Evaluation of the cytotoxic effects and apoptosis inducing of Cuscuta epithymum extract on esophageal squamous cell carcinoma
Автор: Hashemi M., Panahi A., Nosrati R., Oranj G.D., Jafari-shakib R.
Журнал: Сибирский онкологический журнал @siboncoj
Рубрика: Лабораторные и экспериментальные исследования
Статья в выпуске: 4 т.23, 2024 года.
Бесплатный доступ
Background. Esophageal cancer is the eighth most common cancer in the world. The antitumor effects of medicinal plants have been shown as a therapeutic strategy to treat esophageal cancer. This study aimed to evaluate in vitro effects of Cuscuta epithymum extract on the survival and apoptosis of esophageal cancer cell line. Material and Methods. Here, the hydroalcoholic Soxhlet extract of C. epithymum plant was prepared. The cell viability of esophageal cancer cell line KYSE-30 was evaluated by MTT assay after 24 h treatment with concentrations of 50, 100, 200, 400, 600, 800 and 1000 μg/ml of the extract. Then, the apoptotic effect of extract was evaluated by flow cytometry using Propidium Iodide (PI) staining and sub-G1 peak analysis, and Annexin V-FITC/PI staining in cells treated with concentrations of 125, 250, 500 and 750 μg/ml as well as morphological change of healthy cells to apoptotic and necrotic form.
Cuscuta extract, cuscuta epithymum, mtt, esophageal cancer, apoptosis
Короткий адрес: https://sciup.org/140307092
IDR: 140307092 | УДК: 616.329-006.61-08:615.322 | DOI: 10.21294/1814-4861-2024-23-4-77-85
Текст научной статьи Evaluation of the cytotoxic effects and apoptosis inducing of Cuscuta epithymum extract on esophageal squamous cell carcinoma
Esophageal cancer (EC) is the eighth most common cancer and the chance of getting of EC increases with age. EC is the sixth most common cause of cancer-related death in the world [1, 2].Worldwide 5-year overall survival rates range from 15 % to 25 % [3]. According to the morphology of malignant cells and the kind of cells involved, EC can be classified into several forms. Esophageal adenocarcinoma (EAC) and squamous cell carcinoma (ESCC) are the two most common types of EC. EAC begins in the glandular cells while ESCC develops from the squamous epithelial cells in the esophagus [4, 5]. ESCC is the predominant histological type of EC worldwide [6, 7]. When the esophageal mucosa is exposed to associated risk factors such as tobacco and alcohol consumption, poor diet, lack of excise, and obesity, epithelial cells proliferate abnormally and eventually develop into invasive EC [8].
EC is extremely malignant cancer and its mortality is increasing rapidly worldwide and its treatment is important. Chemotherapy, esophagectomy, and radiotherapy are the most common approaches for EC therapy [9]. Although esophagectomy and postoperative chemotherapy are considered the main choices for EC treatment, the surgical technique is very invasive and is associated with high rates of mortality and morbidity, and several side effects are may raise from chemotherapy [10, 11]. Accordingly, the cytotoxic and anti-apoptotic effects of natural compounds and medicinal plants with low adverse effects is currently receiving great attention for EC treatment [12, 13].
Cuscuta spp., also known as dodder is one of the medicinal plants consists of 100-170 species that assigned to the Convolvulaceae family [14]. Cuscuta are mainly yellow, orange, red or rarely green parasitic plants. C. epithymum is one of the species of Cuscuta, which is named in different countries with the names of Kashout, Pittimo, Aftimoon,, crop parasite, etc [15, 16]. C. epithymum is a parasitic plant with several secondary metabolites such as glycosides, saponins, quercetin, tannins, steroids, and kaempferol [16, 17]. C. epithymum have been frequently used in traditional medicine due to main biological and pharmacological activities, such as antioxidant, anti-bacterial, antifungal, and anticonvulsant activities, urease inhibition, and cytotoxicity [14, 18]. This plant is routinely used for treatment of insanity (Iran), diabetes (Morocco), burn injuries (India), psychometric disorders (India), liver disorders (India), vision improvement (Greece), and rheumatism (China) [17, 18]. Recent studies attribute the anti-cancer effects of the extract of this plant [19].
Given the above evidence, in this study, we have evaluated in vitro anticancer effects of hydroalcoholic extracts of C. epithymum on the survival and apoptosis of EC KYSE-30 cell line.
This study aimed to evaluate in vitro effects of Cuscuta epithymum extract on the survival and apoptosis of esophageal cancer cell line.
Material and Methods
Preparation of Cuscuta extract
-
C. epithymum plant was collected from Namin (Ardabil province) and were identified by the research herbarium of University of Mohaghegh Ardabili, Ardabil, Iran (Voucher No. 42,082). The plants were air-dried at room temperature and 50 g of root powder was mixed with 500 mL of 70 % hydroalcoholic
solvent by a Soxhlet extraction method. Then, the resulting mixture was evaporated in several stages by rotary evaporator and the extract was dried in the room temperature away from light and stored at 4 °C until use [17, 20].
Cell lines
ESCC cell line (KYSE-30) and HDF nan-cancerous cell line were achieved from National Bank of Iran, Pasteur Institute, Iran. The cells were cultured in 75 cm2 flasck (BioIdea, Iran) in RPMI-1640 medium (Gibco; Darmstadt, Germany) supplemented with 10 % FBS and 1 % penicillin/streptomycin (pen/strep) and incubated in a humidified condition at 37 °C and 5 % CO2. The media was changed twice a week. When cultures reached confluence, the cells were detached from the culture flask by 0.25 %Trypsin-EDTA solution (Gibco; Darmstadt, Germany).Cell counts were performed by Trypan blue (Sigma-Aldrich; Munich, Germany) exclusion method using haemocytometer slide.
Cell viability assay
The Cytotoxicity of C.epythimum extract on KYSE-30 cells was investigated using MTT method. Briefly, 5×103 cells/well were cultured in RPMI-1640 medium with 10 % FBS and 1 % pen/strep and incubated 24 h at 37 ℃ and 5 % CO2 in 96-well plates. Then, cells were treated with concentrations of 1000, 800, 600, 400, 200, 100, and 50 µg/ml of C. epithymum extract for 24 h. Then, the culture media were exchanged with fresh medium and added a 20 μL of 5 mg/mL MTT solution (Sigma-Aldrich; Munich, Germany) to each well and a further incubation was carried out ~ 4 h. Then, MTT was replaced with 100 μL of DMSO (Gibco; Darmstadt, Germany) as a formazan solvent to dissolve formazan crystals at room temperature. After the crystals were dissolved, the absorbance of each well of the plate was detected at 570 and 630 nm using a microplate reader (BioTeK, USA) [21].
Propidium iodide assay
Cell staining using the fluorescent dye propidium iodide (PI) and assessing the Sub-G1 Peak by flow cytometry are used to assess cell viability or DNA content in cell cycle analysis. For this purpose, 5 × 104 cells were seeded in a 24-well plate and incubated for 24h. After 24 h, cells were treated with concentrations of 250, 500, and 750 µg/ml of C. epithymum extract and were again incubated for 24h. The cells were harvested and washed in PBS, fixed in cold 70 % ethanol and left at 4 ℃ for 2 h. Then, cells were washed twice with PBS and resuspended cell pellet in 1 ml of hypotonic buffer containing PI reagent (Sigma-Aldrich; Munich, Germany) (100 µg/ml of PI in 0.1 % Triton X-100) and RNase A (Sigma-Aldrich; Munich, Germany) and were incubated for 30 min at 37 °C. Then it was analyzed by FAC Scan flow cytometer [22].
Apoptosis assay
Evaluation of apoptotic and necrotic cells was done by Annexin V-FITC/PI kit staining method (IQ-products; Netherlands). In this reaction, Annexin-V binds to phosphatidylserine (PS) in a specific and calcium-dependent manner, which is used to detect early apoptosis. Separation of early apoptosis from lately apoptosis is done with the help of PI dye reaction. To perform this test, about 5 × 105 cells/ well were cultured in 6-well plates containing RPMI-1640 medium and incubated overnight at 37 ℃ and 5 % CO2. Cells were exposed to concentrations of 125, 250, 500 and 750 µg/ml of C. epithymum extract and incubated for 24 h. Then, the adherent KYSE-30 cells were trypsinized with trypsin/EDTA and washed with serum-containing media and collected cells by centrifugation at 1500 g for 5 mins. The cells pellets were resuspended in 90 µL of 1X Annexin V binding buffer. 10 µl of Annexin V-FITC solution and 10 µl of PI solution were added to the solution, gently mixed and incubated at room temperature in the dark for 20 mins. Finally, the reaction suspension was replaced with 400 µl of 1X binding buffer and analyzed by FAC Scan flow cytometer. To set up compensation and quadrants of flow cytometry, unstained cells, cells stained with Annexin V-FITC without PI, and cells stained with PI alone (no Annexin V-FITC) were used as controls [23].
Assessment of changes in cellular morphology
The morphological changes of the treated cells were investigated by overnight treatment of 5 × 105 cells with 250 and 500 µg/ml C. epithymum extract in 6-well plate using an inverted microscope compared to untreated cells. In addition, apoptotic and necrotic cells morphology were investigated using fluorescent microscope after Annexin V-FITC/PI staining.
Statistical analysis
Statistical analyzes were performed using GraphPad Prism version 8 software. One-way variance test and Tukey’s multiple comparisons test were used to perform comparative tests. P-value<0.05 was also considered as the level of significance and the results were reported as mean ± standard deviation (SD).
Results
Effect of C. epithymum on the viability of KYSE-30 cells
In vitro cytotoxicity of different concentrations of hydroalcoholic extract of C. epithymum on KYSE-30 cells by MTT assay demonstrated that the cytotoxicity of this extract was evaluated in a dose-dependent manner after 24 h since the concentration of 1000 µg/ml showed the most cytotoxic effects (67.7 %) on the esophageal squamous cell line. According to the results of a dose-escalating experiment, IC50 of hydroalcoholic extract of C. epithymum on KYSE-30 cell line was obtained 646 µg/ml. However, in HDF nan-cancerous cell line, the different concentration of extract was not shown significant difference in term of cytotoxicity (p>0.05) (Fig. 1).
Effect of C. epithymum on cell cycle in KYSE-30 cells
The flow cytometry results of PI assay showed a significant increase of the sub-G1 percentage in cells
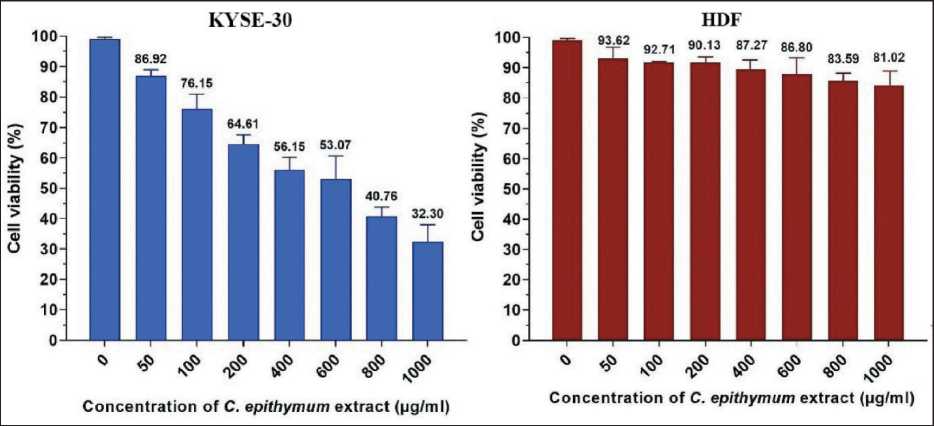
Fig. 1. Cell viability assessments of concentrations of 50–1000 µg/ml of C. epithymum extract in KYSE-30 and HDF cell lines after 24 h of incubation at 37 °C. Data are shown as mean ± SD in triplicate. Note: created by the authors
Рис. 1. Оценка жизнеспособности клеток при концентрации 50–1000 мкг/мл экстракта C. epithymum в клеточных линиях KYSE-30 и HDF после 24 ч инкубации при 37 °C. Данные представлены как среднее значение ± SD в трехкратном повторении. Примечание: диаграммы выполнены авторами
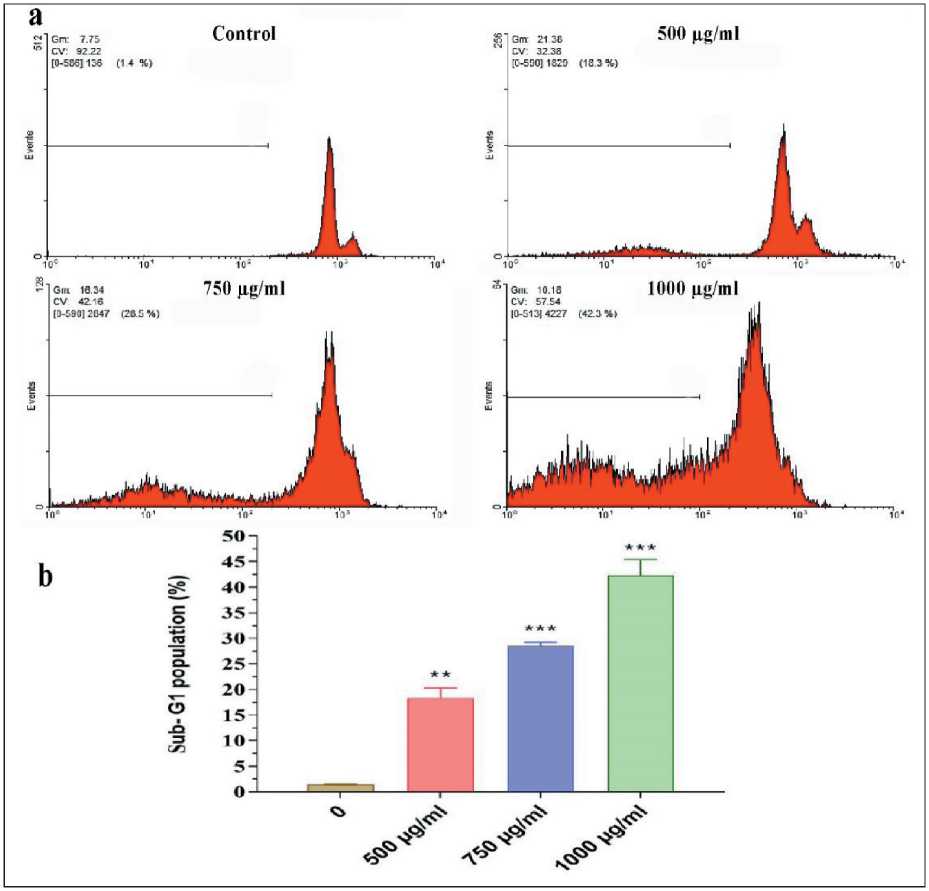
Fig. 2. a) Flow cytometry cell cycle histograms show a significant increase of the sub-G1 area of KYSE-30 cells treated with 500, 750, and 1000 µg/ml of C. epithymum extract for 24 h compared to the control group; b) Sub-G1 population percentage of cells treated with same concentration of C. epithymum extract for 24 h. (**– p <0.01 and *** – p <0.001 compared to the control group) Note: created by the authors
Рис. 2. a) гистограммы клеточного цикла, полученные с помощью проточной цитометрии, показывают значительное увеличение области суб-G1 клеток KYSE-30, обработанных 500, 750 и 1000 мкг/мл экстракта C. epithymum в течение 24 ч, по сравнению с контрольной группой; б) процент популяции суб-G1 клеток, обработанных той же концентрацией экстракта C. epithymum в течение 24 ч (значимость различий по сравнению с контрольной группой: ** – p<0,01 и *** – p<0,001).
Примечание: диаграммы выполнены авторами treated with 500, 750, and 1000 µg/ml of C. epithymum extract for 24 h compared to the control group (p<0.01 and p<0.001). Fig. 2a, b shows the flow cytometry histograms and sub-G1 population percentage in different concentration of C. epithymum extract.
Apoptosis analysis
Based on Annexin V-FITC/ PI double staining and results of flow cytometry, treatment of KYSE-30 cells with 125, 250, 500, and 750 µg/ml caused a higher apoptosis rate than that of untreated cells. As revealed in dot plot of four quadrants (Q1-Q4) images observed by flow cytometry assay (Fig 3a to 3f), there was more viable cells in the untreated group (3b) compared to the treated cells (Q3). In the untreated group, apoptotic and necrotic cells were about 2.5 %, while after the treatment of the cells, an increase in the apoptotic cells was observed in a dose-dependent manner. At the concentration of 125 µg/ml, about 9 % of the cells had undergone apoptosis (early and late apoptosis) (Q2 + Q4) and at the concentration of 250, 500, and 750 µg/ml the apoptotic cells (early and late apoptosis) were 17, 33, and 45 %, respectively, which was significant compared to the untreated group (p<0.001).
Morphological analysis
Morphological assessment of KYSE-30 cell line treated with 250 and 500 µg/ml hydroalcoholic extract of C. epithymum after 24 h by an inverted microscope, represented the membrane bubbles, formation of apoptotic bodies, cell aggregation, and dissociation of cell connections. Fluorescence microscopic images of Annexin V-FITC/PI double staining showed the apoptotic and necrotic cells morphology after treatment with 250 and 500 µg/ml of C. epithymum extract (Fig. 4). Despite normal morphology and the uniformly green cell membrane of the live cells, early apoptotic cells indicated by green to yellow. Cells with green membrane and red nucleus were late apoptotic cells, and orange to red cells indicated disintegrated morphology of necrotic cells (dead cells). The quantitative average percentage of early and late apoptosis, and necrosis in KYSE-30 cells treated with different concentrations of C. epithymum extract was shown in Fig. 5.
Discussion
Nowadays, with the increasing acceptance and public interest in the use of constituents of medicinal plants (herbal medicine) as natural alternatives to chemical-based treatments. Regarding this concern, many studies have shown their cytotoxicity potential as a therapeutic strategy to control the progress of various cancers via apoptosis or necrosis [24, 25]. In the present study, the effect of hydroalcoholic extract
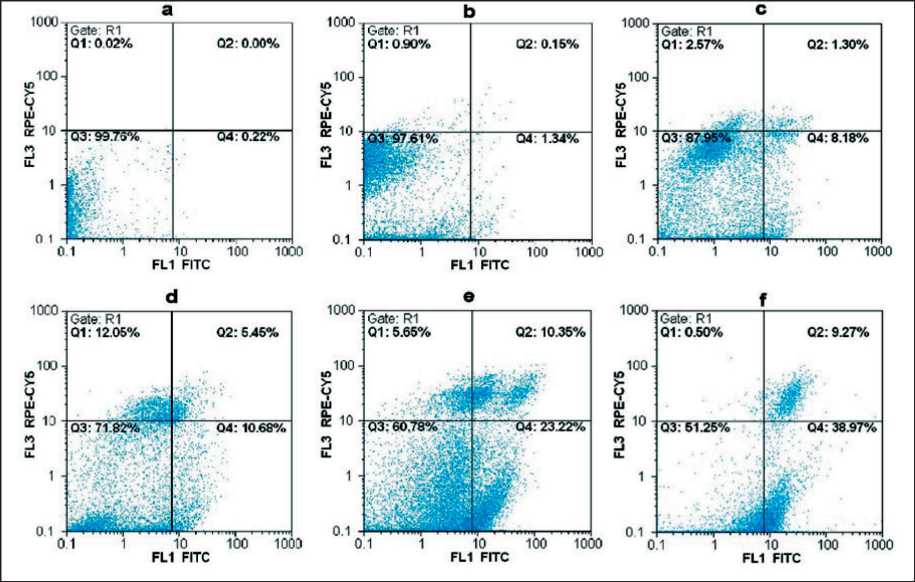
Fig. 3. Flow cytometry analysis of apoptosis of KYSE-30 induced with different concentrations of C. epithymum extract using Annexin-FITC/PI. Dot plots show apoptosis ratios of a) unstained control cell, b) untreated cell and stained with Annexin/PI, c) 125 µg/ml, d) 250 µg/ml, e) 500 µg/ml, and f) 750 µg/ml of C. epithymum extract. The Q1 quadrant represents unviable cells (PI+/AV-). The Q2 quadrant shows late apoptosis or necrosis (PI+/AV+). The Q3 quadrant represents viable cells (PI-/AV-). The Q4 quadrant represents cells in early apoptosis (AV+/PI-). Note the remarkable increase in apoptotic cells number (Q2+Q4) in the C. epithymum extract groups. Note: created by the authors
Рис. 3. Выраженность апоптоза KYSE-30, индуцированного различными концентрациями экстракта C. Epithymum, оценена методом проточной цитометрии с использованием Аннексина-FITC/PI. Коэффициенты апоптоза в неокрашенной контрольной клетке (a), в необработанной клетке, окрашенной Аннексином/PI (b), в клетках, обработанных экстрактом C. epithymum в различных концентрациях: 125 мкг/мл (c), 250 мкг/мл (d), 500 мкг/мл (e), 750 мкг/мл (f). Квадрант Q1 показывает нежизнеспособные клетки (PI+/AV-); Q2 – поздний апоптоз или некроз (PI+/AV+); Q3 – жизнеспособные клетки (PI-/AV-); Q4 – клетки на раннем апоптозе (AV+/PI-). Отмечается значительное увеличение числа апоптотических клеток (Q2 + Q4) в группах, получавших экстракт C. epithymum . Примечание: диаграммы выполнены авторами
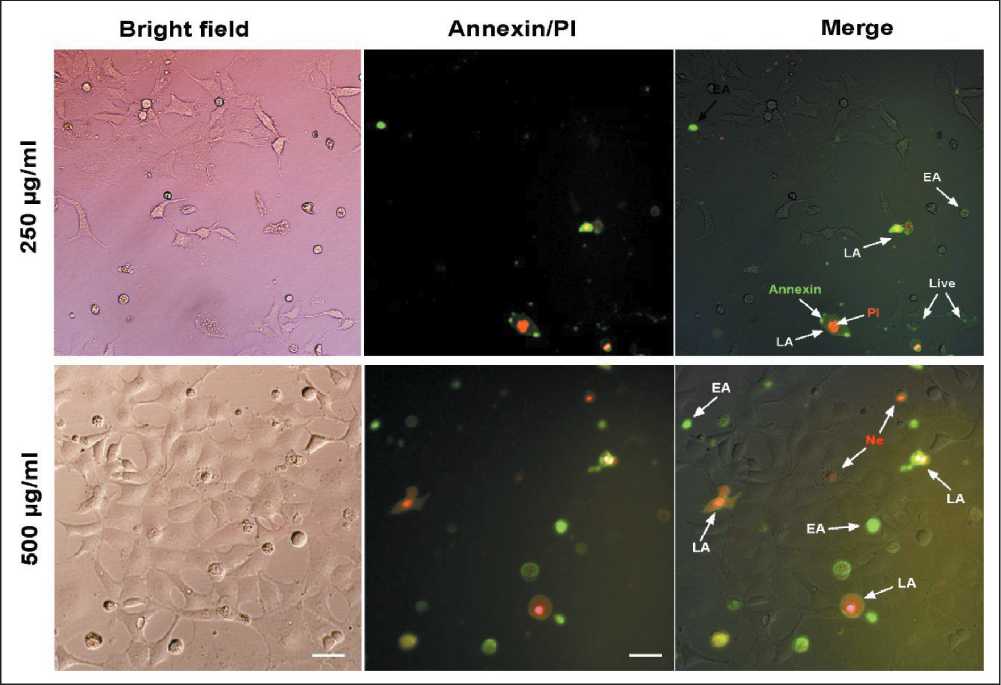
Fig. 4. Microphoto. Fluorescence microscopic images of apoptotic morphology of KYSE-30 cells treated with C. epithymum (250 and 500 μg/mL) for 24 h using Annexin V-FITC/PI staining. The earlier apoptotic cells turn green. Cells stained as membrane green and the nuclei red turn orange indicates the late apoptotic phase. The red stained cells indicate the dead cells. (The magnification is ×200. EA: early apoptosis, LA: late apoptosis, Ne: necrotic cells). Note: created by the authors
Рис. 4. Микрофото. Флуоресцентная микроскопия. Апоптоз клеток KYSE-30, обработанных C. epithymum (250 и 500 мкг/мл) в течение 24 ч, окраска Annexin V-FITC/PI, ×200. При ранней фазе апоптоза (EA) клетки становятся зелеными. Мембраны клетки, окрашенные в зеленый цвет, а красные ядра, становящиеся оранжевыми, указывают на позднюю фазу апоптоза (LA). Некротизированные (Ne) клетки окрашены красным. Примечание: микрофото выполнены авторами
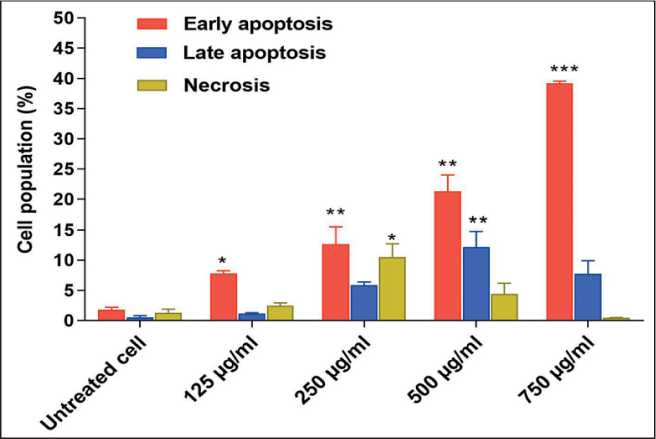
Fig. 5. Bar graph of average percentage of early and late apoptosis, and necrosis in KYSE-30 cells treated with different concentrations of C. epithymum extract. The values are presented as means±SD. (*– p<0.05, ** – p<0.01 and *** – p<0.001 compared to the untreated group). Note: created by the authors
Рис. 5. Средней процент раннего и позднего апоптоза и некроза в клетках KYSE-30, обработанных экстрактом C. epithymum различной концентрации. Значения представлены как среднее значение ± SD. (значимость различий по сравнению с контрольной группой: * – p<0,05, ** – p<0,01, *** – p<0,001). Примечание: диаграмма выполнена авторами
of C. epithymum on the in vitro survival and apoptosis of KYSE-30 esophageal squamous carcinoma cells was investigated. After treating the cells with different concentrations of C. epithymum extract, a significant decrease in cell viability and a significant increase in the number of apoptotic cells were observed in a dose-dependent manner.
C. epithymum has been used in traditional medicine for many years and recently, its anti-tumor properties have been reported. Its therapeutic and protective effects arise from bioactive compounds such as flavonoids, alkaloids, and saponins. However, the apoptosis induction of esophageal cancer cells has not been investigated so far. Herein, we confirmed a significant toxic effect of its extract on the viability of KYSE-30 cells with the MTT assay. In previous studies, the toxicity of the extract of this species and other species of Cuscuta has been shown against different types of cancer. In a study reported by Ghazanfari et al,. the cytotoxic effect of Cuscuta extract was found on the viability of lymphoma and melanoma cancer cells in a dose- and time-dependent manner since its cytotoxicity was 80 % and 81 % on SK-MEL-3 and Raji cell lines with a IC50 value of 3.53 mg/ml and 2.95 mg/ml at24 h, respectively [26]. In another study, the hydroalcoholic extracts of C. chinensis significantly reduced the viability of HT29, Hela and MDA-MB-468 cells as well as the hydroalcoholic extract of C. epitymum could significantly decreased the viability of MDA-MB-468 cells (IC50 = 340 µg/ml) [27]. Moradzadeh et al,. also showed that the hydroalcoholic extract of C. campestris could inhibited the cell viability with values of IC50 = =23.9 µg/ml for HL60 and IC50 = 60.3 µg/ml for NB4 cells after 72 h post-treatment [28]. The comparison of previous findings and our received IC50 values (646 µg/ml), suggesting that the epithelial cells of breast adenocarcinoma (MDA-MB-468) are more sensitive than the esophageal epithelial cells, melanoma and Burkitt's lymphoma to the Cuscuta extract.
Among the various alcoholic extracts of C. epithymum , methanolic and dichloromethane extracts have been shown the high toxicity, so that the lowest IC50 for 4T1 and MDA-MB-231 cells was obtained at a concentration of 72.83 µg/ml and 24.53 µg/ml from methanolic extract after 48 h [29]. The difference between the results of present study and these studies related to the type of extraction method, duration of treatment, and target cell line. In this study, we used the hydroalcoholic extract that it has been found an almost similar output with alcoholic extracts, however, a low cytotoxicity has been reported for hydraulic extract [30]. It has been demonstrated that most anticancer properties of Cuscuta species related to the bioactive compounds. Therefore, in the future studies, their cytotoxicity effects of the different fragments of species of this plant should been investigated.
Apoptotic cells are recognized either on the basis of their reduced DNA-associated fluorescence as cells with diminished DNA content (sub-G1) or morphologic changes. Here, a significant increase in the percentage of sub-G1 in flow cytometry histograms and DNA fragmentation was observed in the toxicity caused by the hydroalcoholic extract of C. epithymum in esophageal cancer cell line, which indicated the in vitro anticancer properties of C. epithymum extract. In addition, a significant increase in the percentage of early and late apoptotic cells after
Список литературы Evaluation of the cytotoxic effects and apoptosis inducing of Cuscuta epithymum extract on esophageal squamous cell carcinoma
- Liu C.Q., Ma Y.L., Qin Q., Wang P.H., Luo Y., Xu P.F., Cui Y. Epidemiology of esophageal cancer in 2020 and projections to 2030 and 2040. Thorac Cancer. 2023; 14(1): 3-11. https://doi.org/10.1111/1759-7714.14745.
- Uhlenhopp D.J., Then E.O., Sunkara T., Gaduputi V. Epidemiology of esophageal cancer: update in global trends, etiology and risk factors. Clin J Gastroenterol. 2020; 13(6): 1010-21. https://doi.org/10.1007/s12328-020-01237-x.
- Yang J., Liu X., Cao S., Dong X., Rao S., Cai K. Understanding Esophageal Cancer: The Challenges and Opportunities for the Next Decade. Front Oncol. 2020; 10. https://doi.org/10.3389/fonc.2020.01727.
- Zhang Y. Epidemiology of esophageal cancer. World J Gastroenterol. 2013; 19(34): 5598-606. https://doi.org/10.3748/wjg.v19.i34.5598.
- Li J., Xu J., Zheng Y., Gao Y., He S., Li H., Zou K., Li N., Tian J., Chen W., He J. Esophageal cancer: Epidemiology, risk factors and screening. Chin J Cancer Res. 2021; 33(5): 535-47. https://doi.org/10.21147/j.issn.1000-9604.2021.05.01.
- Wang Q.L., Xie S.H., Wahlin K., Lagergren J. Global time trends in the incidence of esophageal squamous cell carcinoma. Clin Epidemiol. 2018; 10: 717-28. https://doi.org/10.2147/CLEP.S166078.
- Sheikh M., Roshandel G., McCormack V., Malekzadeh R. Current Status and Future Prospects for Esophageal Cancer. Cancers (Basel). 2023; 15(3): 765. https://doi.org/10.3390/cancers15030765.
- Yang Y.M., Hong P., Xu W. W., He Q.Y., Li B. Advances in targeted therapy for esophageal cancer. Signal Transduc Target Ther. 2020; 5(1). https://doi.org/10.1038/s41392-020-00323-3.
- Bollschweiler E., Plum P., Mönig S.P., Hölscher A.H. Current and future treatment options for esophageal cancer in the elderly. Expert Opin Pharmacother. 2017; 18(10): 1001-10. https://doi.org/10.1080/14656566.2017.1334764.
- Abbas G., Krasna M. Overview of esophageal cancer. Ann Cardiothorac Surg. 2017; 6(2): 131-6. https://doi.org/10.21037/acs.2017.03.03.
- Borggreve A.S., Kingma B.F., Domrachev S.A., Koshkin M.A., Ruurda J.P., van Hillegersberg R., Takeda F.R., Goense L. Surgical treatment of esophageal cancer in the era of multimodality management. Ann N Y Acad Sci. 2018; 1434(1): 192-209. https://doi.org/10.1111/nyas.13677.
- Ying J., Zhang M., Qiu X., Lu Y. The potential of herb medicines in the treatment of esophageal cancer. Biomed Pharmacother. 2018; 103: 381-90. https://doi.org/10.1016/j.biopha.2018.04.088.
- An J., An S., Choi M., Jung J.H., Kim B. Natural Products for Esophageal Cancer Therapy: From Traditional Medicine to Modern Drug Discovery. Int J Mol Sci. 2022; 23(21). https://doi.org/10.3390/ijms232113558.
- Noureen S., Noreen S., Ghumman S.A., Batool F., Bukhari S.N.A. The genus Cuscuta (Convolvolaceac): An updated review on indigenous uses, phytochemistry, and pharmacology. Iran J Basic Med Sci. 2019; 22(11): 1225-52. https://doi.org/10.22038/ijbms.2019.35296.8407.
- Ahmad A., Tandon S., Xuan T.D., Nooreen Z. A Review on Phytoconstituents and Biological activities of Cuscuta species. Biomed Pharmacother. 2017; 92: 772-95. https://doi.org/10.1016/j.biopha.2017.05.124.
- Pourhadi M., Niknam Z., Ghasemi R., Zomorrod M. S., Niazi V., Faizi M., Zali H., Mojab F. Cuscuta epithymum Murr. crude extract pre-conditioning inhibits cell apoptosis in glutamate-induced cytotoxic condition. 2022. https://doi.org/10.21203/rs.3.rs-1950388/v1.
- Abedini M. R., Paki S., Mohammadifard M., Foadoddini M., Vazifeshenas-Darmiyan K., Hosseini M. Evaluation of the in vivo and in vitro safety profile of Cuscuta epithymum ethanolic extract. Avicenna J Phytomed. 2021; 11(6): 645-56. https://doi.org/10.22038/AJP.2021.18529.
- Chabra A., Monadi T., Azadbakht M., Haerizadeh S.I. Ethnopharmacology of Cuscuta epithymum: A comprehensive review on ethnobotany, phytochemistry, pharmacology and toxicity. J Ethnopharmacol. 2019; 231: 555-69. https://doi.org/10.1016/j.jep.2018.10.016.
- Mishra S., Alhodieb F.S., Barkat M.A., Hassan M.Z., Barkat H.A., Ali R., Alam P., Alam O. Antitumor and hepatoprotective effect of Cuscuta reflexa Roxb. in a murine model of colon cancer. J Ethnopharmacol. 2022; 282. https://doi.org/10.1016/j.jep.2021.114597.
- Katiyar N.S. A Study on Hepatoprotective and Anti-Inflammatory Activities of Stem Extracts of Cuscuta Reflexa (Roxb) in Rats. Rajiv Gandhi University of Health Sciences. India, 2010.
- Sylvester P.W. Optimization of the tetrazolium dye (MTT) colorimetric assay for cellular growth and viability. Methods Mol Biol. 2011; 716: 157-68. https://doi.org/10.1007/978-1-61779-012-6_9.
- Nair A., Manohar S.M. A flow cytometric journey into cell cycle analysis. Bioanalysis. 2021; 13(21): 1627-44. https://doi.org/10.4155/bio-2021-0071.
- Rajagopal A., Rajakannu S. Cassia auriculata Linn. extracts induce apoptosis and cell cycle arrest of A549 lung cancer cell lines: An in vitro approach. South African Journal of Botany. 2022; 147: 275-85. https://doi.org/10.1016/j.sajb.2022.01.020.
- van Wyk A.S., Prinsloo G. Health, safety and quality concerns of plant-based traditional medicines and herbal remedies. South African Journal of Botany. 2020; 133: 54-62.
- Welz A.N., Emberger-Klein A., Menrad K. Why people use herbal medicine: insights from a focus-group study in Germany. BMC Complement Altern Med. 2018; 18(1): 92. https://doi.org/10.1186/s12906-018-2160-6.
- Ghazanfari T., Naseri M., Shams J., Rahmati B. Cytotoxic effects of Cuscuta extract on human cancer cell lines. Food Agric Immunol. 2013; 24(1): 87-94. https://doi.org/10.1080/09540105.2011.648608.
- Jafarian A., Ghannadi A., Mohebi B. Cytotoxic effects of chloroform and hydroalcoholic extracts of aerial parts of Cuscuta chinensis and Cuscuta epithymum on Hela, HT29 and MDA-MB-468 tumor cells. Res Pharm Sci. 2014; 9(2): 115-22.
- Moradzadeh M., Hosseini A., Rakhshandeh H., Aghaei A., Sadeghnia H.R. Cuscuta campestris induces apoptosis by increasing reactive oxygen species generation in human leukemic cells. Avicenna J Phytomed. 2018; 8(3): 237-45.
- Firoozan J., Khodaie L., Mohammadi A., Fazljou S. M., Torbati M., Mohammadi Q., Mansoori B., Moghadam S.B.,, Baradaran B. Dichloromethane EXTRACT of Cuscuta epithymum inhibits triple-negative breast cancer development via inducing apoptosis and suppression of migration. J Biochem Tech. 2020; 11(1): 92-9.
- Bhagat M., Arora J.S., Saxena A.K. In vitro and in vivo antiproliferative potential of Cuscuta reflexa Roxb. J Pharm Res. 2013; 6(7): 690-5. https://doi.org/10.1016/j.jopr.2013.06.005.
- Behbahani M. Evaluation of in vitro anticancer activity of Ocimum basilicum, Alhagi maurorum, Calendula officinalis and their parasite Cuscuta campestris. PLoS One. 2014; 9(12). https://doi.org/10.1371/journal.pone.0116049.


