Enzyme activity, hormone concentration in tree shrew (Tupaia belangeri) during cold acclimation
Автор: Zhang Lin, Zhu Wanlong, Wang Zhengkun
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 3 т.8, 2012 года.
Бесплатный доступ
Environmental factors play an important role in the seasonal adaptation of body mass and thermogenesis in wild small mammals. The tree shrew (Tupaia belangeri), is a unique species of small mammals which is origin of island in the Oriental realm. The present study was to test the hypothesis that ambient temperature was a cue to induce adjustments in body mass, energy intake, metabolism, uncoupling protein 1 (UCP1) in brown adipose tissue (BAT), and other biochemical characters of T. belangeri during cold exposure about 21 days. Our data demonstrate that cold acclimation induced a remarkable increase in body mass, a significant increase in energy intake and metabolic rate, and high expression of UCP1 in BAT of T. belangeri. Cold acclimation induced an increase in cytochrome c oxidase (COX) and Thyroidhormones (T3/T4). These data supported that T. belangeri increased the body mass and increased energy intake and expenditure under cold acclimation. Increased expression of UCP1 was potentially involved in the regulation of energy metabolism and thermogenic capacity following cold acclimation. And it through changes in enzyme activity and hormone concentration under cold acclimation, and suggested temperature changes play an important role in the regulation of thermogenic capacity in tree shrew.
Brown adipose tissue (bat), cold adaptation, cytochrome c oxidase (cox), energy metabolism, thyroidhormones, tupaia belangeri, uncoupling protein 1(ucp1)
Короткий адрес: https://sciup.org/14323673
IDR: 14323673
Текст научной статьи Enzyme activity, hormone concentration in tree shrew (Tupaia belangeri) during cold acclimation
Endothermic animals living in a cold environment maintain normothermia and energy balance through an activation of mechanisms that increase heat production and heat conservation. It has been demonstrated that an animal’s body weight, energy balance and metabolic rate all are affected by temperature (Cooper and Withers,
2002; Lovegrove, 2003; Oufara et al., 1988). Temperature also acts as an environmental zeitgeber for seasonal acclimatization of thermoregulation in rodents (Merritt and Zegers, 1991). Heat production is commonly classified as either obligatory or facultative thermogenesis
(Himms-Hagen, 1990; Lowell and Spiegelman, 2000).
Nonshivering thermogenesis (NST) is an important mechanism for thermoregulatory heat production, particularly in small mammals (Jansky, 1973). Most small mammals increase their capacity of NST in winter or winter-like conditions (Feist and Rosenmann, 1976; Heldmaier et al., 1990; Merritt and Zegerts 1991) through uncoupling respiration in brown adipose tissue (BAT) (Foster and Frydman, 1978; Cannon et al., 1982). The key element for the energy dissipation capacity of BAT is uncoupling protein 1 (UCP1), a 32 kDa protein uniquely expressed in the inner membrane of BAT mitochondria. UCP1 dissipates the proton gradient created by the respiratory chain, thereby accelerating respiration (Lanni et al., 2003; Li et al., 2001b; Lowell and Spiegelman, 2000). Cold temperature, one of the important environment factors, affects thermogenic capacity in some small mammals. BAT is the main site of NST (Ricquier and Bouillaud, 2000); it is the main site of facultative thermogenesis in small rodents and probably most eutherian mammals during the early postnatal period. Furthermore, this tissue is also traditionally regarded as being of particular significance in maintenance of the euthermic state in the cold (Himms-Hagen, 1990; Nedergaard et al., 2001a).
As the terminal enzyme in oxidative phosphorylation in mitochondria, cytochrome c oxidase (COX, complex IV) is involved in mitochondrial energy metabolism (Kadenbach et al., 2000). It has also been demonstrated that several hormones are related to thermogenesis, e.g., thyroidhormones (tri-iodothyronine, T3 and thyroxine, T4) affect adaptive thermogenesis by influencing sever alaspects of energy metabolism, including substrate cycling, ion cycling and mitochondrial proton leaks (Wu et al., 1999; Krotkiewski, 2002). Thyroid-stimulating hormone (TSH or thyrotropin) is a peptide hormone synthesized and secreted by thyrotrope cells in the anterior pituitary gland, which regulates the endocrine function of the thyroid gland (Ronald and McPherson, 2000). TSH stimulates the thyroid gland to secrete the hormones thyroxine (T4) and triiodothyronine (T3) (Figure 1). Potential mechanisms for elevating obligatory thermogenesis include decreased carrier protein affinities for T4, increasing mass-specific aerobic enzyme capacity, ion cycling, and mitochondrial proton leak (Lanni et al., 2003; Tomasi and Mitchell, 1996).
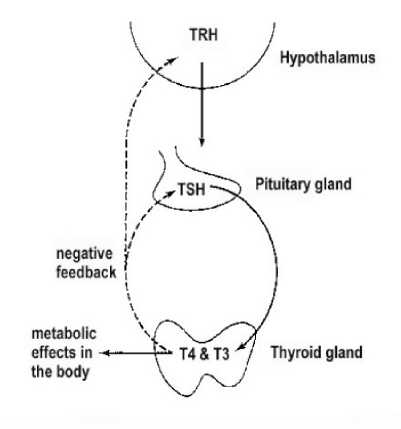
Figure. 1 The relationship of Thyroid-stimulating hormone and thyroidhormones
The tree shrew, Tupaia belangeri belongs to Scandebtia Tupaiidae. It is a unique species of small mammals in the Oriental realm and widely geographical distribution at Southern China, India, and Southeast Asia. T. belangeri is the widest of distribution and lives at the highest of latitude in their family, Yunnan-Kweichow Plateau mostly were its northern limit (Wang et al., 1991) and their habitat always was terrestrial, arboreal, mountainous forest and shrub areas. Early studies in our lab show that the physiological characters of T. belangeri show for example resting metabolic rate (RMR), nonshivering thermogenesis (NST) and the metabolism (Wang et al., 1994; 1995; Zhang et al., 2001) of T. belangeri showed robust seasonal cycles. Furthermore, administration of exogenous melatonin at physiological doses induced seasonal cycles of the thermogenesis in T. belangeri (Wang et al., 2000) and that thermogenesis in tree shrews was increased during cold exposure (Wang et al., 1995; Li et al., 2001b; Zhang et al., 2011; 2012a; 2012b).
In our laboratory early studies showed that the Tree shrews’ thermogenesis capacity will increased under cold acclimation (Wang et al., 1994; Zhang et al., 2001), here we measure thermogenic properties integratively from organismal to molecular levels including BAT mass, mitochondrial protein (MP) content, tri-iodothyronine (T 3 ), thyroxine (T 4 ) and cytochrome c oxidase (COX) activity from under cold acclimation T. belangeri and explore the potential role of COX, T 3 and T 4 in the regulation of thermogenesis. We hypothesize and predict that, similar to other small mammals, Tree shrews will change their thermogenesis by increase enzyme activity and hormone concentration under cold acclimation.
MATERIALS AND METHODS
Animals
The tree shrew, T. belangeri were captured (25°25'~26°22' N, 102°13'~102°57' E, 679 m in altitude) around boskage at Luquan County, Yunnan Province, China. After being captured, tree shrews were transported to the School of Life Science of Yunnan Normal University, Kunming, China (1910 m in altitude). Animals (24 males, 26 females), all healthy adults, each tree shrew was housed individually in a wire cages (40 cm × 40 cm × 40 cm) with no bedding, and were provided with natural illumination and 85%~92% relative humidity for 3 days, then experiment. The cold-exposed animals were maintained under 5±10C and 12L:12D (light : dark, lights on 08:00) photoperiod which contained 0 d (control), 7 d,14 d, and 21 d groups. There have 8 animals in every group. All pregnant, lactating or young individuals were excluded. They were fed the mixed food; the food mixture contained the following ingredients in proportion (by weight): 90 parts cornmeal, 5 parts milk, and 5 parts sugar, add a little water. The tree shrews were fed once daily at 12:00 h., additionally, with ratio of 3:1 every two days interval, we feed appropriate apples, pears and other fruits.
Energy budget
Energy budget were calculated by food equity (Rosenmann and Morrison, 1974), animal was house individually in a metabolic cage (20×15×15 cm3) without nest materials where food was provided in excess of the animals’ need and water was provided ad lib. After one week stablization, energy budget were measured and calculated. Animals were feeding fix quantify and time (AM: 10:00–11:00), next day weighted animal body mass, collected food uneaten, faeces. Residual food and faeces (about 1 g, precision: 0.0001 g) were dried in vacuum airier to get the consistent mass, the caloric value of the samples food were measured by YX-ZR/Q automatism calorimeters instrument (U-therm Industry Co. Ltd. Changsha, China). The calorie of the diet fed to these animals was 17.96±0.6 kJ/g. Gross energy intake was calculated by the equation: Gross energy intake (kJ/day) =Dry food intake (g/day) × caloric value (kJ/g) of dry food (Drozdz, 1975).
Metabolic rate
The level of RMR of T. belangeri , were determined in every experimental week before and after the breakfast using AD ML870 open respirometer. All the experimental animals’ sets were incubated in breath chamber with volume of 500ml with flow rate 200ml/min. The temperature was maintained 30 oC (The thermal neutral zone (TNZ) of T. belangeri was 30 - 35 oC, (Wang et al., 1994)) by SPX-300 artificial climatic engine (±0.5 X ) for 1.5 hrs and the metabolic rates were measured using ML206 gas analyzer. The animal’s body mass was measured by weight loss method and temperature was determined by portablethermometer before and after the experiment. The method used for calculating the metabolic rate is detailed in Hill (Hill 1972).
Maximum NST was defined as the maximum metabolic response to norepinephrine (NE) and was induced by a subcutaneous injection of NE at 30ºC (TNZ, thermal neutral zone). The mass-dependent dosage of NE (Shanghai Harvest Pharmaceutical Co. Ltd.) was calculated according to body weight (0.8 mg/Kg). Two continuous stable maximal recordings were used to calculate maximum NST. Oxygen consumption reached peak values within 15–30min after NE injection.
Mitochondria respiration
On day after metabolic measurement, the animals were killed sucrose-buffered medium, cleaned of any adhering tissue, blotted, and weighed, followed by homogenization for the isolation of mitochondria (1/15, w/v) with medium A (containing 250mM sucrose, 10mM TES, 1mM EDTA, 64 AM BSA, pH 7.2) (Cannon and Lindberg, 1979). The liver was weighed and followed by homogenization for the isolation of mitochondria
(1:15, w/v) with medium A (containing 250mM sucrose, 10mM TES, 1mM EDTA, 0.1% BSA, pH 7.2) (Cannon and Lindberg, 1979). The supernatant was then centrifuged at 8740g for 10min at 4 ºC, and the resulting pellet was resuspended (1:1, w/v) with ice-cold medium B (containing 100mM KCl, 20mM TES, 1mM EGTA, pH 7.2) and subsequently used for Western blotting. The protein content of mitochondria was determined by the Folin phenol method with bovine serum albumin as standard (Lowry et al., 1951). The state 3 and state 4 of mitochondrial respiration of liver and BAT were measured by Hanstech Oxy-Lab Chloroab 2 oxygen electrode.
Enzyme activity and hormone concentration
The COX activity of liver and BAT mitochondria was measured with the polarographic method using oxygen electrode units (Hansatech Instruments Ltd., England) according to Sundin et al. (1987), the α-glycerophosphate oxidase (α-PGO) was determined polarographically according to Steffen and Roberts (Steffen and Roberts, 1977). Thyroxin 5’-deiodinase (T 4 5’-D II) activityin BAT was assayed as previously described (Leonard et al. 1983). Serum thyroid stimulating hormone (TSH), triiodothyronine (T3) and thyroxine (T4) concentrations were quantified by radioimmunoassay using RIA kits (China Institute of Atomic Energy, Beijing). Antigen for T 3 and T 4 was labeled with 125NaI. The kits were validated for all species tested by cross activity, which showed a parallel linear relationship.
Western bloting
UCP1 content was measured by Western blotting as described previously (Li and Wang, 2005). Total BAT protein (15 mg per lane) was separated in a discontinuous SDS–polyacrylamide gel (12.5% running gel and 3% stacking gel) and blotted to a nitrocellulose membrane (Hybond-C, Amersham). To check for the efficiency of protein transfer, gels and nitrocellulose membranes were stained after transferring with Coomassie brilliant blue and Ponceau red, respectively. Unspecific binding sites were saturated with 5% nonfat dry milk in PBS. UCP1 was detected using a polyclonal rabbit anti-hamster UCP1 (1:5000) as a primary antibody and peroxidase-conjugated goat antirabbit IgG (1:5000) (JacksonImmuno. Inc., USA) as the second antibody. Enhanced chemoluminescence (ECL, Amersham Biosciences, England) was used for detection. UCP1 concentration was determined from area readings using Scion Image Software (Scion Corporation) and was expressed as relative units (RU) (Li and Wang, 2005).
Statistical analysis
Statistical analyses were performed using sigmaplot10.0, SPSS for Windows15.0 statistical package. All data are expressed as the means ± SE, P < 0.01 and P < 0.05 were considered to be statistically significant.
RESULTS
Body mass and energy metabolism
Results presented in Table 1 showed that the body mass in all tree shrews were not markedly affected after cold acclimation in 2 weeks, but it has a significantly difference after 3 weeks ( P <0.05).
Energy intake in T. belangeri increased during cold acclimation after 21 days (Fig. 2). Relation between energy intake and cold acclimation showed significant correlation ( F 3, 24 =14.83, P <0.01). In the three weeks of cold acclimation, the gross energy intake was 22.8% higher than control.
RMR and NST
The RMR (ml O 2 /g h) was significantly affected by low temperature ( F 3, 24 =26.313, P <0.01), and LSD tests showed that the RMR increased markedly and enhanced 55.43% after 3 weeks. The NST (ml O 2 /g h) was significantly affected by low temperature ( F 3, 24 =9.319, P <0.01), and LSD tests showed that the NST significantly increased 24.2% after 3 weeks acclimated to cold, respectively (Fig. 3). Using AOCOVA, the result shown the no significant impact of BM on BMR ( F 3, 24 =0.451 , P >0.05) and on NSTmax ( F 3, 24 =0.007, P >0.05).
Liver: wet mass, protein content, mitochondrial respiration, activity of cytochrome C oxidase and α-glycerophosphate oxidase
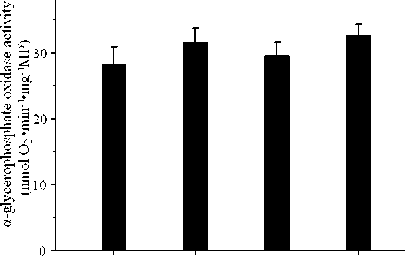
The effect of the thermogenic abilities in liver of tree shrew during cold exposure was shown in Table 1. The increase of liver mass showed significant correlation ( P <0.01) compared 21 days to control group, the mass of liver increased 65.3%; the relative mass (LW/BW) increased 60.7% compared to control group. Content of total protein and content of mitochondrial protein in liver at 21 days increased 30.6% and 33.0% relative to control group, respectively. State 3 (nmol O 2 /mg mitochondrial protein min) and state 4 (nmol O 2 /mg mitochondrial protein min) of mitochondrial respiration in liver increased 69.1% and 99.0% at 21 days of acclimation group, respectively. The activities of COX (ng O 2 /min mg MP) and α-PGO (nmol O 2 /mg min MP) in acclimation group increased 99.2% and 15.2% after 21 days, respectively.
Brown adipose tissue (BAT): wet mass, protein content, Thyroxin 5’-deiodinase (T4 5’-D II), UCP1 and activity of cytochrome C oxidase
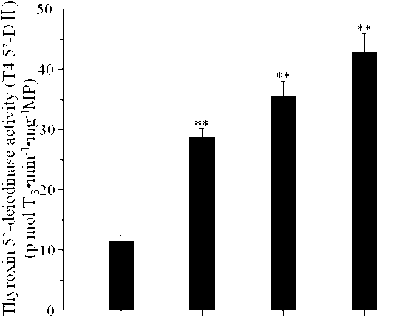
The effect of the thermogenic abilities in BAT of tree shrews during cold exposure were displayed in Table 1. The mass of BAT increased 60.5%; the relative mass (BAT/BW) increased 18.2% after 21 days cold acclimation in acclimation group than control group, and the content of total protein and content of mitochondrial protein in liver increased 49.6% and 67.0%, respectively. State 4 (nmol O2/min mg MP) of mitochondrial respiration in BAT increased 64.3%. Content of UCP1 (mg/100g body mass) increased 42.7%. The activities of COX(ng O2/min mg MP), α-PGO (nmol O2/mg min MP) and T4 5’-D И (p mol Timin'1 •mg-1MP) increased 60.8%, 177.5% and 272.2% after 21 days, respectively (Fig. 4).
Serum T3, T4 and Thyroid stimulating hormone (TSH)
Under cold acclimation, serum T 3 concentrations increased significantly, but T 4 concentration decreased. The level of serum thyroid hormones of T. belangeri were significant effects during cold acclimation (Table 2). Control tree shrews averaged 36.95±3.82 ng/ml serum T4, 0.58±0.05 ng/ml serum T3 and 0.127±0.014 ng/ml serum Thyroid stimulating hormone (TSH). The level of T4 decreased 22.8% in cold acclimation group at 21 days compared with control group; while the level of T3 increased 187.9%, T3/T4 increased 211.8% in cold acclimation group at 21 days compared with control group, the level of TSH increased 17.3% (Table 2).
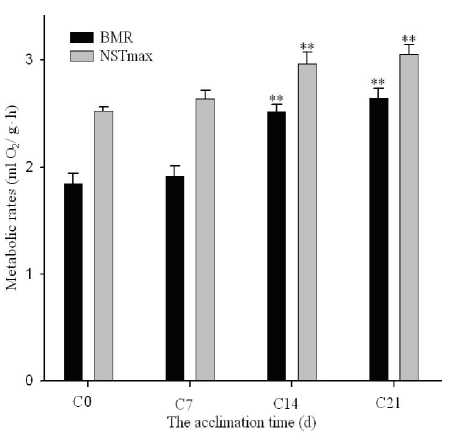
Figure 2. Effects of cold acclimation on BMR and NSTmax of Tree shrews.
Data are means ±SEM. C0=before cold acclimation; C7, C14, and C21 = days of cold acclimation.
** Vs. control ( P < 0.01).
40 -1
CO C7 C14 C21
Hie acclimation tune (d)
Figure 3. Effects of cold acclimation on α-glycerophosphate oxidase activity content in Tree shrews. Data are means ± SEM. C0=before cold acclimation; C7, C14, and C21 = days of cold acclimation.
CO С" C14 C21
The acclimation time (d)
Figure 4. Biochemical parameters of seedling under different pH and iron dust exposure after 15 day of sowing.
Table 1 Effects of cold acclimation on mitochondrial protein (MP) and cytochrome C oxidase (COX) in the Tree shrews (means ± SE)
|
Parameters |
C0 |
C7 |
C14 |
C21 |
Increasing ratio(%) |
|
Sample |
8 |
8 |
7 |
6 |
|
|
Mass (g) |
122.1±3.8 |
125.6±2.7 |
127.5±5.4 |
130.6±5.0* |
6.96 |
|
Brown adipose tissue (BAT) |
|||||
|
Mass (g) |
0.43±0.03 |
0.48±0.04 |
0.59±0.03 |
0.69±0.02 |
60.5 |
|
% body mass |
0.44±0.03 |
0.48±0.05* |
0.49±0.03* |
0.52±0.07** |
18.2 |
|
TP (mg·g-1) |
23.4±1.2 |
29.7±2.0* |
30.7±1.4** |
35.0±3.1** |
49.6 |
|
MP (mg·g-1) |
9.1±0.9 |
10.6±0.9 |
13.4±1.7* |
15.2±1.6** |
67.0 |
|
COX (ng O 2 ·min-1 ·mg-1MP) |
975±76 |
1088±99.4 |
1280±118.9 |
1568±148 |
60.8 |
|
Liver |
|||||
|
Msaa (g) |
4.38±0.23 |
5.83±0.32* |
6.51±0.51** |
7.24±0.23** |
65.3 |
|
% body mass |
3.69±0.8 |
4.64±0.17* |
5.11±0.42** |
5.93±0.61** |
60.7 |
|
TP (mg·g-1) |
95.2±4.9 |
108.5±6.2* |
118.4±3.7** |
124.3±5.4** |
30.6 |
|
MP (mg·g-1) |
31.5±2.2 |
32.7±1.5 |
36.1±2.2* |
41.9±3.1** |
33.0 |
|
COX (ng O 2 ·min-1 ·mg-1MP) |
78.0±6.1 |
109.8±8.6** |
138.7±6.4** |
155.4±6.3** |
99.2 |
* Vs. control ( P < 0.05); ** vs. control ( P < 0.01). C0=before cold acclimation; C7, C14, and C21 = days of cold acclimation.
|
Parameters |
C0 C7 C14 C21 Increasing ratio (%) |
|
Sample |
88 7 6 |
|
TSH (ng·ml-1) |
0.127±0.01 0.147±0.012 0.183±0.012* 0.149±0.006 17.3 4 |
|
T 3 (ng·ml-1) T 4 (ng·ml-1) |
0.58±0.05 1.07±0.01** 1.35±0.05** 1.67±0.10** 187.9 36.95±3.82 35.88±2.73 32.54±1.60 28.54±2.34* -22.8 |
|
T 3 /T 4 (×100) |
0.017±0.00 0.4031±0.002 0.049±0.004* 0.053±0.003* 1 * * * 211.8 |
* Vs. control ( P < 0.05); ** vs. control ( P < 0.01). C0=before cold acclimation; C7, C14, and C21 = days of cold acclimation.
DISCUSSION
Ambient temperature plays an important role in mediating animals’ physiology and behaviors. It is known that variation in body mass of small mammals is relative to adaptation to geographical and climatological events. It has been demonstrated that several small mammals respond to winter-associated environmental cues by reducing body mass, such as Siberian hamsters, prairie voles (Microtus ochrogaster), and meadow voles (Microtus pennsylvanicus) (Peacock et al., 2004). However, our present results showed that cold temperature is an important environmental cue that has T. belangeri to increase their body mass significantly; different species in small mammals had different space-time reaction to optimal physiological level. Body mass increased in winterlike conditions is considered to be an adaptive mechanism for the reduction of absolute heat loss when stress occurs. There are two opposite evolutionary response about the energy budget of small animals in winter. The hibernating mammals increase the body mass by storing the energy in the form of lowering metabolic rate in autumn (Nagy et al., 1995). This means, T. belangeri storing the energy by developing their body mass in order to survive in cold environment. Body mass in T. belangeri gradually decreased during the cold acclimation, the maximal difference appeared in 7 days, after 7 days, body mass start to rise, which might indicate that E. miletus has used to be the new environmental conditions and adjusted to a new physiological level in cold acclimation. However, an increase in body mass will decrease the ratio of surface-to-volume, which can decrease heat loss, and thus reduce energy consumption, which is support the heat dissipation limitation hypothesis (Duarte et al., 2010).
The variations in body mass were associated with changes in energy intake and expenditure. It is evident that many winter-active small mammals enhance RMR and NST for survival in the cold (Lovegrove, 2003). It is evident that many winteractive small mammals enhance BMR and NST for survival in the cold (Lovegrove, 2003). In the present study, BMR and NST increased significantly during cold acclimation, which was consistent with other rodents, such as short-tailed voles (McDevitt and Speakman, 1994), root voles ( Microtus oeconomus )(Wang et al., 1996), Mongolian gerbils ( Meriones unguiculatus )(Li and Wang, 2005), and Siberian hamsters ( Phodopus sungorus )(Wiesinger et al. 1990).
The changes in thermogenesis at the whole animal level were further supported by other biochemical markers examined in the present study, including the mitochondrial protein content, COX activity, and UCP1 content. Further, liver, as an important energy-expending organ, is considered to make a large contribution to BMR (Selman et al., 2001). Liver thermogenesis accounts for 20 - 25% of RMR (Couture and Hulbert, 1995), interspecific differences in metabolic intensity are linked with differences in mitochondrial densities, oxidative capacities and mitochondrial proton leaks. A strong correlation between metabolic rate and mitochondrial respiration and leak has been reported (Porter and Brand, 1993; Brookes et al., 1998). In our study, liver COX activity under cold acclimation was significantly increased (after cold acclimation, the activities of COX and α-PGO increased 99.2% and 15.2%, respectively), which was consistent with the changes in BMR. These data suggested that changes of BMR could be at least partially due to changes of mitochondrial respiration of the liver. Conversely, liver COX activity might potently underlie the changes of BMR induced by photoperiodic changes.
Tree shrews showed increased NST in winter as adaptation to seasonal declines in environmental temperature. Enhancement of thermogenic capacity is generally indicated by increases in serum thyroid hormone levels (Tomasi and Mitchell, 1996; Li et al., 2001a), BAT COX activity (Heldmaier and Buchberger, 1985) and UCP1 expression (Li et al., 2001b; von Praun et al., 2001; Jakus et al., 2002). UCP1 mRNA expression and production in BAT may be indicative of the thermogenic capacity (Cannon and Nedergaard, 2004), and the thermoregulatory role of UCP1 has been emphasized in UCP1-deficient mice, whose resistance to cold is impaired (Nedergaard et al., 2001b). In our study, BAT COX activity under cold acclimation was significantly increased (after cold acclimation, the activities of
COX increased 272.2%), which was consistent with the changes in NST, and the UCP1 content increased markedly in the cold condition. The cold-induced increase in BAT UCP1 expression was also found in Siberian hamsters (von Praun et al., 2001), Mongolian gerbils, and ground squirrels ( Spermophilus dauricus ) (Li et al., 2001a). The increased energy expenditure in the cold can be compensated by increasing in energy intake and mobilizing of the body reserves. Data from the present study showed that T. belangeri could increase energy intake by 22.8% after 28 days. Cold-induced hyperphagia is a common feature in other rodents, such as rats, mice (Bing et al., 1998), and Alaskan collared lemmings ( Dicrostonyx groenlandicus ) (Maier and Feist, 1991).
A growing body of evidence suggests that thyroid hormone is an important determinant of basalmetabolic rate (Kim, 2008), endogenous variation of thyroxine levels appears to correlate with metabolic rate in both endotherms (Tomasi, 1991) and ectotherms (Steyermark et al., 2005). The function of thyroid hormones is to simulate cell to produce thermogenesis (McNab, 1992), the change of the level of thyroid hormones can reflect the thermogenesis characters in low temperature (Tomasi and Mitchell, 1994). During cold acclimation, T4 decreased or hold a same level, but T3 increased, it is adapt to the increased of thermogenesis (Jordan, 1995), such as Mesocricetus auratus (Tomasi and Horwitz, 1987), Simodon hispidus (Tomasi and Mitchell, 1994). Because of the utilizing T4, T4 decreased during cold acclimation (Tomasi, 1991), this changes come down to complicated physiological regulative mechanism (Tomasi and Horwitz, 1987), and T4 can be converted to T3 in peripheral tissues by deiodinases, such as Type II iodothyronine 5P- deiodinase (DII) in BAT (Lanni et al., 2003). TSH production is controlled by thyrotropin-releasing hormone (TRH), which is manufactured in the hypothalamus and transported to the anterior pituitary gland via the superior hypophyseal artery, where it increases TSH production and release. The level of thyroid hormones (T3 and T4) in the blood has an effect on the pituitary release of TSH; when the levels of T3 and T4 are low, the production of TSH is increased, and, on the converse, when levels of T3 and T4 are high, TSH production is decreased. In our study, BAT T4 5’-D И activity under cold acclimation was significantly increased (after cold acclimation, the activities of T4 5’-D И increased 272.2%), the level of TSH increased 17.3%, the level of T4 decreased 22.8% compared 21days to control group, and the level of T3 increased 187.9% compared 21days to control group, T3/ T4 rate increased 211.8%.
Environmental cues play an important role in the mediation of seasonal adaptation of body mass, thermogenesis, and energy intake in wild small mammals. In our present study, we found that several physiological, hormonal and biochemical variables indicative of thermogenic capacity, such as BMR, COX activity and thyroidhormones, the changes of thermogenic capacity of cold stress, suggesting that low temperature changes may provide cues altering adaptive thermogenesis, and our data show that variations in the activity of thyroid hormones (T3/T4) and the metabolic biochemical markers of tissues were strongly correlated with variations of BMR in Tree shrews, suggesting that those features may play an important role in the determination of BMR variations. We need to know further the differences in proton conductance, uncoupling proteins (UCPs), adenine nuclide translocase (ANT) contents, and the membrane composition in it, all of which can affect the basal proton leak of tissues (Shabalina et al., 2006; Brookes et al., 1998). These data suggest that temperature changes play an important role in the regulation of thermogenic capacity in Tree shrews.
ACKNOWLEDGMENTS
We are grateful to all the members of Animal physiological ecology group and Yunnan biological energy and environmental biotechnology innovation team for their help on the experiment. This study was financially supported by the NSFC (No.31071925), the Basic Program of Yunnan Province (No.2007C0005Z1), and the Basic Program of Yunnan Province Education Department (No.ZD2009007) to Z-K Wang. We also thank the suggestions of the other anonymous reviewers, and helped by Yunnan biological energy and environmental biotechnology innovation team. We also thank the suggestions of the other anonymous reviewers.
Список литературы Enzyme activity, hormone concentration in tree shrew (Tupaia belangeri) during cold acclimation
- Bing C, Frankish HM, Pickavance L, Wang Q, Hopkins DF, Stock MJ, Williams G. 1998. Hyperphagia in cold exposed rats is accompanied by decreased plasma leptin but unchanged hypothalamic NPY. American Journal of Physiology 274: 62-68.
- Brookes PS, Buckingham JA, Tenreiro AM, Hulbert AJ, Brand MD. 1998. The proton permeability of the inner membrane of liver mitochondria from ectothermic and endothermic vertebrates and from obese rats: correlations with standard metabolic rate and phospholipid fatty acid composition. Comparative of Biochemical and Physiology 119B: 325-334.
- Cannon B, Lindberg O. 1979. Mitochondria from brown adipose tissue: isolation and properties. Methods Enzymol 55: 65-78.
- Cannon B, Nedergaard J. 2004. Brown adipose tissue: function and physiological significance. Physiol Rev 84: 277-359.
- Cannon B, Hedin A, Nedergaard J. 1982. Exclusive occurrence of thermogenin antigen in brown adipose tissue. FEBS Lett 150: 129-32.
- Cooper CE, Withers PC. 2002. Metabolic physiology of the numbat (Myrmecobius fasciatus). Journal of Comparative Physiology 172B: 669-675.
- Couture P, Hulbert AJ. 1995. Relationship between body mass, tissue metabolic rate, and sodium pump activity in mammalian liver and kidney. American Journal of Physiology 268: R641-R650.
- Drozdz A. 1975. Metabolic cages for small rodents. Pp. 346-351. In Grodzinski W, Klekowski RZ, Duncan A, eds. Methods for Ecological Bioenergetics. Oxford: Blackwell Scientific Press.
- Duarte LC, Vaanholt LM, Sinclair RE, Gamo Y, Speakman JR. 2010. Limits to sustained energy intake XII: is the poor relation between resting metabolic rate and reproductive performance because resting metabolism is not a repeatable trait. J Exp Biol 213: 278 -287.
- Feist DD, Rosenmann M. 1976. Norepinephrine thermogenesis in seasonally acclimatized and cold acclimated red-backed voles in Alaska. Canadian Journal of Physiology and Pharmacology 54(2): 146-153.
- Foster DO, Frydman ML. 1978. Nonshivering thermogenesis in the rat. II. Measurements of blood flow with microspheres point to brown adipose tissue as the dominant site of the calorigenesis induced by noradrenaline. Canadian Journal of Physiology and Pharmacology 56: 110-122.
- Heldmaier G., Buchberger A. 1985. Sources of heat during nonshivering thermogenesis in Djungarian hamsters: a dominant role of brown adipose tissue during cold adaptation. J Comp Physiol 156B: 237-245.
- Heldmaier G, Klaus S, Wiesinger H. 1990. Seasonal adaptation of thermoregulatory heat production in small mammals. Pp. 235-243. In Blighand J, Voightm K. eds. Thermoreception and Temperature Regulation. Heidlberg: Springer-Verlag.
- Hill RW. 1972. Determination of oxygen consumption by use of the paramagnetic oxygen analyzer. J Appl Physiol 33: 261-263.
- Himms-Hagen J. 1990. Brown adipose tissue thermogenesis: role in thermoregulative, energy regulation and obesity. Pp. 327-414. In Schonbaum E, Lomax P. eds. Physiology and Biochemistry. Pergamon Press Inc, USA.
- Jakus PB, Sipos K, Kispal G, Sandor A. 2002. Opposite regulation of uncoupling protein 1 and uncoupling protein 3 in vivo in brown adipose tissue of cold-exposed rats. FEBS Lett 519: 210-214.
- Jansky L. 1973. Non-shivering thermogenesis and its thermoregulatory significance. Biological Reviews 48: 85-132.
- Jordan D. 1995. Temperature regulation in laboratory rodents. Journal of Anatomy 186(Pt1): 228.
- Kim B. 2008. Thyroid hormone as a determinant of energy expenditure and the basal metabolicrate. Thyroid 18: 141-144.
- Kadenbach B, Hüttemann M, Arnold S, Lee I, Bender E. 2000. Mitochondrial energy metabolism is regulated via nuclear-coded subunits of cytochrome c oxidase1. Free Radical Biology and Medicine 29: 211-221.
- Krotkiewski M. 2002. Thyroid hormones in the pathogenesis and treatment of obesity. Eur J Pharmacol 440: 85-98.
- Lanni A, Moreno M, Lombardi A, Goglia F. 2003. Thyroid hormone and uncoupling proteins. FEBS Lett 543: 5-10.
- Leonard JL, Mellen SA, Larsen RP. 1983. Thyroxine 5'-deiodinase activity in brown adipose tissue. Endocrinology 112: 1153-1155.
- Li QF, Sun RY, Huang CX, Wang ZK, Liu XT, Hou JJ, Liu JS, Cai LQ, Li N, Zhang SZ, Wang Y. 2001a. Cold adaptive thermogenesis in small mammals from different geographical zones of China. Comparative Biochemistry and Physiology 129A: 949-961.
- Li QF, Liu XT, Huang CX, Sun RY, Lin QS. 2001b. Thermogenic capacity and expression of uncoupling protein gene of brown adipose tissue from mongolian gerbils Meriones unguiculatus during cold acclimation. Acta Zoologica Sinica 47: 388-393.
- Li XS, Wang DH. 2005. Regulation of body weight and thermogenesis in seasonally acclimatized Brandt's voles (Microtus brandti). Hormones and behavior 48: 321-328.
- Lovegrove BG. 2003. The influence of climate on the basal metabolic rate of small mammals: a slow-fast metabolic continuum. Journal of Comparative Physiology 173B: 87-112.
- Lowell BB, Spiegelman BM. 2000. Towards a molecular understanding of adaptive thermogenesis. Nature 404: 652-660.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. 1951. Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265-75.
- Maier HA, Feist DD. 1991. Thermoregulation, growth, and reproduction in Alaskan collared lemmings: role of short day and cold. Am J Physiol 261: R522-R530.
- McDevitt RM, Speakman JR. 1994. Limits to sustainable metabolic rate during transient exposure to low temperatures in short-tailed field voles (Microtus agrestis). Physiological Zoology 67: 1103-1116.
- McNab KB. 1992. A statistical analysis of mammalian rates of metabolism. Functional Ecology 6: 672-679.
- Merritt JF, Zegerts DA. 1991. Seasonal thermogenesis and body-mass dynamics of Clethrionomys gapperi. Canadian Journal of Zoology 69: 2771-2777.
- Nagy TR, Gower BA, Stetson MH. 1995. Endocrine or relates of seasonal baby mass dynamics in the collared lemming Dicrostonyxgroen landicus. American Zoologist 35: 246-258.
- Nedergaard J, Golozoubova V, Matthias A, Asadi A, Jacobsson A, Cannon B. 2001a. CP1: the only protein able to mediate adaptive non-shivering thermogenesis and metabolic inefficiency. Biochem Biophys Acta 1504: 82-106.
- Nedergaard J, Golozoubova V, Matthias A, Shabalina I, Ohba KL, Ohlson K, Jacobsson A, Cannon B. 2001b. Life without UCP1: mitochondrial, cellular and organismal characteristics of the UCP1-ablated mice. Biochemical Society Transactions 29: 756-763.
- Oufara S, Barré H, Rouanet JL, Minaire Y. 1988. Great adaptability of brown adipose tissue mitochondria to extreme ambient temperature in control and cold-acclimation gerbils as compared with mice. Comp Biochem Physiol 90B: 209-214.
- Peacock WL, Król E, Moar KM, McLaren JS, Mercer JG, Speakman JR. 2004. Photoperiodic effects on body mass, energy balance and hypothalamic gene expression in the bank vole. J Exp Biol 207: 165-177.
- Porter RK, Brand MD. 1993. Body mass dependence of H+ leak in mitochondria and its relevance to metabolic rate. Nature 362: 628-630.
- Ricquier D, Bouillaud F. 2000. Mitochondrial uncoupling proteins: from mitochondria to the regulation of energy balance. J Physiol 529: 3-10.
- Ronald S, McPherson FA. 2000. Wildmann's Clincal Interpretation of Laboratory Tests. 11th ed. Davis Company, Philadelphia.
- Rosenmann M, Morrison P. 1974. Maximum oxygen consumption and heat loss facilitation in small homeotherms by He-O2. Am J Physiol 226: 490-495.
- Selman C, Lumsden S, Bunger L, Hill WG, Speakman JR. 2001. Resting metabolic rate and morphology in mice (Mus musculus) selected for high and low food intake. J Exp Biol 204: 777-784.
- Shabalina IG, Kramarova TV, Nedergaard J, Cannon B. 2006. Carboxyatractyloside effects on brown-fat mitochondria imply that the adenine nucleotide translocator isoforms ANT1 and ANT2 may be responsible for basal and fatty-acid-induced uncoupling respectively. Biochemical Journal 399: 405-414.
- Steffen JM, Roberts JC. 977. Temperature acclimation in the Mongolian gerbil (Meriones unguiculatus): biochemical and organ weight changes. Comp Biochem Physiol 58B: 237-243.
- Steyermark AC, Miamen AG, Feghahati HS, Lewno AW. 2005. Physiological and morphological correlates of among-individual variation in standard metabolic rate in the leopard frog Rana pipiens. Journal of Experimental Biology 208: 1201-1208.
- Sundin U, Moore G, Nedergaard J, Cannon B. 1987. Thermogenin amount and activity in hamster brown fat mitochondria: effect of cold acclimation. American Journal of Physiology 252: R822-832.
- Tomasi TE, Horwitz BA. 1987. Thyroid function and cold acclimation in the hamster, Mesocricetus auratus. American Journal of Physiology 252: 260-267.
- Tomasi TE, Mitchell DA. 1994. Seasonal shifts in thyroid function in the cotton rat (Sigmodon hispidus). Journal of Mammalogy 75(2): 520-528.
- Tomasi TE, Mitchell DA. 1996. Temperature and photoperiod effects on thyroid function and metabolism in cotton rats (Sigmodon hispidus). Comparative Biochemistry and Physiology 113A: 267-274.
- Tomasi TE. 1991. Utilization rates of thyroid hormones in mammals. Comparative Biochemistry and Physiology 100A: 503-516.
- von Praun C, Burkert M, Gessner M, Klingenspor M. 2001. Tissuespecific expression and cold-induced mRNA levels of uncoupling proteins in the Djungarian hamster. Physiological and Biochemical Zoology 74: 203-211.
- Wang DH, Sun RY, Wang ZW, Liu JS. 1999. Effects of temperature and photoperiod on thermogenesis in plateau pikas (Ochotona curzoniae) and root voles (Microtus oeconomus). Journal of Comparative Physiology 169B: 77-83.
- Wang DH, Sun RY, Wang ZW, Liu JS, Chen Z. 1996. Adaptive thermogenic properties during cold exposure in root voles (Microtus oeconomus). Acta Zoologica Sinica 42(4): 369-397.
- Wang YX, Li CY, Ma SL. 1991. The classification and ecology of treeshrew. Kunming: Yunnan Scientic and Technological Press.
- Wang ZK, Sun RY, Li QF. 1994. Characteristics of the resting metabolic rate of the treeshrews. Journal of Beijing Normal University (Natural Science) 30(3): 408-414.
- Wang ZK, Li QF, Sun RY. 1995. Characteristics of the nonshivering thermogenesis and cellular respiration in the tree shrews. Zool Res 16 (3): 239-248.
- Wang ZK, Li QF, Sun RY. 2000. Effects of exogenous metatonin on the adaptive thermogenesis in the tree shrews (Tupaia belangeri). Acta Zool Sin 46 (2): 54-159.
- Wiesinger H, Klaus S, Heldmaier G, Champigny O, Ricquier D. 1990. Increased nonshivering thermogenesis, brown fat cytochrome-c oxidase activity, GDP binding, and uncoupling protein mRNA levels after short daily cold exposure of Phodopus sungorus. Can J Physiol Pharmacol 68: 195-200.
- Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM. 1999. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98: 115-124.
- Zhang L, Wang R, Zhu W, LP, Cai J, Wang Z, Sivasakthive S, Lian X. 2011. Adaptive thermogenesis of the liver in tree shrew (Tupaia belangeri) during cold acclimation. Animal Biology 61: 385-401.
- Zhang L, Liu P, Zhu W, Cai J, Wang Z. 2012a. Variations in thermal physiology and energetics of the tree shrew (Tupaia belangeri) in response to cold acclimation. Journal of Comparative Physiology 182B: 167-176.
- Zhang L, Zhang H, Zhu W, Li X, Wang Z. 2012b. Energy metabolism, thermogenesis and body mass regulation in tree shrew (Tupaia belangeri) during cold acclimation and rewarming. Comparative Biocheimal and Physiology 162A: 437-442
- Zhang WX, Wang ZK, Xu WJ. 2001. The Effects of Cold Acclimation on the metabolism of energy in treeshrews (Tupaia belangeri). Acta Theriologica Sinica 22(2): 123-129.


