Endoscopic layout of esophageal-intestinal anastomosis in relation to versions of rehabilitation of alimentary tract after gastrectomy
Автор: Toigonbekov Aivar, Akhunbaev Stalbek, Umetov Maksat, Borbashev Tilek
Журнал: Бюллетень науки и практики @bulletennauki
Рубрика: Медицинские науки
Статья в выпуске: 7 т.6, 2020 года.
Бесплатный доступ
This study offers findings of endoscopic research of patients with stomach cancer after surgery of gastrectomy with different versions of small intestine plastic surgery. Total number of patients exposed to the research is 130, divided into 3 groups. Findings: veracious decrease of esophagitis (p
Stomach cancer, gastrectomy, endoscopic examination
Короткий адрес: https://sciup.org/14117784
IDR: 14117784 | УДК: 616.36-006-036.22(575.2) | DOI: 10.33619/2414-2948/56/19
Текст научной статьи Endoscopic layout of esophageal-intestinal anastomosis in relation to versions of rehabilitation of alimentary tract after gastrectomy
Бюллетень науки и практики / Bulletin of Science and Practice
UDC 616.36-006-036.22(575.2)
The stomach cancer disease is still one of the most common illnesses in the world and has many challenges in modern oncology. Today, SC morbidity ranks fourth as to the frequency of the cases, following lung-tumors, breast-tumors, and tumors of the large intestine [1].
According to data of stats department of the NCOH of MH of the KR the stomach cancer goes 3rd in the structure of malignant tumors in Kyrgyzstan. There are 727 patients with this pathology newly registered during 2016 in the KR.
Nowadays, stomach cancer surgery has become a golden standard [1]. Regardless of progress in stomach surgery, there are still various post-gastrectomy diseases, which dramatically affect the quality of patients’ lives. And this depends on a version of small-intestine plastic surgery. The vast majority of patients become disabled because of complete removal of the stomach and changes in normal anatomic-physiological processes in alimentary tract. Just because of this, over the last decades the surgery of stomach cancer has changed its upward trend of radicalism in surgery decisions and reduction of post-surgery complications and mortality, to more attention paid to functional results [1; 2].
The main pathologic syndrome after gastrectomy is reflux esophagitis caused by bile discharge into the esophagus at the adductor loop, with frequency of around 37.0% to 90.0% cases [0]. This is the most commonly occurring and serious functional sequelae, which directly depend on method of reconstruction of the alimentary tract.
Inevitable resection of the cardiac pulp during gastrectomy procedure with erosion of all its sphincter components (muscular, valve and diaphragm mechanisms, His’ corner with esophageal-diaphragmatic ligament) is the essential factor. At the same time stem vagotomy, sympathetic denervation, trophic devascularization with atony of esophageal walls play a huge role [3; 4].
Research objectives: main post-gastrectomy complications’ prophylaxis based on the analysis of different types of small-intestine plastic surgery.
Research materials and methods
This research is based on analysis of results of medical care and monitoring of 130 patients with malignant tumors of stomach, receiving treatment in the abdominal oncology department of NCO of MH of the KR during 2012-2017 years. All patients were divided into 3 groups in relation to the method of reconstruction of alimentary tract.
Group №1 included 54 patients with stomach cancer of Т 1 - 4 N 0 - 2 М О . After gastrectomy in front of mesentery of transverse colon we form esophageal-enteric anastomosis of jejunum initial loop (35-40sm), then we supply the esophagus with the chosen loop of small intestine for anastomosis procedure to form an anastomosis in the way as designed by M. I. Davydov. 15 sm lower of esophageal-intestinal anastomosis we form inter-intestinal anastomosis between the afferent and abductor loops side to side, with ligation of afferent loop above the Brown’s fistula by A. A. Shalimov (Figure 1).
Group №2 comprised of 53 patients after gastrectomy with formation of the small-intestine plastic surgery by Roux (Figure 2).
Group №3 contained 23 patients with stomach cancer of Т1-4 N0-2 МО. They have taken gastrectomy with formation of small-intestinal reservoir on an uncrossed loop (uncut-Roux). After gastrectomy in front of mesenteries of transverse colon we form esophageal-enteric anastomosis by use of jejunum initial loop (35-40sm), then we supply the esophagus with the particular chosen loop of small intestine for anastomosis procedure to form an anastomosis by the type of M.I. Davydov. Afterwards, as lower as 5-7 sm to esophageal-enteral anastomosis we form a small-intestine reservoir of 12-15 sm from adductor and abductor guts [3].
We use separate nodal serous-muscular nylon sutures between the discharge and leading loop to form the first row of the posterior wall of the reservoir. At the next stage, no less than 0.5 cm lower from the previous seam, the gaps of the adductor and abductor loops are opened over a length of 8-10 cm by electrocautery. After careful hemostasis by the use of individual nodal nylon sutures, we form the inner row and outer row of the back side of the reservoir. Then, we form a two-row frontal side of the reservoir by nodal nylon as well. Right after that, below the reservoir the adductor loop is tied with a lavsan thread and covered with individual nodal serous-muscular sutures. Then, 5-7cm lower we construct inter-intestine anastomosis between adductor and abductor loops side to side. A thin nasogastric tube is installed in the reservoir to track for hemostasis and decompression of the reservoir (Figure 3).
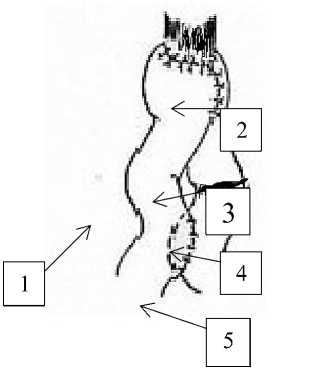
Figure 1. Forming of esophagus-intestinal anastomosis with ligation of afferent loop: 1-abductor loop 2-esophagal-intestine anastomosis 3-afferent loop 4-ligation of afferent loop 5- inter-intestine anastomosis
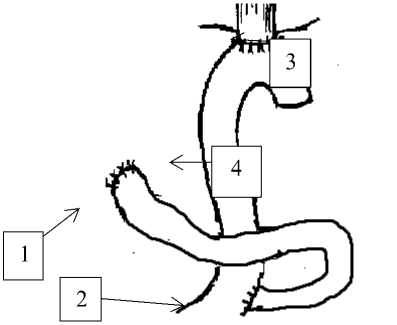
Figure 2 forming of esophageal-intestine anastomosis by Roux: anastomosis 3-esophagal-intestine anastomosis 4-abductor loop
1-duodenum 2- inter-intestine
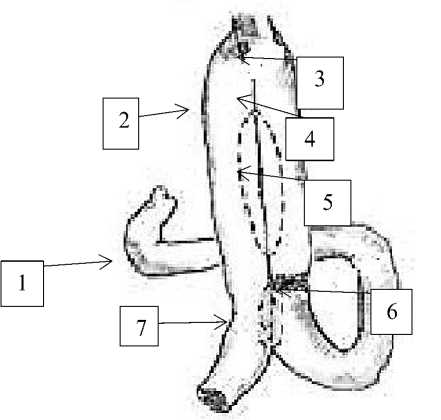
Figure 3. the constructed reservoir after gastrectomy with bondage of abductor loop (uncut-Py): 1-duodenum 2-abdominal loop 3- esophageal-intestinal anastomosis 4-abdominal loop 5-reservoir 6-bondage of gastric loop 7-inter-intestinal anastomosis
In our practice of post-surgery period, after each 3 months we have conducted control testing of patients on esophagoscopy, which is done to examine them for presence of refluxesophagitis and anastomositis.
Methodology of endoscopic research
Endoscopic testing of post-surgery patients was done through the use of a high resolution technical equipment (EG2990/ of “PENTAX”). At the same time, conditions of the esophageal mucosa, anastomosis, proximal small intestine segment, presence of inflammatory and cicatricial changes were detected by eyet. Biopsy with following histological testing is provided if necessary. We used statistical analysis software — “SPSS16”. The reliability of the difference in relative indicators is encoded by the following signs: * — р<0,05 (95,0%); ** — р<0,01 (99,0%); *** — р<0,001 (99,9 %).
Inflammatory and cicatricial changes in the lower thoracic esophagus were classified by Savary-Miller (1978) (Table 1).
Table 1 REFLUX-ESOPHAGITIS CLASSIFICATION
Severity Characteristic of changes
0 Absence of reflux-esophagitis features
I One or more single erosions of mucous membrane with erythema occupying less than 10% of the area of the distal section of esophagus.
II Drain erosive defects to the mucous membrane, occupying 10–50% of the circumference of the distal esophagus.
III Multiple erosive defects occupying almost the entire circumference of the distal esophagus
IV Complicated forms of reflux esophagitis: ulcer, stricture,
Barrett's esophagus
Results and discussions
Table 2
DATA OF ENDOSCOPIC TESTS - 3 MONTHS AFTER SURGERY
|
Group I N=54 |
Group II N=53 |
Group III N=23 |
|
|
Esophagitis of the I phase |
2 (3,7%) |
- |
- |
|
Esophagitis of the II phase |
1 (1,9%) |
- |
- |
|
Esophagitis of the III phase |
1 (1,9%) |
- |
- |
|
Esophagitis of the IV phase |
- |
- |
- |
As we see from the table 2, two (3.7%) patients in the 1st group have esophagitis of the I phase, and one (1.9%) patient is defined as having esophagitis of the II and III phases. Three months later during control testing the esophagitis was not detected. In comparing among groups the statistical unreliability is p>0,005.
Table № 3
DATA OF ENDOSCOPIC TESTS - 6 MONTHS AFTER SURGERY
|
Group I N=54 |
Group II N=53 |
Group III N=23 |
|
|
Esophagitis of the I phase |
14 (25,9%)** |
2 (3,8%) |
- |
|
Esophagitis of the II phase |
7 (13%) |
- |
- |
|
Esophagitis of the III phase |
1 (1,9%) |
- |
- |
|
Esophagitis of the IV phase |
- |
- |
- |
After 6 months of monitoring the indicators of esophagitis have been significantly changed. Thus, the Esophagitis of the I phase in the 1st group (54 patients) is found in 14 (25.9%) patients, what is definitely more common (p<0,005) in relation to the 2nd group, where it is found only in 2 (3.8%) patients, and to 3rd group, where no patient had any symptom of the esophagitis of the I phase.
The esophagitis of the II phase is detected in 7 (13%) patients of the 1st group, but in the II and III groups after 6 months there is no any single case of the esophagitis of the II phase. Only 1 (1,9%) patient from the I group has symptoms of the esophagitis of the III phase.
Table 4
DATA OF ENDOSCOPIC TESTS - 9 MONTHS AFTER SURGERY
|
Group I N=54 |
Group II N=53 |
Group III N=23 |
|
|
Esophagitis of the I phase |
14 (25,9%)* |
4 (7,5%) |
- |
|
Esophagitis of the II phase |
25 (46,3%)*** |
2 (3,8%) |
- |
|
Esophagitis of the III phase |
2 (3,7%) |
- |
- |
|
Esophagitis of the IV phase |
- |
- |
- |
The 9 months endoscopic-tests’ results show us, the esophagitis of the I phase in the 1st group is found in 14 (25,9%) patients and in 4 (7,5%) in the 2nd group. The difference between groups is reliable (p<0,005).
The esophagitis of the II phase veraciously is more often registered (p<0,005) in the group of patients who received loop surgery with bondage of abductor loop (uncut Roux) - 25(46,3%) of patients in comparison to patients having undergone surgery by Roux, where the esophagitis of the II phase is found only in 2(3,8%) sick people.
The esophagitis of the III phase is found in 2 (3,7%) cases in the group I. There is no any case of reflux-esophagitis in groups II and III.
Table 5
DATA OF ENDOSCOPIC TESTS - 12 MONTHS AFTER SURGERY
|
Group I N=54 |
Group II N=53 |
Group III N=23 |
|
|
Esophagitis of the I phase |
6 (11,1%) |
5 (9,4%) |
3 (13%) |
|
Esophagitis of the II phase |
34 (63%)*** |
3 (5,7%) |
- |
|
Esophagitis of the III phase |
5 (9,3%) |
- |
- |
|
Esophagitis of the IV phase |
1 (1,9%) |
- |
- |
After 1 year of dynamic monitoring, according to the data above , the esophagitis of the I phase is presented in all 3 groups: 6(11,1%) patients in the 1st group; 5(9,4%) patients in the 2nd group; and 3(13%) patients in the 3rd group. The difference between groups is not veracious (p<0,005).
The esophagitis of the II phase of patients received loop surgery with bondage of abductor loop (uncut Roux) is found in 34 (63%) cases. This number is significantly higher in comparison to the number of patients having received plastic surgery of small-intestine by Roux, which is only 3 (5,7%) patients. This data is reliable (p<0,005).
The esophagitis of the III and IV phases is determined in 5(9,3%) and in 1 (1,9%) patients of the group I resp.
Thus, we can state the post-gastrectomy reflux-esophagitis is directly connected to the method of formation of alimentary tract tube after gastrectomy. Our research states that formation of a small-intestine reservoir on the uncut Roux and provides great opportunities of prophylaxis of postgastrectomy syndrome. This is supported by one-year endoscopic monitoring results.
Findings
Surgery of a small-intestine with formation of reservoirs during radical gastrectomy due to cancer issues might prevent progress of reflux-esophagitis, and that definitely increases quality of patients’ lives.
Formation of small-intestine reservoirs on uncut Roux can be considered as a method of choice taken by patients in radical gastrectomy.
Список литературы Endoscopic layout of esophageal-intestinal anastomosis in relation to versions of rehabilitation of alimentary tract after gastrectomy
- Ferlay J., Soerjomataram I., Dikshit R., Eser S., Mathers C., Rebelo M.,.. Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012 // International journal of cancer. 2015. V. 136. №5. P. E359-E386. DOI: 10.1002/ijc.29210
- Давыдов М. И. Тер Ованесов М. Д. Современная стратегия хирургического лечения рака желудка // Современная онкология. 2000. Т. 2. №1. С. 4-12.
- Конюхов Г. В. Варианты тонкокишечной пластики при гастрэктомии по поводу рака: дисс.. канд. мед. наук. М., 2006. 102 с.
- Бондарь Г. В., Думанский Ю. В., Попович А. Ю., Бондарь В. Г. Рак желудка: профилактика, диагностика и лечение на современном этапе // Онкологiя. 2006. Т. 8, №2. С. 171-175.


