Evaluation of sclerostin as a new biomarker in the diagnosis of osteoporosis
Автор: Al-Masoody A.K., Naser S.A., AL-Khafaji M.N., Al-Fahham A.A.
Журнал: Гений ортопедии @geniy-ortopedii
Рубрика: Оригинальные статьи
Статья в выпуске: 5 т.31, 2025 года.
Бесплатный доступ
Background Sclerostin is a glycoprotein mostly produced by osteocytes; it has a key function in bone metabolism and the pathophysiology of osteoporosis. Objectives The aim of this study is to evaluate the potential use of sclerostin as a new biomarker in the diagnosis of osteoporosis. Methods This case-control cross-sectional study was carried in Najaf, in Iraq. Seventy patients diagnosed with osteoporosis were involved in the study. The control group consisted of 40 apparently healthy persons identified during the same period. Body Mass Index (BMI) categories were classified according to the world health organization classification. Serum sclerostin levels were determined by a sandwich ELISA technique. Results The mean sclerostin concentration in patients was 7.9 ± 2.3 ng/mL, much greater than that measured in the control group 2.88 ± 1.22 ng/mL. The univariate logistic regression analysis shows a significant association between high sclerostin levels and the likelihood of having osteoporosis, with an odds ratio of 1.66 and a p-value of < 0.034. The results also indicated that sclerostin reported a sensitivity of 78 % and specificity of 82 % (p-value 0.029). Conclusions This study indicated a strong association between high serum sclerostin levels and having osteoporosis risk, suggesting its potential as a bone health biomarker. Further research on larger sample is required to confirm its diagnostic value.
Короткий адрес: https://sciup.org/142246012
IDR: 142246012 | УДК: 616.71-007.234-071 | DOI: 10.18019/1028-4427-2025-31-5-625-631
Текст научной статьи Evaluation of sclerostin as a new biomarker in the diagnosis of osteoporosis
Введение . Склеростин — гликопротеин, вырабатываемый преимущественно остеоцитами и играющий ключевую роль в метаболизме костной ткани и патофизиологии остеопороза.
Цель работы — оценить потенциальное использование склеростина в качестве нового биомаркера диагностики остеопороза.
Материалы и методы . Перекрестное исследование «случай – контроль» проведено в Наджафе (Ирак). В исследовании приняли участие 70 пациентов с диагнозом остеопороз, контрольная группа состояла из 40 здоровых людей. Индекс массы тела (ИМТ) соответствовал классификации Всемирной организации здравоохранения. Уровни склеростина в сыворотке крови определяли методом сэндвич-ИФА.
Результаты . Средняя концентрация склеростина у пациентов составила (7,9 ± 2,3) нг/мл, что значительно выше, чем в контрольной группе, — (2,88 ± 1,22) нг/мл. Однофакторный логистический регрессионный анализ показал значимую связь между высоким уровнем склеростина и вероятностью развития остеопороза с отношением шансов 1,66 и значением p < 0,034. Чувствительность склеростина составила 78 %, а специфичность — 82 % ( p = 0,029).
Заключение . Данное исследование выявило тесную связь между высоким уровнем склеростина в сыворотке крови и риском развития остеопороза, что свидетельствует о его потенциале в качестве биомаркера здоровья костей. Для подтверждения его диагностической ценности необходимы дальнейшие исследования на более крупной выборке.
INTRODUCTION
Sclerostin is a glycoprotein mostly produced by osteocytes; it has a key function in bone metabolism. As such, sclerostin holds critical assumptions for the understanding and treatment of diseases related to bone. The pathway involves Wnt/ β -catenin and the regulation by micro RNAs that sclerostin has to create in itself an interaction, therefore creating complexity which needs future attention [1]. Further studies on the structure and physiology of sclerostin will not only deepen the knowledge about bone but also help in creating new ways of treating issues related to bones, like osteoporosis. It works as an inhibitor for the growing process of bones by blocking a certain type of signaling linked to Wnt/ β -catenin that is very important for producing new bone cells [2]. The implementation of sclerostin measurement in clinical practice comes with various benefits. It permits better patient stratification according to their fracture risk, which can consequently inform more individualized treatment strategies. For instance, the level of sclerostin could direct the choice of pharmacologic therapies that work by enhancing bone density and lowering fracture risk [3]. These individualized treatment plans are most important in postmenopausal women, where there is a higher prevalence of fractures related to osteoporosis [4]. Besides, the levels of sclerostin give judgments about bone formation over resorption; thus, it has potential as a monitoring biomarker for treatment. By evaluating changes in sclerostin levels over time, it can help at least for sure interventions and hence improve indexing treatments [5]. J. Delgado-Calle et al. highlighted the role of the sclerostin–LRP4 interaction in bone metabolism, suggesting that sclerostin suppresses Wnt/ β -catenin signaling through this pathway. This mechanism is crucial for understanding the way by which sclerostin controls bone remodeling and demonstrates that therapeutic modulation of this pathway may offer novel strategies for the treatment of osteoporosis [6]. Regulatory micro RNAs can also be one of the routes through which miR-218 influences sclerostin, hence affecting the differentiation of osteoblasts. Therefore it shows the subtle intricate role sclerostin plays within the larger parameters of bone biology. A study by M.Q. Hassan et al. reported that miR-218 enhances osteoblast differentiation through down-regulation of sclerostin, therefore promoting Wnt signaling pathway activity [1]. The cross-talk between miR-218 and sclerostin not only gives a greater insight into osteobiology but indeed opens up prospective pharmacological targets for driving bone formation processes in pathological states characterized by reduced bone mass [7]. The bone formation effects of sclerostin are just one small aspect of its physiology. High levels of sclerostin, and therefore low skeletal mass, are often seen in postmenopausal women and so underscore the involvement of sclerostin in osteoporosis [8]. This was one of the objectives tested clinically in a trial like that of R.R. Recker et al., which checked whether blosozumab, an anti-sclerostin monoclonal antibody, could increase bone mineral density in such patients. The findings revealed that at both the spine and hip, blosozumab substantially increased bone mineral density maturing the concept of sclerostin as a negative regulator of bone formation with an optimistic therapeutic approach for managing osteoporosis [9]. The various signaling pathways that involve sclerostin also point to its multiple roles in maintaining healthy bones. Those are the interactions from which one might derive insight aimed at crafting fresh intervention strategies leveraging targets on sclerostin to boost bone mass and reduce osteoporotic infection dangers [10]. There remains a gap in knowledge of the role of sclerostin in bone biology. Thus, till now, the exact molecular mechanisms that govern the regulation of sclerostin expression under different physiological conditions have not been fully clarified. Also, though well established, the contribution of other potential interacting partners to the sclerostin-LRP4 interaction should also be explored [11]. The future should bring studies that uncover new microRNAs and signaling pathways involved in regulating networks for controlling sclerostin expression and activity. Longitudinal studies on bone health after treatment with anti-sclerostin therapies like blosozumab in varied populations would help fill this gap. Studies outside osteoporosis, like metastatic bone disease, will give us broader information on the role of sclerostin in skeletal health [9].
The aim of this work is to evaluate the potential use of sclerostin as a new biomarker in the diagnosis of osteoporosis.
MATERIALS AND METHODS
Patients and data collection
This case-control cross-sectional study was carried out at Al-Najaf General Hospital, in Najaf, in Iraq, from February 2024 to September 2024. Seventy patients diagnosed with osteoporosis were involved in the study. The control group consisted of 40 apparently healthy persons identified during the same period. Information about the age and body mass index (BMI) was collected directly from the patients. BMI categories were classified according to the World Health Organization (WHO) classification [12]. Serum sclerostin levels were determined by a technique of sandwich ELISA using the Human SOST Quantikine Immunoassay kit (Rand D, USA). Human SOST Quantikine Immunoassay Kit is a sandwich ELISA for the quantitative determination of sclerostin (SOST) in human serum or plasma. Samples were added to microplate wells that had been pre-coated with capture antibody to ensure attachment of sclerostin from the samples followed by detection with enzyme-linked antibodies. After washing away unbound components, color substrate was added and color development was observed; intensity of color is proportional to concentration of sclerostin in the sample. Finally, the reaction was stopped and the absorbance was measured at 450 nm standard curve used to determine levels in tested samples.
Statistical Analysis
Data was analyzed using SPSS Statistics software, version 25.0 (SPSS, Chicago). The Kolmogorov – Smirnov test was utilized to check the normality of parametric data. Those data that demonstrated normal distribution were expressed as mean ± standard deviation and were subjected to the independent t-test for comparison. The predictive ability of sclerostin in predicting relapses among patients with osteoporosis can be tested by applying the receiver operating characteristic (ROC) curve. A p-value of less than 0.05 was considered statistically significant.
RESULTS
The demographic comparison as shown in Table 1 indicates that there was no statistical difference between the two groups of patients and controls with regard to age ( χ ² = 4.79, p = 0.18) or gender distribution ( χ ² = 0.33, p = 0.56), meaning that these variables were well matched between the two samples. However, there was a very marked difference in the classification of BMI χ ² = 19.04, p = 0.000 with underweight participants significantly more in number in the study group; this accounted for about 37 % of the osteoporosis patients and only about 5 % of the controls
Table 1
Distribution of patients (osteoporosis) and control groups by their demographic data
|
Items |
Rating |
Patient ( n = 70) |
Control ( n = 40) |
Chi Square ( p -value) |
||
|
Freq. |
% |
Freq. |
% |
|||
|
Age |
21–30 |
14 |
20.00 |
13 |
32.5 |
4.79 (0.18) |
|
31–40 |
25 |
35.71 |
17 |
42.5 |
||
|
41–50 |
16 |
22.86 |
4 |
10 |
||
|
52–60 |
15 |
21.43 |
6 |
15 |
||
|
Mean ± SD |
46.33 ± 12.47 |
|||||
|
Gender |
Male |
31 |
44.29 |
20 |
50 |
0.33 (0.56) |
|
Female |
39 |
55.71 |
20 |
50 |
||
|
BMI |
Underweight |
26 |
37.14 |
2 |
5 |
19.04 (0.000) |
|
Normal |
22 |
31.43 |
28 |
70 |
||
|
Overweight |
18 |
25.71 |
8 |
20 |
||
|
Obese |
4 |
5.71 |
2 |
5 |
||
As shown in Figure 1, it was found that the patients’ group had a significantly raised level of serum sclerostin as compared to the controls ( p < 0.000). The mean sclerostin concentration in the patients was 7.9 ± 2.3 ng/mL, much greater than that measured in the control group (2.88 ± 1.22 ng/mL). This striking difference indicates that increased expression of sclerostin may be intimately linked with the pathophysiology of the disease under study; perhaps it reflects altered bone metabolism or impaired osteogenic signaling among the patients of this group.
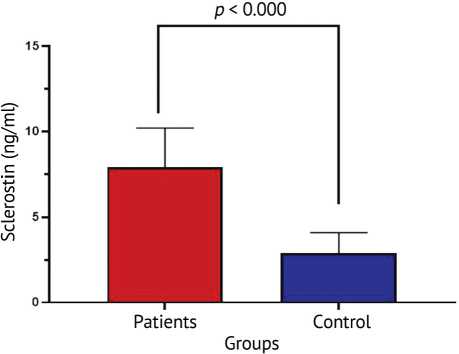
Fig. 1. Measurement of serum sclerostin (ng/mL) between patients and control groups
The univariate logistic regression analysis shows a significant association between high sclerostin levels and the likelihood of having osteoporosis, with an odds ratio (OR) of 1.66 and a p-value of < 0.034; 95 % confidence interval (CI) just includes 1.0 (0.92–1.79), the p-value appears to be statistically significant. So, it allows us to conclude sclerostin is relevant as a possible risk factor. From these results, we can infer that increased sclerostin levels may lead to osteoporosis, underlining its potential role in risk prediction and clinical evaluation.
The analysis of diagnostic performance has revealed sclerostin to have a strong potential as a biomarker for osteoporosis with an area under the curve (AUC) of 0.82 which is fairly good. At a cut-off value of 5.8 sclerostin reported a sensitivity of 78 % and specificity of 82 % which means it can fairly well identify those individuals who have osteoporosis from those who do not. The p -value obtained (0.029) also adds to the evidence in favor of the reliability of sclerostin in this case. These findings strengthen the potential clinical application of sclerostin evaluation as a non-invasive biomarker in the future for detecting and assessing the risk of osteoporosis at an early stage (Fig. 2).
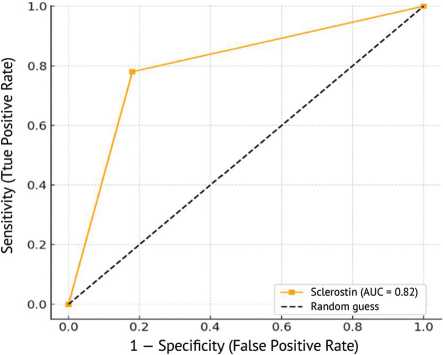
Fig. 2. ROC curve for sclerostin in the diagnosis of osteoporosis
DISCUSSION
The present study findings resonate well with the strong association between low BMI and osteoporosis; thus, in this specific population, low BMI could potentially be considered a risk factor when assessed clinically for bone health [12]. Many previous studies have associated BMI with osteoporosis. Thus, J.S. Walsh et al. reported significant correlation between BMI and osteoporosis, suggesting possible causes like increased loading and higher aromatase activity [13]. Another study conducted by J.T. Lloyd et al. in the USA showed that every unit of increase in BMI was associated with 0.0082 g/cm2 increase in BMD [14]. Also, D.T. Felson et al. showed the guarding effect of elevated body weight on bone mineral density (BMD) values in different places, mainly in bones that bear weight [15]. In a likely manner, an Asian study found good links between body weight, BMI, height, and being osteoporotic at various anatomical sites [16]. The potential of sclerostin as an osteoporosis biomarker has been discussed in several scenarios. Studies report that the sclerostin level has positive correlation with BMD in postmenopausal women, implying its relevance in assessing fracture risk [17]. Also, the link of sclerostin with metabolic disease markers makes it plausible that it could be a dual biomarker for bone and metabolic diseases in postmenopausal women [18–19]. The only real action that sclerostin performs is to primarily inhibit the Wnt signaling pathway, essential for bone formation. High sclerostin levels have then been associated with low osteoblastic activity and defective bone formation; thus, they are useful towards evaluating severity of osteoporosis [10]. Sclerostin's involvement in bone metabolism highlights its possible application as a diagnostic tool, more so in postmenopausal women, who are about to develop an increased risk of osteoporosis because of estrogen deficiency [20]. Osteoporosis has a very complicated pathophysiology, involves processes at the level of bone remodeling which are controlled by several molecular factors on the activity of sclerostin. Sclerostin is an inhibitor of bone formation which is a glycoprotein produced by osteocytes, it exerts its actions by blocking Wnt signaling pathway, one of the most important regulators of metabolism in bones [21]. The regulation of sclerostin should be very important concerning the maintenance of bone density; high levels have been associated with osteoporosis; elevated sclerostin is also involved in repression of bone formation what makes it an attractive potential therapeutic target for anti-sclerostin therapy that has currently emerged as a very promising novel interdisciplinary approach targeted toward enhancement of bone formation treatment for osteoporosis [10]. The UK National Osteoporosis Guideline Group (NOGG) recommended the incorporation of sclerostin in clinical practice as a biomarker for the diagnosis and management of osteoporosis. The measurement of sclerostin may improve understanding of individual bone health, that is, BMD assessed conventionally [22]. In this way, treatment could be tailored more on an individual basis for specific high-risk postmenopausal women [23]. Evidence shows that a combined assessment of sclerostin and BMD can give a holistic view regarding the diagnosis and treatment of osteoporosis [10]. Sclerostin assessment will not replace conventional BMD measurements as a linchpin for diagnosing osteoporotic disease but can be added as a biomarker to refine diagnosis and monitoring of treatment [2]. The possible role of sclerostin in guiding clinical choices gets more backing from studies that link its levels to how patients with osteoporosis respond to treatment [21]. The combined use of sclerostin and BMD measurements is likely to provide a comprehensive view in osteoporosis diagnosis and management evaluation [10]. BMD is one essential component in diagnosing osteoporosis, and the incorporation of biomarkers like sclerostin may refine diagnostic precision as well as treatment monitoring [24]. Further, the implied clinical utility of sclerostin based on its level studies relating to treatment response in osteoporotic patients has been underscored by investigations [21]. The importance of sclerostin does not limit its involvement merely to the diagnostic aspect but rather plays a pivotal role in mechanistic understanding related to the coupling within bone remodeling between osteoclasts and osteoblasts. Sclerostin inhibition will be a completely new way that could stimulate bone formation specifically without influencing resorption [25]. Study of the Sirt1-sclerostin route further underlines sclerostin’s role as a likely marker and target, implying that changes in this pathway might allow new methods for osteoporosis treatment [26].
CONCLUSION
This study indicates that high serum sclerostin levels are greatly linked to higher risk of osteoporosis, underlining its possible role as a beacon for bone health check. Sclerostin also showed a near link, hinting at a likely tie between metabolic state and bone density. These findings need more investigation in bigger varied studies to confirm the real importance of this biomarker in osteoporosis diagnosis and care.
Ethics approval The protocol in this study was approved by the ethical committee of the Medical College at the University of Kufa (No. 188 in 2025).
Consent to participate Before collection of samples, the patients involved in the protocol were asked to sign consent for their participation.
Funding The researchers rely only on their own financial support.


