Elucidating the Role of Secondary Metabolite and Reactive Oxygen Species in High-Temperature Stress on Medicinal Plants
Автор: Pooja Tamta, Babita Patni
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 4 т.16, 2020 года.
Бесплатный доступ
Worldwide warming is anticipated to have a typically terrible impact on plant development because of the harmful impact of excessive temperatures on plant improvement. Plants' diversity and productivity are adversely suffering from abiotic environmental factors. Thermal /heat stress is now becoming the main concern for plants everywhere, Major reason behind this is sudden changes in weather and climate which affects medicinal plants around the world and will eventually cause the destruction of some key species. The growing risk of climatological extremes such as very excessive temperatures might result in a catastrophic lack of crop productiveness and bring about extensive famine. Within the boom situation of plant life, several secondary metabolites are produced with the aid of them to serve a ramification of cell capabilities vital for physiological approaches, and the latest growing proof has implicated stress reaction. The medicinal plants comprise bioactive PSNPs, which perform a key role in plant life with the altering environment and stress condition. Within past decades, various studies advertised the healing properties and biological activities of medicinal plants. Excessive levels of pressure in medicinal vegetation exploitation caused by abiotic stress outcomes with the production of ROS inside the cell chambers of a plant cell which ultimately have a tremendous effect on secondary metabolite production. In this article, we have focused on what is the role of secondary metabolite and ROS generation in protecting the plant under the high stressful condition of heat.
RPSMs, plant secondary metabolites, heat stress, ROS, RNS, oxidative stress
Короткий адрес: https://sciup.org/143173858
IDR: 143173858
Текст научной статьи Elucidating the Role of Secondary Metabolite and Reactive Oxygen Species in High-Temperature Stress on Medicinal Plants
PLANT SECONDARY METABOLITES
Plant secondary metabolites are a peculiar supply for medication diet components flavors and industrially essentials. Environmental factors viz., temperature, humidity, candlepower, water minerals, and co2 impact the enlargement of a plant and secondary metabolite manufacturing. Secondary metabolites play a big role within the variation of plants to the changing environment. Plants have an almost limitless ability to synthesize these metabolites. Phytochemicals have proven to cure many ailments and especially lifestyle diseases. This will help to chop back the side effect of synthetic treatment of the chemical remedies. Plant-life is multifaceted chemical factories that produce a huge variety of structurally numerous natural compounds that don't appear to be immediately involved in the conventional boom development or replica however are ideas to be required inside the variation with their environment. Those secondary metabolites generally incorporate over one useful organization and from time to time showcase a couple of functionalities and bioactivity secondary metabolites derive their synthesis from confined products of initial metabolism (Crozier et al., 2006). Foremost not unusual unavoidable interplay going on in plant groups is that the plant surroundings interact. Outside elements quantitatively have an effect on the plant's metabolic approaches via their outcomes on plant development boom rates and partitioning of assimilates into essential metabolites. These factors may trigger abrupt activation of qualitative changes in secondary metabolite production (Laughlin, 1993; Lommen et al., 2008; Pérez-Estrada et al., 2000). Abiotic elements frequently have a mainly huge effect on the biosynthetic stages and quality of secondary metabolites in plant life (Coley, 1987).
ROLE OF ROS AND RNS IN THEMANIPULATION OF A PLANT SYSTEM
Researchers observed an association among lipid configuration and oxidative stress, in the meantime, the next content material of MDA (Malondialdehyde - a response cease fabricated from the lipid oxidation via ROS) and an advanced formation of “ox-lipids” through the endoplasmic reticulum have been determined after thermal exposure (Narayanan, 2016). In element, the excessive temperature can also motive an upsurge in ROS improvement particularly in the chloroplasts, mitochondria, and peroxisomes (Apel, Hirt, 2004). These ROS are greater reactive forms of oxygen in their basal state (O 2 ), like singlet oxygen (O), superoxide radical (•O 2 -), radical (HO•), and peroxide (H 2 O 2 ), which can be shaped evidently throughout rather active approaches like photosynthesis and respiration. ROS can purpose oxidative injury to molecules like nucleic acids, proteins, and lipids, which in the end have an effect on metabolic movements and additionally the integrity of organelles.
Most of the high damages resulting from ROS is lipid peroxidation (LPO), an elaborate procedure of oxidation of polyunsaturated fatty acids that occurs each in animals and plant life, and outcomes within the formation and propagation of lipid radicals, the rearrangement of double bonds of unsaturated lipids and additionally the capability destruction of membrane lipids (Hariyadi, Parkin, 1991). Underneath favorable environmental situations for plant metabolism and increase, the excess of these oxidative damages is averted due to ROS manufacturing is counterbalanced through antioxidant mechanisms that remove those compounds. In standard, the plant antioxidant device consists of non-enzymatic additives like ascorbate, glutathione, tocopherols (vitamin E) and carotenoids; and an elaborate enzyme device containing, as an example, (SOD), guaiacol peroxidase, ascorbate peroxidase, glutathione reductase, and catalase. SOD is that the primary enzyme to carry out ROS detoxing, changing the superoxide radical into H2O2, that's converted into H2O by CAT, APX, and guaiacol peroxidase enzymes. The APX additionally acts in the ascorbate-glutathione cycle collectively with GR and numerous different enzyme additives to regenerate ascorbate and decreased glutathione (Gupta et al., 2015). warmness exposure may additionally cause improved production of reactive nitrogen species (RNS), which consist of the gaseous gasoline radical (NO) and numerous other compounds that result from their reaction with distinct molecules, like peroxynitrite (ONOO-), peroxynitrous acid (HONO2), and additionally the gas radical (•NO2).
In addition to ROS, whilst stress exposure subsequently finally ends up in the deregulated synthesis or exacerbated manufacturing of RNS, those molecules will have poisonous outcomes on cells due to the fact they are visiting react with numerous cellular components like thiols, enzyme cofactors, proteins, nucleotides, and lipids, which subsequently cease inside the so-referred to as a nitrosative strain. Especially for lipids, RNS can cause LPO like ROS, and as a consequence have an effect on the membrane shape via nitration reactions prompted especially through NO derivatives like •NO 2 and ONOO-. NO, and related molecules can also furthermore perform post-translational modifications through numerous mechanisms like S-nitrosylation, nitration, and binding to metal centers, targeting tyrosine residues of proteins, thiols, DNA, and lipids, affecting the metabolism and phenomenon.
In citrus plant (Citrus aurantium L .), it turned into observed that the exposure at 42°C for 5 hours caused an endless growth within the NO content material related to considerable leaf damage and electrolyte leakage, membrane damages (Ziogas et al 2013).
However, it's important to take a look at that, irrespective of the deleterious impact of ROS and RNS accumulation over an extended-time period length of heat publicity, the quick increase in the content material of those molecules for the duration of the primary duration of a strain called oxidative and nitrosative rush for ROS and RNS commonly can act as a part of the signaling route for activating adjustment mechanisms in species tolerant to excessive temperatures so on allowing their survival under such conditions (Suzuki, Mittler, 2006; Fancy et al , 2017).
PHENOLOGICAL CHANGES:
Excessive temperature may additionally modify the whole phenological length through decreasing the life span the life cycles of plant life correspond to seasonal cues whether trade alters the plant life and ecosystems. Much essential medicinal vegetation is beneath extensive risk of danger from those phonological modifications. Phenological activities for medicinal vegetation are adapted to a globally warm climate is probably taken into consideration as (I) Budburst and Leaf unfolding, (ii) Flowering and placing fruit, (iii) Autumn or season leaf drop, and (iv) The associated approaches of winter hardening and Breaking as heating progresses it will have an effect on the appearance of spring and consequently the duration of the season.
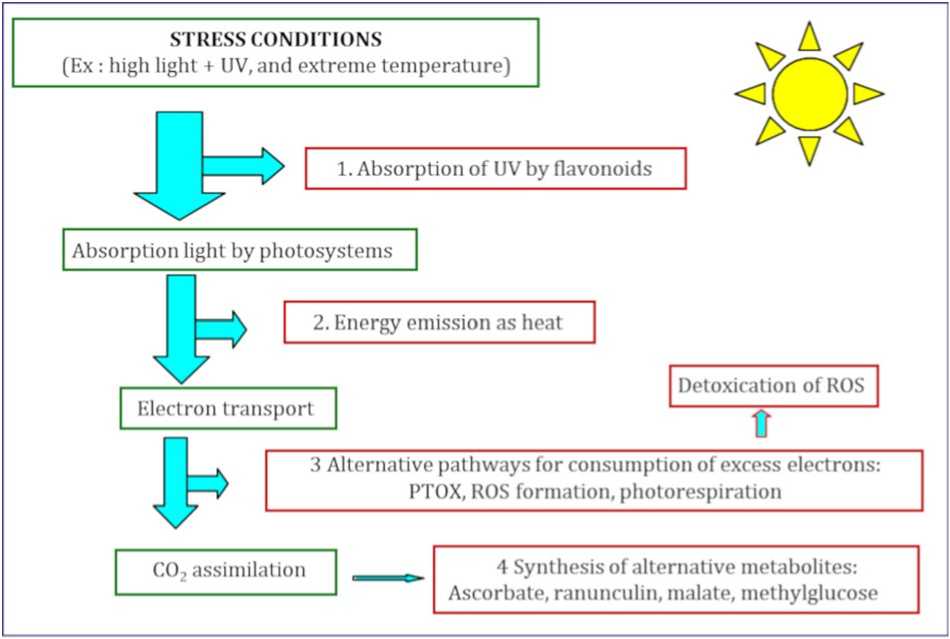
Figure 1 Energy Flow in the Leaf under Stress and Light Conditions in Alpine Plants. (Sterb et al. , 2019)
Senescence/Abiotic stresses
Activation of nuclear genes participating in protection against high light
Action of B-cyclocitral Modification of expression of genes encoding chloroplast proteins
Non-enzymatic lipid peroxidation which contributes to execution phase of plant cell death
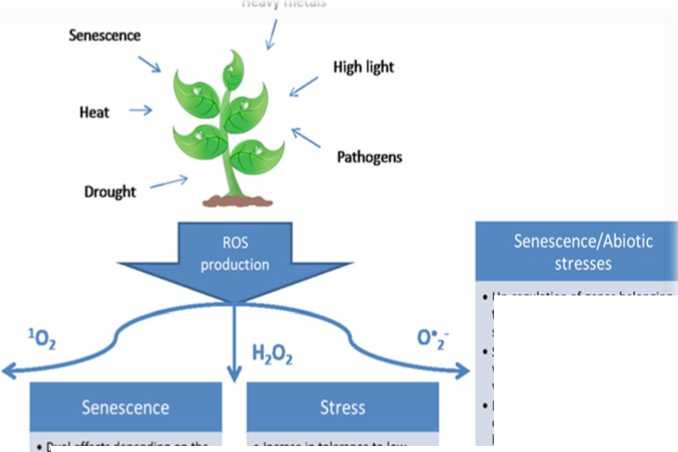
Dual effects depending on the concentration
-
• Induction of expression of NAC TF and SAGs
-
• Acceleration of senescence via induction of ORS1 and ATAF1 TF
-
• Delay of senescence by upregulating JUB1 TF
-
• Incrase in tolerance to low light and heat stress via modulation of AO
-
• Increase of drought tolerance by activation of stress responsive genes
-
• Protection against pathogens by increase in activity of Class III peroxidases
Up-regulation of genes belonging to categories functioning in abiotic stress responses
Signalling role during the early wound response in activation of wound responsive proteins
Participation in plant responses to cadmium stress in an interplay between AO and NO
-
• Possible role in down-regulation о photosynthesis-associated genes during senescence
-
• Acceleration of senescence which contributes to cell death
Figure 2 Possible roles of ROS during senescence and abiotic stresses. (Jajic et al. , 2015).
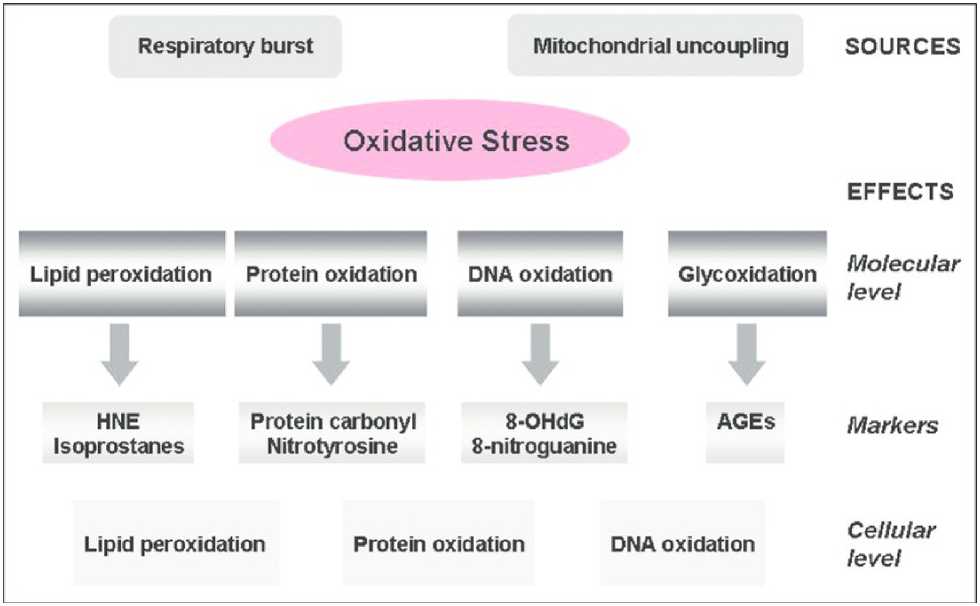
Figure 3 - Sources and effects of oxidative stress on a molecular and cellular level. (Gella & Durany, 2009).
OXIDATIVE STRESS RESPONSE:
Exceeding temperature is cytotoxic in nature. The mitochondrion is a primary site as well as the main target of ROS initiation. Major aspects are as follow (Wang CH, et al . 2013)
1-Elicits oxidative damage,
-
2- Provoke the mitochondrial maintenance of equilibrium
3-Cell dysfunction knockdowns.
-
4- Imbalance the concentration of target proteins, lipids, polysaccharides, and DNA, and increase the rate of cellular death.
5-oxidative detriment alters the ETC (electron transport chain), the Adenosine Tri Phosphate synthesis, the uncoupling respiration, and the morphological conformation of biomolecules, and this is the result due to the failure of the mitochondrial antioxidant system which unable to maintain the stable phase of free radicals.
-
6- Hence because of the above-stated phenomenon Heat shock response fails to repair impaired proteins system. In the end, a final determination is –heat stress is usually associated with apoptosis and necrosis in green tissues and cell organ’.
CONCLUSION
MAPs in higher and lower Himalaya which includes alpine regions face harsh situations related to their swiftly converting environments, and some researchers have raised issues concerning the viable losses of local plant inhabitant and genetic collection in the one's regions. The potential loss of MAP species from the results HT strain goes to very own fundamental ramifications at the livelihoods of significant numbers of susceptible populations throughout the globe. HT stress has terrible outcomes on plant secondary metabolite improvement and advancement through disrupting the stability of assorted proteins, carbs, lipids, and major primary metabolites.
There is remarkable growth within the consumption and need for medicinal vegetation reported in the ultimate decade. Scientific studies on MAPs is commencing new horizons within the ability of drugs and different herbal merchandise. Even though we have little data (20%) about total vegetative flora all over but still it is reported that the world is dependent on 60% of their need for drugs chiefly on plants. The vital oil, flavors, fragrances of aromatic flora at the commercial level, contributes to the economic system of developing nations. Warmness induces accumulation of HSPs which cease protein degradation and it additionally creates a state of metabolic imbalance and consequently the build-up of poisonous by usingmerchandise, like ROS, which ultimately influences vegetative and reproductive improvement and drops the yield amount.
The outcomes of excessive temperature on medicinal vegetation, specifically, have no longer been properly-studied and aren't always completely understood. As the situation reveals, global climate change could raise the abiotic factors mainly extreme temperature which can become a more pressing issue for the herbal community, potentially affecting users, harvesters, and makers of MAP species
So it's very necessary to review the impact of stresses on MAP's and its potential, ecological status, and thoroughly. The results and outcomes of the study will guide the steps.
Список литературы Elucidating the Role of Secondary Metabolite and Reactive Oxygen Species in High-Temperature Stress on Medicinal Plants
- Agostini-Costa, T. D. S., Vieira, R. F., Bizzo, H. R., Silveira, D., & Gimenes, M. A. (2012). Chromatography and its applications. In: Dhanarasu S (ed) Plant secondary metabolites, In Tech Publisher, Rijeka, Croatia, pp. 131-164.
- Apel, K., & Hirt, H. (2004). REACTIVE OXYGEN SPECIES: Metabolism, Oxidative Stress, and Signal Transduction. Annual Review of Plant Biology, 55(1), 373–399.
- Ashraf, M, & Athar, H, & Harris, P. & Kwon, T.R.. (2008). Some Prospective Strategies for Improving Crop Salt Tolerance. Advances in Agronomy. 97. 45-110. 1
- Ashraf, M., Iqbal, M., Rasheed, R., Hussain, I., Riaz, M. & Arif, M. (2018). Environmental Stress and Secondary Metabolites in Plants. In: Plant Metabolites and Regulation Under Environmental Stress. pp. 153-167 10.1016/B978-0-12-812689-9.00008-X.
- Belhadj Slimen, I., Najar, T., Ghram, A., Dabbebi, H., Ben Mrad, M., & Abdrabbah, M. (2014). Reactive oxygen species, heat stress and oxidativeinduced mitochondrial damage. A review. International journal of hyperthermia, 30(7), 513-523.
- Coley, P. D. (1987). Interspecific variation in plant antiherbivore properties: the role of habitat quality and rate of disturbance. New phytologist, 106, 251-263.
- Crozier, A., Clifford, M. N., & Ashihara, H. (Eds.). (2006). Plant Secondary Metabolites. Blackwell Publishing, 382 p.
- Edreva, A., Velikova, V., Tsonev, T., Dagnon, S., Gürel, A., Aktaş, L., & Gesheva, E. (2008). Stressprotective role of secondary metabolites: diversity of functions and mechanisms. Gen Appl Plant Physiol, 34(1-2), 67-78.
- Fancy, N. N., Bahlmann, A. K., & Loake, G. J. (2017). Nitric oxide function in plant abiotic stress. Plant, Cell & Environment, 40(4), 462-472.
- Fang, X, Yang, CMAQ, Yang, L, Chen, X (2011) Genomics grand for diversified plant secondary metabolites. Plant Divers Resour 33, 53–64
- Gella, A., & Durany, N. (2009). Oxidative stress in Alzheimer disease. Cell adhesion & migration, 3(1), 88-93.
- Gupta, D. K., Palma, J. M., & Corpas, F. J. (Eds.). (2016). Redox state as a central regulator of plant-cell stress responses. Springer. pp. 1-386
- Hansen, J., Ruedy, R., Sato, M., Lo, K. (2010). Global surface temperature change. Rev. Geophys. 48 (4), RG4004.
- Hariyadi, P., Parkin, K.L. (1991) Chilling-induced Oxidative stress in cucumber fruits. Postharvest Biol. Techn. 1, 33–45
- Hartley et al., 2000 Hartley‐Whitaker, J., Ainsworth, G., & Meharg, A. A. (2001). Copper‐and arsenateinduced oxidative stress in Holcus lanatus L. clones with differential sensitivity. Plant, Cell & Environment, 24(7), 713-722.
- Hopkins D. P., Cameron D. D., Butlin R. K. (2017). The chemical signatures underlying host plant discrimination by aphids. Sci. Rep. 7, 8498.
- Jajic, I., Sarna, T., & Strzalka, K. (2015). Senescence, Stress, and Reactive Oxygen Species. Plants, 4(3), 393–411.
- Kliebenstein, D. J. (2013). Making new molecules— evolution of structures for novel metabolites in plants. Current opinion in plant biology, 16(1), 112-117.
- Kurepin, L. V., Ivanov, A. G., Zaman, M., Pharis, R. P., Hurry, V., & Hüner, N. P. (2017). Interaction of glycine betaine and plant hormones: protection of the photosynthetic apparatus during abiotic stress. In Photosynthesis: Structures, mechanisms, and applications . Springer, Cham. pp. 185-202
- Lambers, H. (1993). Rising CO2, secondary plant metabolism, plant-herbivore interactions and litter decomposition. In CO2 and Biosphere Springer, Dordrecht. pp. 263-271
- Lambers, H., Van der Werf, A., & Bergkotte, M. (1993). Respiration: the alternative pathway. Methods in Comparative Plant Ecology. Chapman & Hall, London, 140-144.
- Laughlin, 1993; Laughlin, J. C. (1994). Agricultural production of artemisinin—a review. Transactions of the Royal Society of Tropical Medicine and Hygiene, 88, 21-22.
- Levine L. H., Kasahara H., Kopka J., Erban A., Fehrl I., Kaplan F. (2008). Physiologic and metabolic responses of wheat seedlings to elevated and super-elevated carbon dioxide. Adv. Space Res. 42, 1917–1928.
- Li, Z., & Sharkey, T. D. (2013). Molecular and pathway controls on biogenic volatile organic compound emissions. In Biology, controls and models of tree volatile organic compound emissions . Springer, Dordrecht. pp. 119-151
- Lommen, W. J. M., Bouwmeester, H. J., Schenk, E., Verstappen, F. W. A., Elzinga, S., & Struik, P. C. (2008). Modelling processes determining and limiting the production of secondary metabolites during crop growth: the example of the antimalarial artemisinin produced in Artemisia annua. Acta Horticulturae, 765, 87-94.
- Loreto, F., & Schnitzler, J. P. (2010). Abiotic stresses and induced BVOCs. Trends in plant science, 15(3), 154-166.
- Narayanan, S., Tamura, P. J., Roth, M. R., Prasad, P. V., & Welti, R. (2016). Wheat leaf lipids during heat stress: I. High day and night temperatures result in major lipid alterations. Plant, Cell & Environment, 39(4), 787–803.
- Pérez-Estrada, L. B., Cano-Santana, Z., & Oyama, K. (2000). Variation in leaf trichomes of Wigandia urens: environmental factors and physiological consequences. Tree Physiology, 20(9), 629-632.
- Ramakrishna, A., & Ravishankar, G. A. (2011). Influence of abiotic stress signals on secondary 426 metabolites in plants. Plant Signaling & Behavior, 6(11), 1720 1731.
- Raupach, M. R., Marland, G., Ciais, P., Le Quéré, C., Canadell, J. G., Klepper, G., & Field, C. B. (2007). Global and regional drivers of accelerating CO2 emissions. Proceedings of the National Academy of Sciences of the United States of America, 104, 10288– 10293.
- Singsaas, E. L. (2000). Terpenes and the thermotolerance of photosynthesis. New Phytologist, 146(1), 1-2.
- Siwko, M. E., Marrink, S. J., de Vries, A. H., Kozubek, A., Uiterkamp, A. J. S., & Mark, A. E. (2007). Does isoprene protect plant membranes from thermal shock? A molecular dynamics study. Biochimica et Biophysica Acta (BBA)-Biomembranes, 1768(2), 198-206.
- Streb P., Cornic G., Bligny R. (2019) How do plants cope with alpine stress? Encyclopédie de l'environnement URL: https://www.encyclopedieenvironnement. org/en/life/how-do-plants-copewith-alpine-stress/ (accessed 22 November 2020)
- Suzuki, N., & Mittler, R. (2006). Reactive oxygen species and temperature stresses: a delicate balance between signaling and destruction. Physiologia plantarum, 126(1), 45-51.
- Wang, C. H., Wu, S. B., Wu, Y. T., & Wei, Y. H. (2013). Oxidative stress response elicited by mitochondrial dysfunction: implication in the pathophysiology of aging. Experimental biology and medicine, 238(5), 450-460.
- Ziogas, V., Tanou, G., Filippou, P., Diamantidis, G., Vasilakakis, M., Fotopoulos, V., & Molassiotis, A. (2013). Nitrosative responses in citrus plants exposed to six abiotic stress conditions. Plant Physiology and Biochemistry, 68, 118–126.


