (2004). Human αA-and αB-crystallins bind to Bax and Bcl- XS to sequester their translocation during staurosporine-induced apoptosis. Cell Death & Differentiation, 11(5), 512-526. https://doi.org/10.1038/sj.cdd.4401384
- Mehlen, P., Kretz‐Remy, C., Preville, X., & Arrigo, A. P. (1996). Human hsp27, Drosophila hsp27 and human alphaB‐crystallin expression‐mediated increase in glutathione is essential for the protective activity of these proteins against TNFalpha‐induced cell death. The EMBO journal, 15(11), 2695-2706. https://doi.org/10.1002/j.1460-2075.1996.tb00630.x
- Morrison, L. E., Hoover, H. E., Thuerauf, D. J., & Glembotski, C. C. (2003). Mimicking phosphorylation of αB-crystallin on serine-59 is necessary and sufficient to provide maximal protection of cardiac myocytes from apoptosis. Circulation research, 92(2), 203-211. https://doi.org/10.1161/01.RES.0000052989.83995.A5
- Morozov, V., & Wawrousek, E. F. (2006). Caspasedependent secondary lens fiber cell disintegration inαA-/αB-crystallin double-knockout mice. https://doi.org/10.1242/dev.02262
- Nagaraj, R. H., Nahomi, R. B., Mueller, N. H., Raghavan, C. T., Ammar, D. A., & Petrash, J. M. (2016). Therapeutic potential of α-crystallin. Biochimica et biophysica acta, 1860(1 Pt B), 252–257. https://doi.org/10.1016/j.bbagen.2015.03.012
- Nahomi, R. B., Oya-Ito, T., & Nagaraj, R. H. (2013). The combined effect of acetylation and glycation on the chaperone and anti-apoptotic functions of human α-crystallin. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease, 1832(1), 195-203. https://doi.org/10.1016/j.bbadis.2012.08.015
- Nath, M., Sluzala, Z. B., Phadte, A. S., Shan, Y., Myers, A. M., & Fort, P. E. (2022). Evidence for Paracrine Protective Role of Exogenous αA-Crystallin in Retinal Ganglion Cells. Eneuro, 9(2). https://doi.org/10.1523/ENEURO.0045-22.2022
- Pasupuleti, N., Matsuyama, S., Voss, O., Doseff, A. I., Song, K., Danielpour, D., & Nagaraj, R. H. (2010). The anti-apoptotic function of human αA-crystallin is directly related to its chaperone activity. Cell death & disease, 1(3), e31-e31. https://doi.org/10.1038/cddis.2010.3
- Phadte, A. S., Sluzala, Z. B., & Fort, P. E. (2021). Therapeutic potential of α-crystallins in retinal neurodegenerative diseases. Antioxidants, 10(7), 1001. https://doi.org/10.3390/antiox10071001
- Piri, N., Kwong, J. M., Gu, L., & Caprioli, J. (2016). Heat shock proteins in the retina: focus on HSP70 and alpha crystallins in ganglion cell survival. Progress in retinal and eye research, 52, 22-46. https://doi.org/10.1016/j.preteyeres.2016.03.001
- Ruebsam, A., Dulle, J. E., Myers, A. M., Sakrikar, D., Green, K. M., Khan, N. W., ... & Fort, P. E. (2018). A specific phosphorylation regulates the protective role of αA-crystallin in diabetes. JCI insight, 3(4). https://doi.org/10.1172%2Fjci.insight.97919
- Schaefer, H., Marcus, K., Sickmann, A., Herrmann, M., Klose, J., & Meyer, H. E. (2003). Identification of phosphorylation and acetylation sites in αacrystallin of the eye lens (mus musculus) after twodimensional gel electrophoresis. Analytical and bioanalytical chemistry, 376, 966-972. https://doi.org/10.1007/s00216-003-1983-1
- Sharma, K. K., & Santhoshkumar, P. (2009). Lens aging: effects of crystallins. Biochimica et Biophysica Acta (BBA)-General Subjects, 1790(10), 1095-1108. https://doi.org/10.1016/j.bbagen.2009.05.008
- Takemoto, L. J. (1997). Changes in the C-terminal region of alpha-A crystallin during human cataractogenesis. The International Journal of Biochemistry & Cell Biology, 29(2), 311-315. https://doi.org/10.1016/S1357-2725(96)00111-2
- Wang, F., Chen, X., Li, C., Sun, Q., Chen, Y., Wang, Y., ... & Zhang, H. (2014). Pivotal role of augmented αB-crystallin in tumor development induced by deficient TSC1/2 complex. Oncogene, 33(34), 4352-4358. https://doi.org/10.1038/onc.2013.401
- Webster, K. A. (2003). Serine phosphorylation and suppression of apoptosis by the small heat shock protein αB-crystallin. Circulation research, 92(2), 130-132. https://doi.org/10.1161/01.RES.0000056967.51841.21
- Whiston, E. A., Sugi, N., Kamradt, M. C., Sack, C., Heimer, S. R., Engelbert, M., ... & Gregory, M. S. (2008). αB-crystallin protects retinal tissue during Staphylococcus aureus-induced endophthalmitis. Infection and immunity, 76(4), 1781-1790. https://doi.org/10.1128/iai.01285-07
- Xia, X. Y., Wu, Q. Y., An, L. M., Li, W. W., Li, N., Li, T. F., ... & Xue, C. Y. (2014). A novel P20R mutation in the alpha-B crystallin gene causes autosomal dominant congenital posterior polar cataracts in a Chinese family. BMC ophthalmology, 14, 1-7. https://doi.org/10.1186/1471-2415-14-108
- Ying, X., Peng, Y., Zhang, J., Wang, X., Wu, N., Zeng, Y., & Wang, Y. (2014). Endogenous α-crystallin inhibits expression of caspase-3 induced by hypoxia in retinal neurons. Life sciences, 111(1-2), 42-46. https://doi.org/10.1016/j.lfs.2014.07.008


