Evaluation of biopsychosocial model for pain management in post-oral cancer therapy patients
Автор: Patil K., Kaggare Puttaraju M., Solayappan E.
Журнал: Сибирский онкологический журнал @siboncoj
Рубрика: Опыт работы онкологических учреждений
Статья в выпуске: 4 т.23, 2024 года.
Бесплатный доступ
Background. Approximately 50 % of cancer patients have pain in their daily lives, which is multifaceted sensation goes beyond basic biochemical signal of pain. Oral mucositis is one of the negative consequences with intense pain, discomfort, challenges in speaking and eating. These components collectively influence patient's total quality of life across mental, biological, social aspects. Biopsychosocial model (BPS) is an effective technique for understanding and addressing conceptualization and treatment of pain in cancer patients. The aim of this study is to assess efficacy of the BPS in managing post-cancer distress and potential to improve quality of life for individuals with cancer. Material and Methods. This study evaluated 30 cancer patients who completed radiotherapy and were referred from cancer hospital. The examination encompasses three distinct categories: biological, psychological, and social components. The biological aspect was documented based on mucosal lesions and VAS scores for individuals; followed by photo-biomodulation was given. Palliative care was provided in psychological aspect through implementation of exercise, meditation, music therapy. The social component encompasses community engagement, social activities, counseling services for family members. Patients were categorized into 3 groups - A, B, C. All three components were carefully evaluated and one-month follow-up was done.
Oral cancer, radiotherapy, photo biomodulation, biopsychosocial model, quality of life
Короткий адрес: https://sciup.org/140307079
IDR: 140307079 | УДК: 616.31-006.6-08+616.8-009.7+616.89 | DOI: 10.21294/1814-4861-2024-23-4-117-124
Текст научной статьи Evaluation of biopsychosocial model for pain management in post-oral cancer therapy patients
Globally, head and neck cancer (HNC) is the seventh most prevalent type of cancer, with around 660,000 new cases and 325,000 deaths occurring each year. The prevalence of HNC is projected to increase by up to 30 % annually by year 2030. This upward trajectory has been observed in both wealthy and developing nations. Considering the extensive variety of adverse effects that occur after therapeutic intervention, it is crucial to evaluate treatment results for patients with head and neck cancer (HNC) [1, 2].
Cancer treatments result in a range of adverse effects, with the most prevalent ones being cancer-related fatigue (CRF) and pain. CRF is described as a “distressing persistent subjective sense of physical, emotional, and/or cognitive tiredness or exhaustion related to cancer or cancer treatment that is not proportional to activity that disrupts normal daily functioning” with abnormal operation [3].
According to the biopsychosocial paradigm, pain and disability are complex and influenced by the interplay of biological, psychological, and social factors [4]. It is commonly acknowledged that chronic pain can lead to feelings of depression, anxiety, sleep disturbances, and difficult interpersonal situations. However, it is less frequently recognized that these factors might also make individuals more susceptible to develop chronic pain. Depression, anxiety, post-traumatic stress, inadequate coping skills, and exaggeration of negative outcomes are all psychological factors associated with the emergence of chronic pain. Sociocultural variables associated with chronic pain include low educational achievement, cultural deprivation, and little social support. Genetics, age, sex, sleep, hormones, and endogenous opiate systems are all biological factors that have a crucial impact [5, 6].
The aim of this study to evaluate the effectiveness of the biopsychosocial model in treating post-cancer fatigue and explore its potential for enhancing the quality of life for cancer patients.
Material and Methods
30 patients of 20 males and 10 females were referred from cancer hospital and those who met the selection criteria followed by the study was evaluated. This study was approved from our institutional ethics committee JSSDCH IEC 21/2022. Prior to evaluating each patient, written consent was obtained individually. Inclusion criteria: age 18 year and older; patient diagnosed with head and neck cancer; patients’ diagnosis was confirmed with histopathological report; patients who have undergone radiotherapy; patients with oral mucositis post radiotherapy; conscious patient; patients with undisturbed orientation. Exclusion criteria: co-morbidities related to fatigue symptoms; patient under antidepressant and antiepileptics; bipolar disorder; stroke; pregnancy.
This evaluation research consists of three components: biological, psychological, and social. The biological aspect entailed the measurement of the VAS score, which spanned from 0 (indicating the absence of pain) to 10 (representing the most intense pain). The patients received Low Level Laser Therapy (photo biomodulation) at a wavelength of 660nm for 2–3 minutes every other day to treat mucosal changes including erosion, ulceration, sloughing, and depapil-lation of the tongue. Three contact mode sessions have been conducted, targeting a total of eight spots in the buccal mucosa and tongue, six points in the lip, and three points in the palate (Fig. 1).
The psychological aspect of the evaluation involved the use of WHO questionnaire assessment criteria. These criteria encompassed various factors such as trait anxiety, state anxiety, depressive symptoms, distress, body image, social problems, physical problems, financial problems, sexual problems, sleep, and appetite. These factors were assessed prior to the administration of palliative care. Music therapy was employed as an intervention in either a passive or interactive manner, including the patient in the creation of live music. This can be implemented either
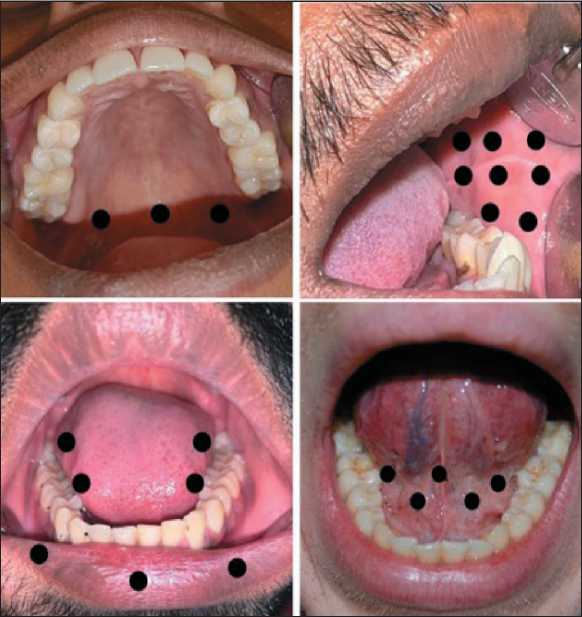
Fig. 1. Depicting intraoral reference points according to site. Note: created by the authors
Рис. 1. Контрольные точки в полости рта, на которые проводились сеансы НИЛИ.
Примечание: фотографии выполнены авторами independently or as part of a multimodal program. The breathing exercise was also modified, which facilitated the regulation of the breathing cycle and ultimately enabled control over the pace and depth of breathing after meditation.
Within the social aspect, the patient engages in communication with their family members and attends regular counseling sessions. These sessions aim to provide encouragement and support, thereby reducing stress and fostering a state of mindfulness. This enables the patient to fully embrace the present moment with a sense of self-assurance, ultimately leading to an enhanced quality of life (Fig. 2). The patients were categorized into groups A, B, and C based on the three components of the BPS model (Table 1).
The statistical analysis was conducted using SPSS software version 22.0. Post-hoc and ANOVA tests were performed to compare the three groups (Groups A, B, and C) based on their Biological, Psychological, and Social components. Descriptive statistics was to evaluate mean and standard deviation. A P value less than 0.05 was deemed to be statistically significant.
Results
The table presents the data collected from three different groups (A, B, and C) over the course of three visits. Initially, Group A had 19 participants, which decreased to 15 participants during the second visit and further declined to 12 participants by the final visit. In contrast, Group B started with 8 participants, which dropped to 5 during the second visit, but slightly increased to 6 participants by the final visit. Meanwhile, Group C showed beginning with 3 participants at the initial visit, rising to 10 participants in the second visit, and reaching 12 participants in the final visit. Overall, all patients during the final visit, 12 from group A, 6 from group B, 12 from group C showing
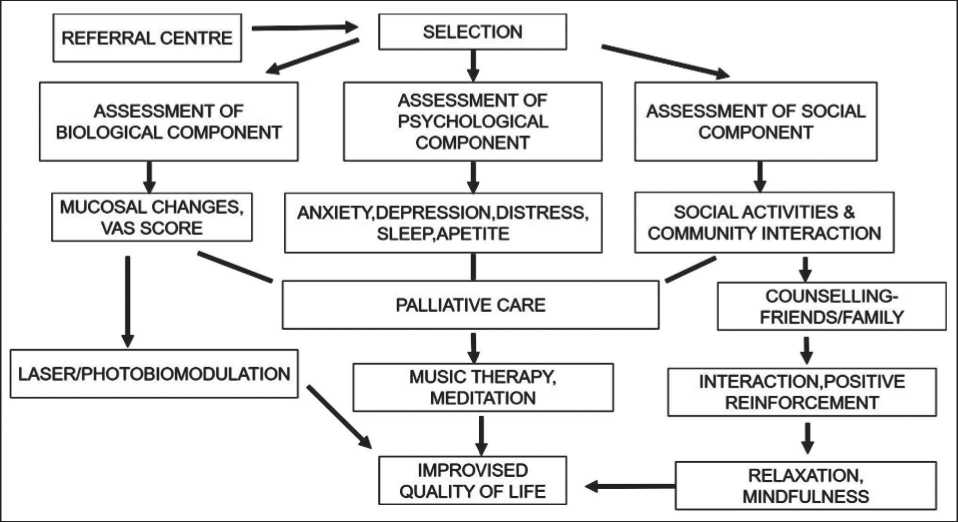
Fig. 2. Illustrating the process from patient selection to the management of biological, psychological, and social components.
Note: created by the authors
Рис. 2. Схема применения биопсихосоциальной модели контроля над болью. Примечание: рисунок выполнен авторами
Table 1/Таблица 1
Примечание: таблица составлена авторами.
Note: created by the authors.
Table 2/Таблица 2
|
Groups/Группы |
Initial visit/Первичный визит |
Second visit/Второй визит |
Final visit/Финальный визит |
|
A |
19 |
15 |
12 |
|
B |
8 |
5 |
6 |
|
C |
3 |
10 |
12 |
|
Total/Всего |
30 |
30 |
30 |
Примечание: таблица составлена авторами.
Note: created by the authors.
Frequency distribution of number of patients between A, B and C group Распределение пациентов между группами A, B и C
Table 3/Таблица 3
Descriptive statistics showing mean value of each visit between A, B and C Средний уровень баллов по шкале ВАШ на каждом визите в сравниваемых группах
ANOVA test among different groups
Тест ANOVA в сравниваемых группах
|
Visits/ Контрольное обследование |
Mean/ Среднее значение |
VAS Scores/Баллы по шкале ВАШ Standard Deviation/ Стандартное отклонение |
Coefficient of variation/ Коэффициент вариации |
|
First visit/Первый визит (n=30) |
9.234 |
0.732 |
0.085 |
|
Second visit/Второй визит (n=30) |
6.852 |
0.825 |
0.135 |
|
Final visit/Финальный визит (n=30) |
4.167 |
0.633 |
0.152 |
Примечание: таблица составлена авторами.
Note: created by the authors.
Table 4/Таблица 4
|
Cases/Случаи |
Sum of squares/ Сумма квадратов |
DF |
Mean square/ Средний квадрат |
F Value/ Значение F |
p-value |
|
VAS scores/ баллы по шкале ВАШ |
201.589 |
2 |
100.754 |
353.832 |
<0.001 |
|
Residuals |
16.511 |
58 |
0.285 |
– |
– |
Примечание: таблица составлена авторами.
Note: created by the authors.
Table 5/Таблица 5
Post hoc analysis showing difference from first visit to final visit among A, B and C group Различия в показателях между визитами в группах A, B и C
|
Visits/ Контрольное обследование |
Mean difference/ Средняя разница |
SE |
T |
p-value |
|
First visit and second visit/Первый и второй визиты |
1.456 |
0.135 |
10.152 |
|
|
First visit and final visit/Первый и финальый визиты |
3.533 |
0.135 |
26.374 |
<0.001 |
|
Second and final visit/Второй и финальный визиты |
2.213 |
0.131 |
15.221 |
Примечание: таблица составлена авторами.
Note: created by the authors.
full recovery from the disease. Despite the variations within each group, the total number of participants across all groups remained constant at 30 for each visit (Table 2).
The VAS score of patients with oral mucositis was assessed at three distinct time points: initial, second, and final visits. The VAS score is a pain intensity scale ranging from 0 (no pain) to 10 (most severe pain). The average Visual Analog Scale (VAS) score decreased from 9.234 during the initial visit to 4.167 at the final appointment, suggesting a decrease in pain experienced by the patients over time. The coefficient of variation exhibited an increase from 0.085 to 0.152 at the final visit, indicating a rise in relative variance (Table 3). The findings of one-way ANOVA test comparing the effects of three different therapies on the VAS score and mucosal lesions of the patients indicate that the F value for the VAS score is 353.832. The p-value for the VAS score was found to be less than 0.001, indicating statistical significance (Table 4).
A post hoc test was conducted to examine the average values of the variables at three different time points: the initial, second, and final visits. The mean difference represents the discrepancy in the averages of the two groups being compared. The P value indicates the presence of a statistically significant difference between the groups. Since all p-values are below 0.001, the findings indicate that there are statistically significant disparities among the three time periods. This implies that there was a significant variation in the variable between the initial and subsequent visits, as well as between the subsequent visits itself. The largest mean difference was observed between the first and final visits (3.533), followed by the second and final visits (2.213), and then the first and second visit (1.456) (Table 5).
Discussion
Advanced head and neck cancer (HNC) patients in the orofacial, oropharyngeal, and neck regions are significantly impacted by radiation therapy (RT). The psychological model is influenced by various factors like the type and stage of the tumor, the technique and intensity of the therapies, as well as the individual's inherent personal qualities. However, in most patients, complications are associated with significant illness and mortality, which puts additional strain on the healthcare system and may potentially affect patient compliance with cancer treatment plans, leading to decreased self-assurance. The majority of patients may encounter diverse issues that augment their overall medical load and diminish their quality of life (QoL) [7, 8]. Cancer patients frequently experience psychological disorders alongside a high prevalence of pain, burning sensations, and challenges with food intake, all of which significantly impact treatment adherence and overall quality of life.
According to Lu et al. (2016) [9], around 30 to 50 % of cancer patients suffer from psychological problems such as post-traumatic stress disorder (PTSD) and post-traumatic stress syndrome (PTSS), which often go unnoticed.
From blood samples of cancer patients, two discernible biological features are inflammation and anemia. Both characteristics have been linked to cancer-related fatigue in individuals who have survived cancer, due to elevated levels of pro-inflammatory cytokines (such as TNF- and IL6) and a decrease in hemoglobin levels. Cancer-related fatigue patients have a higher susceptibility to sarcopenia, which is a notable decline in skeletal muscle mass that impacts muscular strength and endurance. Neuromuscular fatigability, which refers to the decrease in the ability of the neuromuscular system to generate force during exercise, is frequently observed in cancer patients with cancer-related fatigue (CRF). This phenomenon is likely caused by a specific central factor, as indicated by several studies [10–14].
Photo-biomodulation treatment (PBM), formerly referred to as low-level laser therapy, involves the utilization of lasers or non-coherent light sources such as LEDs to effectively enhance cellular metabolism. This therapy regimen utilizes a non-thermal technique, meaning that it does not involve the application of heat or mechanical force that might potentially damage cells. The power and energy levels employed in this treatment are intentionally kept below the threshold known to induce adverse heating effects or mechanical cellular damage. Research has demonstrated that PBM primarily works by speeding up the production of ATP and causing the immediate release of reactive oxygen species (ROS). Furthermore, PBM induces a redox response by swiftly and temporarily triggering the activation of reactive oxygen species (ROS).
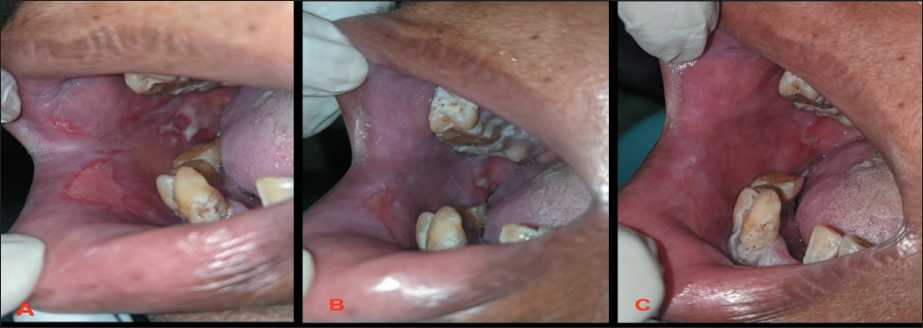
Fig. 3. Expression and dynamics of post-radiation mucositis during treatment and application of the BPS model: 3A – first visit;
3B – after 2 days of first visit; 2C – after 2 days of second visit. Note: created by the authors
Рис. 3. Выраженность и динамика постлучевого мукозита в процессе лечения и применения модели БПС: 3A – первый визит;
3B – через 2 дня после первого визита; 2C – через 2 дня после второго визита. Примечание: фотографии выполнены авторами
Both infrared and red light are likely to enhance ATP production by activating cytochrome c oxidase (CcO), according to the most credible theory proposed by Moradi et al. in 2019 [15].
H. Arora et al. (2008) [16] utilized low-level He-Ne laser therapy to prevent oral mucositis (OM). Their study revealed that patients who underwent LLLT had a significantly reduced occurrence of OM compared to those who did not (p value of 0.019 after 6 weeks). In a study conducted by A. Simes et al. (2009), the researchers examined the impact of three distinct schedules (3 vs 1 sitting each week) and two different laser strengths. After three weekly sessions, they noticed a statistically insignificant improvement in pain reduction (p=0.011). V. Oberoi et al. [17] conducted a comprehensive review and meta-analysis of 18 studies to assess the effects of LLLT on oral mucositis. The research revealed that LLLT significantly reduces the occurrence of severe oral mucositis compared to not receiving LLLT (p=0.001). Similarly, this study demonstrates a statistically significant difference in the average values between the initial visit (9.234) and the final visit (4.167) (Fig. 3).
In the 1990s, a significant number of cancer patients in Australia, North America, and Europe, ranging from over 50 % in Australia to up to two-thirds in North America and Europe, reported using psychological therapies such as meditation, imaginative thinking, mindful living, and hypnotherapy. These therapies are considered popular “alternative therapies” for managing cancer symptoms [18–20]. The main goal of breathing-based relaxation techniques is to enhance mindfulness of the breathing cycle and ultimately achieve mastery over the pace and depth of breathing. Initially, patients in our study are introduced to basic practices that focus on developing awareness of their breathing. However, as the treatment progresses, they also learn to control their breathing. This improvement in breathing control has led to a significant improvement in the patient's quality of life, as observed from their first appointment to their last visit. Patients are advised to concentrate on the rhythm, depth, and sound of their breathing, without trying to control or change it. This practice can help alleviate cancer-related fatigue and enhance their overall well-being. Patients can enhance their practice by acquiring the ability to modify the duration of breaths, the intervals between breaths, and employing other advanced techniques. Diaphragmatic breathing, characterized by slow and deep breaths through the nose, is employed in several breathing techniques [20–22]. This study involved training head and neck cancer patients to initiate swallowing during their relaxed breathing phase using meditation, and to maintain breathing after swallowing with the aid of visual reinforcement. Like the RCT study conducted by Martin et al. (2015) [23], the integration of a systematic approach and respiratory phase-related biofeedback can improve the coordination between respiration and swallowing. The optimal moment to swallow was determined to be during the period of silent respiration in the middle to late stage of exhalation.
The social approach prioritizes symptom management through engagement in regular social activities, rehabilitation, and fostering community support and interactions with colleagues and family members [24]. Hence, coping techniques can serve as a strong indicator of the mental welfare of those who have survived cancer, and it is an important area for caretakers to address and intervene, according to Simoes. Eight cancer survivors who were part of the quantitative study took part in semi-structured qualitative interviews to gain further insight into the factors that affect their psychological well-being. Psychosocial coping techniques can be classified into three primary categories: problem-focused coping, meaning-focused coping, and emotion-focused coping [25]. Specialized clinicians, who have been trained in these frameworks, act as exemplars for biopsychosocial well-being. They understand the significance of coping techniques and their relationship to psychosocial outcomes in the context of illness. Our study involves educating family members about the profound psychological impacts and the crucial role of social awareness. This prepares them to explore coping strategies that contribute to the complete rehabilitation of the patient, ultimately enhancing the patient's overall quality.
Conclusion
This study highlights the significance of comprehending the complex interplay among biological, psychological, and social elements in the initiation and treatment of cancer patients. As far as we know, this is the initial study that links photo biomodulation with
Список литературы Evaluation of biopsychosocial model for pain management in post-oral cancer therapy patients
- Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021; 71(3): 209–49. doi: 10.3322/caac.21660.
- Johnson D.E., Burtness B., Leemans C.R., Lui V.W.Y., Bauman J.E., Grandis J.R. Head and neck squamous cell carcinoma. Nat Rev Dis Primers. 2020; 6(1): 92. doi: 10.1038/s41572-020-00224-3. Erratum in: Nat Rev Dis Primers. 2023; 9(1): 4. doi: 10.1038/s41572-023-00418-5.
- Berger A.M., Mooney K., Alvarez-Perez A., Breitbart W.S., Carpenter K.M., Cella D., Cleeland C., Dotan E., Eisenberger M.A., Escalante C.P., Jacobsen P.B., Jankowski C., LeBlanc T., Ligibel J.A., Loggers E.T., Mandrell B., Murphy B.A., Palesh O., Pirl W.F., Plaxe S.C., Riba M.B., Rugo H.S., Salvador C., Wagner L.I., Wagner-Johnston N.D., Zachariah F.J., Bergman M.A., Smith C.; National comprehensive cancer network. Cancer-Related Fatigue, Version 2.2015. J Natl Compr Canc Netw. 2015; 13(8): 1012–39. doi: 10.6004/jnccn.2015.0122.
- Meints S.M., Edwards R.R. Evaluating psychosocial contributions to chronic pain outcomes. Prog Neuropsychopharmacol Biol Psychiatry. 2018; 87(Pt B): 168–82. doi: 10.1016/j.pnpbp.2018.01.017.
- Edwards R.R., Dworkin R.H., Sullivan M.D., Turk D.C., Wasan A.D. The Role of Psychosocial Processes in the Development and Maintenance of Chronic Pain. J Pain. 2016; 17(9 Suppl): 70–92. doi: 10.1016/j.jpain.2016.01.001.
- Samoborec S., Ruseckaite R., Ayton D., Evans S. Biopsychosocial factors associated with non-recovery after a minor transport-related injury: A systematic review. PLoS One. 2018; 13(6). doi: 10.1371/journal.pone.0198352.
- Epstein J.B., Thariat J., Bensadoun R.J., Barasch A., Murphy B.A., Kolnick L., Popplewell L., Maghami E. Oral complications of cancer and cancer therapy: from cancer treatment to survivorship. CA Cancer J Clin. 2012; 62(6): 400–22. doi: 10.3322/caac.21157.
- Verdonck-de Leeuw I.M., Buffart L.M., Heymans M.W., Rietveld D.H., Doornaert P., de Bree R., Buter J., Aaronson N.K., Slotman B.J., Leemans C.R., Langendijk J.A. The course of health-related quality of life in head and neck cancer patients treated with chemoradiation: a prospective cohort study. Radiother Oncol. 2014; 110(3): 422–8. doi: 10.1016/j.radonc.2014.01.002.
- Lu D., Andersson T.M., Fall K., Hultman C.M., Czene K., Valdimarsdóttir U., Fang F. Clinical Diagnosis of Mental Disorders Immediately Before and After Cancer Diagnosis: A Nationwide Matched Cohort Study in Sweden. JAMA Oncol. 2016; 2(9): 1188–96. doi: 10.1001/jamaoncol.2016.0483. Erratum in: JAMA Oncol. 2016; 2(9): 1244. doi: 10.1001/jamaoncol.2016.1942.
- Kilgour R.D., Vigano A., Trutschnigg B., Hornby L., Lucar E., Bacon S.L., Morais J.A. Cancer-related fatigue: the impact of skeletal muscle mass and strength in patients with advanced cancer. J Cachexia Sarcopenia Muscle. 2010; 1(2): 177–85. doi: 10.1007/s13539-010-0016-0.
- Yavuzsen T., Davis M.P., Ranganathan V.K., Walsh D., Siemionow V., Kirkova J., Khoshknabi D., Lagman R., LeGrand S., Yue G.H. Cancerrelated fatigue: central or peripheral? J Pain Symptom Manage. 2009; 38(4): 587–96. doi: 10.1016/j.jpainsymman.2008.12.003.
- Kisiel-Sajewicz K., Siemionow V., Seyidova-Khoshknabi D., Da-vis M.P., Wyant A., Ranganathan V.K., Walsh D., Yan J.H., Hou J., Yue G.H. Myoelectrical manifestation of fatigue less prominent in patients with cancer related fatigue. PLoS One. 2013; 8(12). doi: 10.1371/journal.pone.0083636.
- Grisold W., Grisold A., Löscher W.N. Neuromuscular complications in cancer. J Neurol Sci. 2016; 367: 184–202. doi: 10.1016/j.jns.2016.06.002.
- Chartogne M., Rahmani A., Landry S., Bourgeois H., Peyrot N., Morel B. Neuromuscular, Psychological, and Sleep Predictors of Cancer- Related Fatigue in Cancer Patients. Clin Breast Cancer. 2021; 21(5): 425–32. doi: 10.1016/j.clbc.2020.12.002.
- Moradi A., Kheirollahkhani Y., Fatahi P., Abdollahifar M.A., Amini A., Naserzadeh P., Ashtari K., Ghoreishi S.K., Chien S., Rezaei F., Fridoni M., Bagheri M., Taheri S., Bayat M. An improvement in acute wound healing in mice by the combined application of photobiomodulation and curcumin-loaded iron particles. Lasers Med Sci. 2019; 34(4): 779–91. doi: 10.1007/s10103-018-2664-9.
- Arora H., Pai K.M., Maiya A., Vidyasagar M.S., Rajeev A. Efficacy of He-Ne Laser in the prevention and treatment of radiotherapy-induced oral mucositis in oral cancer patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008; 105(2): 180–6. doi: 10.1016/j.tripleo.2007.07.043.
- Oberoi S., Zamperlini-Netto G., Beyene J., Treister N.S., Sung L. Effect of prophylactic low level laser therapy on oral mucositis: a systematic review and meta-analysis. PLoS One. 2014; 9(9). doi: 10.1371/journal.pone.0107418.
- Montbriand M.J. Freedom of choice: an issue concerning alternate therapies chosen by patients with cancer. Oncol Nurs Forum. 1993; 20(8): 1195–201.
- Warrick P.D., Irish J.C., Morningstar M., Gilbert R., Brown D., Gullane P. Use of alternative medicine among patients with head and neck cancer. Arch Otolaryngol Head Neck Surg. 1999; 125(5): 573–9. doi: 10.1001/archotol.125.5.573.
- Lazaridou A., Edwards R.R. Relaxation Techniques and Biofeedback for Cancer Pain Management. In: Gulati, A., Puttanniah, V., Bruel, B., Rosenberg, W., Hung, J. (eds) Essentials of Interventional Cancer Pain Management. Springer, Cham. 2018; 463–72. https://doi.org/10.1007/978-3-319-99684-4_50.
- Jerath R., Edry J.W., Barnes V.A., Jerath V. Physiology of long pranayamic breathing: neural respiratory elements may provide a mechanism that explains how slow deep breathing shifts the autonomic nervous system. Med Hypotheses. 2006; 67(3): 566–71. doi: 10.1016/j.mehy.2006.02.042.
- Unseld M., Zeilinger E.L., Fellinger M., Lubowitzki S., Krammer K., Nader I.W., Hafner M., Kitta A., Adamidis F., Masel E.K., Preusser M., Jäger U., Gaiger A. Prevalence of pain and its association with symptoms of post-traumatic stress disorder, depression, anxiety and distress in 846 cancer patients: A cross sectional study. Psychooncology. 2021; 30(4): 504–10. doi: 10.1002/pon.5595.
- Martin-Harris B., McFarland D., Hill E.G., Strange C.B., Focht K.L., Wan Z., Blair J., McGrattan K. Respiratory-swallow training in patients with head and neck cancer. Arch Phys Med Rehabil. 2015; 96(5): 885–93. doi: 10.1016/j.apmr.2014.11.022.
- Register-Mihalik J.K., DeFreese J.D., Callahan C.E., Carneiro K. Utilizing the Biopsychosocial Model in Concussion Treatment: Post- Traumatic Headache and beyond. Curr Pain Headache Rep. 2020; 24(8): 44. doi: 10.1007/s11916-020-00870-y.
- Simões A., de Campos L., de Souza D.N., de Matos J.A., Freitas P.M., Nicolau J. Laser phototherapy as topical prophylaxis against radiation-induced xerostomia. Photomed Laser Surg. 2010; 28(3): 357–63. doi: 10.1089/pho.2009.2486.


