Euhalophyte eryngium maritimum L.: the microstructure and functional characteristics
Автор: Ivanova A.P., Tsonev T.D., Peeva V.N., Maslenkova L.T., Najdenski H.M., Tsvetkova I.V., Babenko L.M., Shcherbatiuk M.M., Sheiko O.A., Kosakivska I.V.
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 3 т.11, 2015 года.
Бесплатный доступ
The microstructure of the leaf surface, lipids composition, pigments spectrum, biological and photosynthetic activity, and hormones status of euhalophyte Eryngium maritimum L., one of the most salt-tolerant plants, were studied. It was shown that the existence in saline and dry soils is provided among others adaptive mechanisms by specific microstructure of leaf, adaxial and abaxial surfaces of which have well-developed cuticle and stomata slit placed below the surface of the epidermis. The presence of a large amount of saturated fatty acids provides decrease of membrane permeability and better resistance against soil salinity. The key role in photosynthetic activity is played by chlorophyll a. At the same time a high amount of carotenoids (as compared with amount of chlorophylls) points out that these pigments have a light-collecting function and could transfer an additional energy to chlorophylls. The data from thermoluminescence analyses showed a possible inhibition of recombination reactions in PS II by the assumed saline concentration in plant tissue. Besides, the fluorescence measurements indicate reduced efficiency of photosynthetic reactions. The high level of active ABA is correlated with salt tolerance and ability to survive and grow in stress conditions. The high level of conjugated form of IAA demonstrated that activity of this hormone is limited.
Eryngium maritimum l, lipids, microstructure, phytohormones, pigments
Короткий адрес: https://sciup.org/14323927
IDR: 14323927
Текст научной статьи Euhalophyte eryngium maritimum L.: the microstructure and functional characteristics
Saline soils occupy 25% of the earth's surface. With optimal amounts salt functions as mineral nutrition, while at high concentrations it becomes a stress factor. High concentrations of salts have toxic effect; adversely affect the water balance of the plant. Root outer cells, which are in direct contact with the salt are damaged. Stem cells conduction system, in which the solution of salts moves to aboveground organs is damaged. Halophytes – plants that are evolutionarily formed in saline soils and are adapted to living in such conditions, can withstand salinity level of 300 mmol. Unlike hlykophytes – plants from unsoiled places, halophytes are adapting to life in excess salt concentration. The aim of our study was to examine the microstructure of the leaf surface, lipids composition, pigment spectrum, biological and photosynthetic activity and hormones status of euhalophyte Eryngium maritimum L., which is one of the most salt-tolerant plants that can accumulate in vacuoles up to 10% of the water content of the whole plant.
MATERIALS AND METHODS
Eryngium maritimum L. is a typical perennial plant of coastal dunes: in open sand at the tops of shores, on the strandline, in foredunes, in more stabilized open sandy turf. It occurs also on shingle and sometimes on coastal wasteground. It is a member of the carrot family (Apiaceae, formerly Umbelliferae), but the flowers are aggregated into a dense, rounded inflorescence rather than the flat-topped umbels so typical of the family (Minasiewicz et al., 2011). It shows a number of xeromorphic adaptations to survive water-loss, including its spiny leaves and bracts to deter grazing animals and its thick, waxy cuticle. The root system is able to grow down a miter or more into the sand. The cuticle probably also protects the plant from the erosive effects of blown sand. Like some other psammophytes (plants of open, sandy habitats) it is able to grow up through accreted sand, the shoot system being stimulated into renewed growth by burial. E. maritimum inhabits at Black Sea and Mediterranean Sea regions. Recently it is subjected to severe habitat fragmentation, due to tourism activities and urban pressures and now is entered in the Red Data Book of Bulgaria. E. maritimum is used as folk remedy for the treatment of various inflammatory disorders and in oncology. It has relatively strong antioxidative activity and possesses antimicrobial activities against bacterial and yeast strains (Meot-Duros et al., 2008). The biological activity of E. maritimum seems to be due of sesquiterpens in its essential oil (Darriet et al., 2014).
Samples of E. maritima were collected in August 2014 from the sand dunes near the Pomorie Lake (soil salinity 330-350 mg salts in 100g soil).
The leaf surface was studied by means of the scanning electron microscope (JEOL JSM-6060 LA). Plant material was fixed in ethyl alcohol at 70°. Fixed samples were dehydrated in ethyl alcohol solution with increasing concentrations. Following the treatment with absolute alcohol, samples were placed on brass objective tables and adhered there using adhesive tape, kept there for some time to reach air-dry condition and covered with a layer of gold in the ion coating chamber to provide conductivity. Structure dimensions on micro-photos were measured using the program UTHSCSA Image Tool 3.0, involving a scale bar set up by the instrument on picture.
For lipid analysis small peaces from the aerial parts were extracted consecutively with chloroformmethanol (2:1) and n-butanol to obtain the total lipophylic and n-butanol extracts. The isolation of the main lipid classes and the analysis of their fatty acid composition were performed using thin-layer and gas chromatographic techniques (Christie, 2010). The antibacterial and antifungal activity was determined according to (Spooner, Sykes, 1972).
Photosynthetic pigments were extracted with 80% aqueous acetone (Wellburn, 1994). Spectra were recorded using a spectrophotometer Spekord M-40 (Germany).
Photosynthetic activity was detected by using methods of chlorophyll fluorescence and thermoluminescence. Thermoluminescence was measured in darkness by using a home-made setup described elsewhere (Zeinalov and Maslenkova, 1996). Dark adapted plant leaves segments were placed on a sample holder and covered with a thin plastic membrane under a weak safe green light. After cooling the sample, an excitation by two single saturated turnover flashes (10 µs half-band width, 1 Hz frequency) was done at low temperature of 1oC, and the sample was being warmed with a constant rate of 0.6oC/s. Thermoluminescence was detected by Hamamatsu HR943-02, (Hamamatsu Photonics, Japan) and registered with computer software. The signals decomposition to B and AG bands was done by using Origin Lab 7.5 (Microcal, USA).
Modulated chlorophyll fluorescence was measured by a FMS fluorimeter (Hansatech Instruments, Norfolk, UK) in dark and light adapted leaves. Samples were dark adapted for 30 minutes before registration the initial (F 0 ) and maximal (F m ) fluorescence and then exposed to 300 μmol m-2 s-1 PPFD white actinic light. After reaching steady state of the fluorescence signal (F), a short saturating pulse was applied followed by far-red illumination and the parameters at light adapted condition F m ' and F 0 ' were measured. The following indices were calculated: maximal quantum yield of PSII F v /F m =(F m -F 0 )/F m , effective quantum yield Φ PSII =(F m '-F)/F m ', nonphotochemical quenching NPQ=(F m - F m ')/F m '.
Extraction of hormones and determination of free and conjugated forms of abscisic acid (ABA) and indole-3-acetic acid (IAA) were performed according to the method (Kosakivska et al., 2014). Qualitative and quantitative analysis of ABA and IAA were performed using high performance liquid chromatography (HPLC) on a liquid chromatograph Agilent 1200 LC system with diode array detector G 1315 B (USA), column Eclipse XDB-C 18, with the parameters 4.6 x 150 mm, size of particles - 5 microns. IAA and ABA were determined at 280 and 254 nm respectively. Elution of hormones was performed at a rate 0,5 ml/min in the solvent system methanol: water: acetic acid (59: 40: 1) in online regime. We use un-labeled IAA and (±) cis-, transABA (Sigma, USA) and the standard addition method of quantification. Chromatograms were calculated using the software ChemStation (version 3.1 V.) in offline mode. Experiments were carried out in three biological and five analytical replicates. Digital materials were processed statistically using the programs MS Excel 2003 and Origin 6.0. Significant differences were assessed by Student's criterion, using a 5% level of significance (P≤0,05).
RESULTS AND DISCUSSION
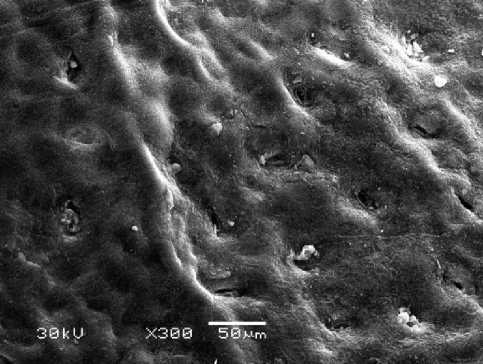
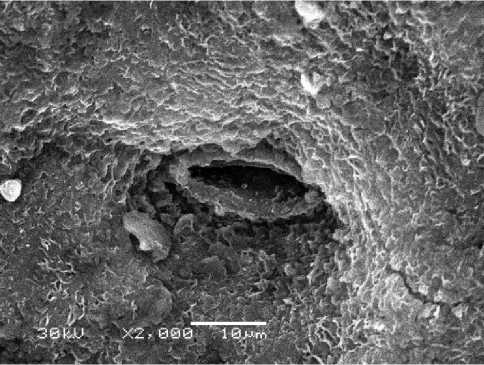
Halophytes grow in a wide variety of saline habitats. As plants, which live in extreme environments, they have to deal with frequent changes in salinity level. This can be done by developing adaptive responses including the synthesis of several bioactive molecules and specific microstructure. Our investigation showed that leaf surface of Е. maritimum is flat, leathery and thorntoothed. Abaxial (internal) surface is covered with stomata having a simple structure. Stomata openings are below the epidermis level (Fig. 1). The number of openings per 1 mm2 reaches 128±4.5. An average diameter of the stoma opening is 11.9±0.74 µm. Cuticle cells on leaf abaxial surface are covered with a considerable layer of loose wax that produces an effect of mat surface (Fig.2). Epidermis of the upper (adaxial) surface is covered with a layer of loose wax that, along with a considerable thickness of a cuticle layer, produces an effect of a glossy surface of the leaf lamina (Fig. 3). Stomata are less embedded in the epidermis surface than it occurs on the abaxial side (Fig. 4). The number of stomata per 1 mm2 is 123± 2.5, diameter of stoma opening – 12.1±1.7 µm. So, the stomata number and their dimensions on the abaxial and adaxial leaf lamina surface do not practically differ. According to the literature data stomata occur on the both leaf surfaces in many angiosperms. In xenomorphic plants the morphological adaptation to dry conditions is achieved through the arrangement of stomata below the epidermis level (Rudall, 2007). Thus, the existence in saline and dry soils of true halophytes E. maritimum was provided among others adaptive mechanisms by specific microstructure of leaf, adaxial and abaxial surface of which have well-developed cuticle and stomata slit placed below the surface of the epidermis.
Table 1 shows that the main fatty acids of E. maritimum leaves are palmitic, linoleic and linolenic, with linoleic 18:2 dominans. The content of 16:1 acid is very low. The amount of the saturated fatty acid is high in comparison with other terrestrial plants. The same was observed in other halophyte plants and seems to be typical for the salt stressed plants (Ivanova et al ., 2000; 2003). The high content of saturated fatty acid leads to decrease of membrane permeability and better resistance of halophytic plants against soil salinity. The membrane fluidity of the plants depends also on the content of the unsaturated linoleic (18:2) and linolenic (18:3) acids. With the increase of salinity there occurs increase in the amount of 18:3 acids in comparison with 18:2.
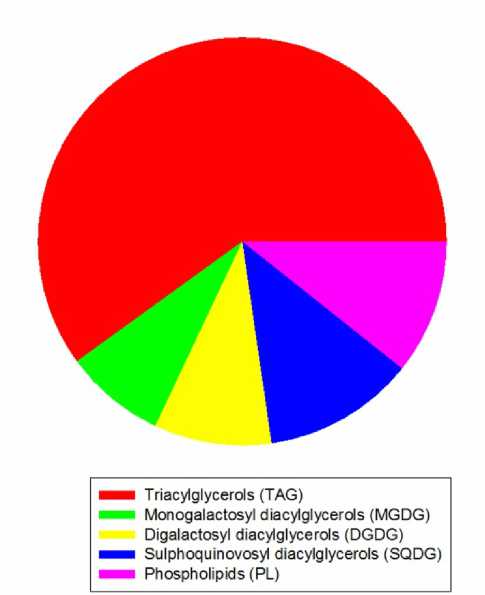
Triacylglycerol (TAG) content is very high (60% of total). TAGs are not membrane constituents, but their content is relatively high in some other halophyte plants from this region. The main glycolipid class is sulphoquinovosyl diacylglycerols (SQDG) – 12%, followed by digalactosyl diacylglycerols (DGDG) – 9,3 % and monogalactosyl diacylglycerols (MGDG) – 8%.
Contrary to the terrestrial plants, the content of phospholipids (PL) – 10,7 %, is relatively low (Fig. 5). Almost half of the fatty acids in PL are saturated. PL are the main lipid constituent in lipid membranes, so a high content of saturated acids leads to decrease of the membrane fluidity and to better resistance to harmful environment. Long-chain fatty acids (20:0 and 22:0) were found only in the TAG. These acids are frequently found in the lipids of roots and probably have a preventive role for the plants. The total lipophilic extract has a moderate activity against Staphylococcus aureus strain, in comparison with the n- butanol extract. Both extracts have not activity against Escherichia coli and Candida albicans. The fractionation of the extracts leads even to loss of some antibacterial activity (Darriet et al., 2014). References data have shown E. maritimum as a source of different compounds with biological activity for medical, pharmaceutical and cosmetically uses. After extraction or fractionation the biological activity of the plant decreases or the biologically active compounds are divided in the fractions. So it is preferable to use the plant in dry state as medicament. The presence of a large amount of saturated fatty acids and natural antioxidants in the plant provides chemical stability of the product.
Table 1. Fatty acid profiles of main lipid classes of E. maritimum
|
Lipids |
Fatty acids (wt % of total) |
|||||||
|
Lipid class |
14:0 |
16:0 |
16:1 |
18:0 |
18:1 |
18:2 |
18:3 |
20:0 |
|
Triacylglycerols (TAG) |
0.6 |
26.5 |
1.8 |
3.5 |
14.1 |
39.0 |
9.4 |
2.5 |
|
Monogalactosyl diacylglycerols (MGDG) |
2.0 |
17.1 |
0.4 |
10.9 |
11.1 |
19.1 |
39.3 |
- |
|
Digalactosyl diacylglycerols (DGDG) |
3.5 |
28.4 |
0.2 |
5.1 |
8.6 |
35.1 |
19.2 |
- |
|
Sulphoquinovosyl diacylglycerols (SQDG) |
3.3 |
27.5 |
0.5 |
5.4 |
5.1 |
30.6 |
27.4 |
- |
|
Phospholipids (PL) |
4.4 |
35.2 |
0.7 |
5.2 |
6.7 |
39.5 |
8.2 |
- |
Table 2. Thermoluminescence B, AG band maximum temperatures (T m ) and intensities (A) after excitation by two flashes from dark-adapted E. maritimum
|
B band T m (°C) |
B band A (%) |
AG band T m (°C) |
AG band A (%) |
|
24 |
56 |
40 |
44 |

-
Figure 2. Е. maritimum stoma on abaxial leaf lamina surface
-
Figure 3. Е. maritimum adaxial leaf lamina surface
-
Figure 4. Е. maritimum stoma on adaxial leaf lamina surface
^^ Triacylglycerols (TAG)
^" Monogalactosyl diacylglycerols (MGDG) Digalactosyl diacylglycerols (DGDG)
^™ Sulphoquinovosyl diacylglycerols (SQDG)
^™ Phospholipids (PL)
Figure 5. Main lipid classes (% of total) in E. maritimum
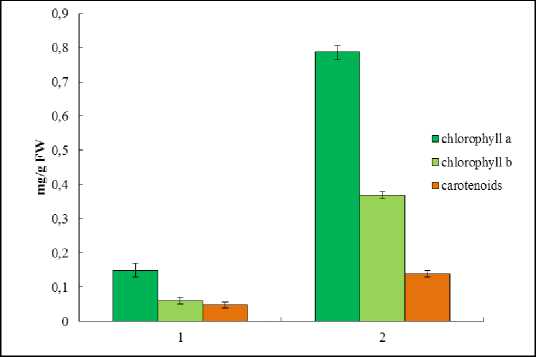
Figure 6. Pigments content in leaves of Е. maritimum (1) and leaves of winter wheat seedlings (2) (mg/g FW).
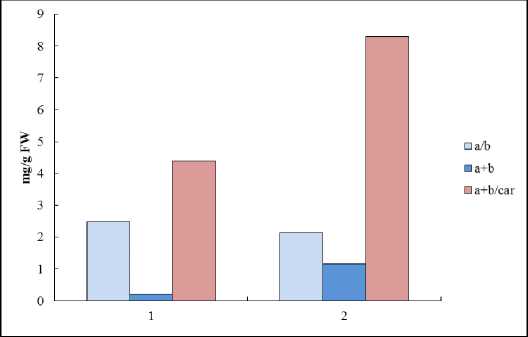
Figure 7. The ratio of pigments classes in leaves of Е. maritimum (1) and leaves of winter wheat seedlings (2) (mg/g FW).

Figure 1. Е. maritimum abaxial leaf lamina surface
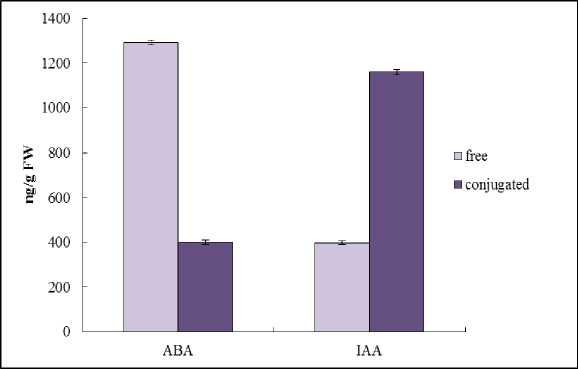
Figure 8. The free and conjugated ABA and IAA content in leaves of Е. maritimum (ng/g FW).
The higher plant pigment complex includes chlorophylls and carotenoids that transfer an additional energy to chlorophylls (light-collecting function) and remove an excessive energy from chlorophylls as well (light-protecting function) (Hormaetxe, 2005). It was shown that in leaf of Е. maritimum the level of chlorophylls was more lower as compared with the same in mesophyte plant Triticum aestivum L. (Fig. 6). Also the content of carotenoids was twice lower than in winter wheat leaf (Babenko et al ., 2014). At whole the ratio between pigments was similar to that in winter wheat, but the amount of chlorophylls and carotenoids were lower (Fig. 7). Thus, the key role in photosynthetic activity in true halophyte Е. maritimum plays chlorophyll a . At the same time a high amount of carotenoids (as compared with amount of chlorophylls) point out that these pigments have a light-collecting function and could transfer an additional energy to chlorophylls.
A complex thermoluminescence glow curves obtained as a result of excitation by two single-turnover flashes of leaf segments were decomposed and analyzed. The thermoluminescence emission from leaves demonstrated a B band with temperature of the maximum of 24°C, and AG emission band at 40°C, where the contribution of the bands to the total emission was found to be almost equal (table 2). The suppressed B band intensity could imply a possible inhibition in charge recombination reactions in PS II by the assumed saline concentration in plant tissue.
The value of maximum efficiency of Photosystem II (the ratio F v /F m ) that was observed in E. maritimum is 0.793±0.031 within the range less than 0.8, which indicates probable negative effects of salinity. The operational efficiency of photochemistry of Photosystem II (Φ PSII ), an indicator determining electron transport in PSII, had the low level in this plant species (0.270±0.054). A similar pattern was observed in photochemical fluorescence quenching q P (0.416±0.072), a parameter that gives an indication of the proportion of open reaction centers of PSII. Nonphotochemical fluorescence quenching (NPQ), characterizing the rate constant of energy loss in the form of heat from PSII was relatively high in this plant (1.12±0.30). It probably due to not efficient use of energy for photochemistry.
Phytohormones play a key role in the regulation of growth, development and resistance of plants. ABA is one of the major plant hormones involved in plant adaptation (Wilkinson, Davies, 2002). In plant tissues, it is present in free and conjugated forms (Zheng et al ., 2014). IAA– a natural auxin, the major function of which is the regulation of growth processes. In the bound state IAA loses its activity (Del Pozo et al ., 2005). We have shown that the pool of endogenous ABA was higher than that of IAA and the free form of ABA and conjugated form of IAA prevailed (Fig. 8). The high level of active ABA is correlated with salt tolerance of Е. maritimum , its ability to survive and grow in stress conditions. The high level of conjugated form of IAA is demonstrated that activity of hormone is limited.
CONCLUSION
Thus, the existence in saline and dry soils of true halophytes E. maritimum provided among others adaptive mechanisms by specific microstructure of leaf, adaxial and abaxial surfaces of which have well-developed cuticle and stomata slit placed below the surface of the epidermis. The presence of a large amount of saturated fatty acids provides decrease of membrane permeability and better resistance against soil salinity. The key role in photosynthetic activity plays chlorophyll a. At the same time a high amount of carotenoids (as compared with amount of chlorophylls) point out that these pigments have a light-collecting function and could transfer an additional energy to chlorophylls. The data from thermoluminescence bands analyses showed an inhibition in recombination reactions in PSII by saline concentration in plant tissue. Our fluorescence measuring data shown that efficiency of PS II (the ratio Fv/Fm) was less than 0.8, which indicates impact of salinity. The operational efficiency of photochemistry of Photosystem II (ΦPSII), an indicator determining electron transport in PSII, had low level. Similar changes in photochemical fluorescence quenching (qP), a parameter that gives an indication of the proportion of open reaction centers of PSII. The high level of active ABA correlates with salt tolerance and ability to survive and grow in stress conditions. The high level of conjugated form of IAA demonstrates that activity of hormone is limited.
ACKNOWLEDGEMENT
This work was completed in the frames of bilateral project between Bulgarian Academy of Sciences and National Academy of Sciences of Ukraine (20142018).
Список литературы Euhalophyte eryngium maritimum L.: the microstructure and functional characteristics
- Babenko L.M., I.V. Kosakivska., Yu.A. Akimov, D.O. Klymchuk, T.D. Skaternya. (2014) Effect of temperature stresses on pigment сontent, lipoxygenase activity and cell ultrastructure of winter wheat seedlings. Genetics and Plant Physiology, 4 (1-2), 117-125
- Blight E.G., Dyer W.J. (1959) A rapid method of total lipid extraction and purification. Cаn. J. Biochem. Physiol., 37, 911-917
- Christie W.W., Han X. (2010) Lipid Analysis Isolation, Separation, Identification and Lipidomic Analysis. Oily Press, Elsevier, P. 448
- Darriet F., Andreani S., De Cian Marie-Cécile, Costa J., Muselli A. (2014) Chemical variability and antioxidant activity of Eryngium maritimum L. essential oils from Corsica and Sardinia. Flavour and Fragrance Journal. 29, 3-13
- Del Pozo J.C., Lopez Mataz M.A., Ramirez-Parra E., Gutierrez C. (2005) Hormonal Control of the Plant Cell Cycle. Physiol. Plant., 123, 173-183
- Hormaetxe K., Becerril J.M., Fleck I., Pintó M., García-Plazaola J.I. (2005) Functional role of red (retro)-carotenoids as passive light filters in the leaves of Buxus sempervirens L.: increased protection of photosynthetic tissues? J. Exp. Bot., 56,.2629-2636
- Ivanova A., Nechev J., Stefanov K. (2000) Lipid composition of some halophyte plants from the Black Sea coast of Bulgaria Compt. rend. Acad. Bulg. Sci., 53, 83-86
- Ivanova A., Khozin-Goldberg I., Kamenarska Z., Nechev J., Cohen Z., Popov S., Stefanov K. (2003) Lipophylic Compounds from Euphorbia peplis L. - a Halophytic Plant from the Bulgarian Black Sea Coast. Z. Naturforsch., 58, 783-788
- Kosakivska I.V., Voytenko L.V., Likhnyovskiy R.V., Ustinova A.Y. Effect of temperature on accumulation of abscisic acid and indole-3-acetic acid in Triticum aestivum L. seedlings. Genetics and Plant Physiology, 4 (3-4), 2014, 117-125
- Minasiewicz J., Borzyszkowska S., Żółkoś K., Bloch-Orłowska J., Afranowicz R. (2011) Population genetic structure of the rare species Eryngium maritimum L. (Apiaceae) in the Gulf of Gdansk: implications for conservation and management Biodiv. Res. Conserv., 24, 39-48
- Meot-Duros L., Le Flochb G., Magné C. (2008). Radical scavenging, antioxidant and antimicrobial activities of halophytic species J. of Ethnopharmacology, 116, 258-262
- Rudall P. J. (2007) Anatomy of flowering plants, Camb. Univ. Press. Cambridge, P. 159
- Spooner F., Sykes G. (1972) Laboratory assessment of antibacterial activity. In J.R. Norris and D. Ribbons (Eds.), Methods in microbiology, London, New York: Academic press, 7B, 216-217
- Wellburn A. J. (1994) The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol., 144, 307-313
- Wilkinson S., Davies W.J. (2002) ABA-Based Chemical Signalling: The Coordination of Responses to Stress in Plants. Plant Cell Environ., 25 (1), 195-210
- Zeinalov Y., Maslenkova L. (1996). A computerized equipment for thermoluminescence investigations. Bulg. J. Plant Physiol., 22, 88-94
- Zheng-Yi Xu, Yun-Joo Y., Inhwan H. (2014) ABA conjugated and their physiological roles in plant cells. In: Abscisic acid: Metabolism, Transport and Signaling. Springer, Dordrecht. P. 77-87


