Expression of circadian genes in dietary stress induced Drosophila melanogaster
Автор: Sanjay V., Malathi R.
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 1 т.21, 2025 года.
Бесплатный доступ
Circadian rhythm, the 24-hour cycles in physiological processes, are governed by circadian genes. In Drosophila melanogaster (fruit flies), these genes regulate various biological functions, including metabolism, sleep-wake cycles, and aging. This study explores the expression of circadian genes under different dietary stress conditions, including high fat, high sugar, high alcohol, high protein, and starvation diets. In Drosophila , Period, Timeless, Clock, and Cycle are the primary circadian genes, which play crucial roles in maintaining these rhythms. The regulatory mechanisms involve feedback loops where proteins encoded by these genes interact to regulate their own expression and that of other genes. Dietary stress can significantly impact circadian gene expression, leading to disruptions in the circadian clock and metabolic pathways. High fat and high sugar diets, for instance, can induce metabolic dysregulation and obesity, while high alcohol intake affects liver function and metabolism. Starvation and high protein diets also alter metabolic pathways, potentially impacting aging and lifespan. This study investigates these impacts at the molecular level, highlighting the intersection between dietary stress, circadian gene expression, and aging signaling pathways.
Circadian rhythm, drosophila melanogaster, high sugar diet, high starvation diet, hyperglycemia, transcription based feedback loops
Короткий адрес: https://sciup.org/143183769
IDR: 143183769
Текст обзорной статьи Expression of circadian genes in dietary stress induced Drosophila melanogaster
Circadian Rhythm (CR) is the general term for any modifications to behaviour, thought, or body that occurs within a period in organism, including mammals and cyanobacteria. An organism's daily activities are regulated by its internal clock to match the day/night cycle of its environment (Young & Kay, 2001). Although CR is a natural occurrence, it can be impacted by a variety of elements, including redox cycles, diet, temperature, and light (Johnston et al., 2016). The suprachiasmatic nucleus (SCN), which controls a variety of neuronal and hormonal pathways, houses the mammalian core clock and establishes synchronization involving the external tissues and internal clock (Albrecht, 2012) The Drosophila melanogaster Central clock is located in the organ of Bolwig, which is located inside the cerebral cortex, and it remains active over the whole life cycle. An auto-regulatory feedback system composed of circadian genes and their products aids in sustaining a rhythmic oscillation that lasts 24 hours. CR controls a variety of physiological and behavioral processes in Drosophila, including eclosion, temperature sensitivity, and courting behavior (Kaneko et al., 2012). Thus, metabolic and physiological abnormalities in Drosophila melanogaster are caused by any disruption in CR, whether as a result of faulty genes or external stimuli. Mammals with similar phenotypes have higher rates of cancer, aging, atherosclerosis, neurodegeneration, obesity, and diabetes, among other illnesses (Barnea et al., 2010;
Diabesity, which is a term used to describe the interdependence of obesity and Type 2 diabetes is distinguished by a decline in liver glucose production, insulin-mediated glucose transport, and peripheral tissue glucose metabolic process (Kahn et al., 2006). According to the previous study, 9% of the human population is at higher risk of obesity and type 2 diabetes (T2D) if they consume a high-fat diet (Heinrichsen et al., 2014). Similar to this, a diet that is heavy in fat or sugar causes hyperglycemia in Drosophila melanogaster (Song et al., 2017). The flies fed a diet heavy in sugar exhibited hyperglycemia in a manner akin to that of flies fed HFD (Heinrichsen & Haddad, 2012) and mutant flies with insulin-producing cells (IPCs) (Rulifson et al., 2002a; Song et al., 2010) . Much research has to be done, despite a wealth of literature suggesting a connection decreased CR and problems in Physiology, behavior, and metabolism in a variety of species (Johnston, 2014; Maury et al., 2010). Previous studies have also suggested that diabetes is a result of disruptions to the y clock, and that individuals with diabetes also have abnormalities in their circadian rhythm (Kadono et al., 2016; Lee et al., 2015). Although there is evidence linking circadian rhythm to the overall status of diabetes, it is yet unknown if irregularities in the circadian rhythm cause metabolic illness to begin, develop, or worsen (Javeed & Matveyenko, 2018). Some data in Drosophila melanogaster suggests that whereas Mutants of the insulin peptide and the insulin receptor exhibit fragmented sleep and dysrhythmia in foxo or shaggy mutants (Martinek et al., 2001a; Metaxakis et al., 2014; Zheng et al., 2007).
Core circadian genes and their functions
Konopka found that every mutation he found corresponded to a single gene, which he named period. These mutations included those that had rhythms that were entirely deleted, shortened (to 19 hours), or prolonged (to 28 hours) in terms of periodicity. While per was the sole known circadian gene for a considerable amount of time, gradual advancements research conducted in the 1990s resulted in the discovery of timeless (Sehgal et al ., 1995), clock (Allada et al ., 1998a), and cycle (Rutila et al ., 1998), which together with per constitute the primary transcriptional feedback loop that generates circadian rhythms. Late 1990s and early 2000s, cryptochrome, often known as cry, was identified as a circadian photoreceptor (Emery et al ., 1998). Additional findings included the kinases doubletime (Martinek et al ., 2001b) as well as vrille, a transcription-activating factor connected to a different feedback loop. (Blau & Young, 1999).
In Drosophila melanogaster , the core circadian genes and their functions are crucial for regulating the biological clock.
-
1. Period ( per ): The per gene is involved in the negative\ feedback mechanism of the circadian rhythm. It encodes the PER protein, which accumulates in the cytoplasm during the night and inhibits its own transcription by forming complexes with other proteins like TIM (Timeless). This regulation controls the timing of the circadian rhythm (Hall, 2003).
-
2. Timeless (t im ): The t im gene works in conjunction with per to regulate circadian rhythms. It encodes the TIM protein, which forms a complex with PER in the cytoplasm. This complex inhibits the activity of the CLOCK-CYCLE transcription factor complex, thereby influencing the expression of clock-controlled genes (Price et al ., 1998).
-
3. Clock ( clk ) and Cycle ( cyc ): The clk and cyc genes encode the CLOCK and CYCLE proteins, respectively, which form a heterodimeric transcription factor complex. This complex activates the transcription from the genes tim and per, thus initiating a positive
feedback loop that drives circadian rhythms (Allada et al ., 1998a).
-
4. Cryptochrome ( cry ): The cry gene encodes the CRYPTOCHROME protein, which is involved in photoreception and resetting the circadian clock in response to light cues. It interacts with the TIM protein to regulate its degradation, thereby influencing the period length of the circadian rhythm (Stanewsky et al ., 1998). The clock molecular mechanism
Transcription-based feedback loops: The idea that the PER protein negatively regulates its own transcription to create an auto regulatory circadian loop was developed in response to the discoveries that both per RNA and protein are expressed cyclically and that rising levels of protein are linked to falling levels of mRNA (Siwicki et al ., 1988; Zerr et al ., 1990). Further research validated this idea by demonstrating that the two mRNAS cycle in phase and that transcription is directly influenced by the direct interaction of the PER and TIM proteins (Gekakis et al ., 1995). The foundation of overt rhythms in Drosophila melanogaster is the negative feed-back loop that is thus produced. As will be shown later, additional components must be included in the loop's maintenance. Per and Tim mRNA levels increase reach their zenith in the early evening during the day. From this juncture over the course of the evening, the two proteins begin to amass, first in the cytoplasm and subsequently in the nucleus (Zheng & Sehgal, 2012). Stabilizing Peroxisomal excretion requires TIM for its transport to the nucleus According to (Jang et al ., 2015), certain importins also regulate the location of the two proteins in the nucleus and this regulation it seems to be time-dependent (Curtin et al ., 1995).
Figure 1 shows the reduction in PER and TIM's mRNA levels caused by the proteins' negative autoregulatory feedback is correlated with their nuclear localization. While PER and TIM is unable to bind DNA, they can control transcription by blocking Clock and Cycle, two of their transcriptional activators. Biochemical evidence supports the PER's sequester of the CLK– CYC complex from DNA and a hypothesis in which it enlists the kinase DBT (aCK1e homolog) to stimulate CLK phosphorylation are both plausible, even if the mechanics of negative feedback remain poorly understood (E. Y. Kim & Edery, 2006; Nawathean et al., 2007) . It is unclear what part TIM plays in unfavorable reviews. Early in the morning sees a large reduction in TIM levels, PER expression has also dramatically dropped by midafternoon, lifting the unfavorable comments and enabling a fresh transcription cycle. Beyond the primary feedback loop mentioned previously, the Drosophila melanogaster clock also has a secondary loop that is synchronized with the primary loop (Cyran et al., 2003) state that the CLK–CYC complex in this loop causes the expression of Clk to be repressed by Vrille (VRI) and transcriptionally activated by PDP1. By giving a rhythmic feedback on Clk expression, PDP1 and VRI maintain the rhythmic expression of Clk mRNA. However, as CLK protein levels do not cycle, the cause of the mRNA cycling is unknown (Houl et al., 2006). According to (Glossop et al., 2003), the second loop is intended to increase the system's precision and stability.
Mammals and insects both have similar transcriptional feedback loop regulation mechanisms, but they also have similar clock gene functions. Human circadian disruption Advanced Sleep Phase Disorder has been associated with bovine Per2 and CK1d, homologs of genes originally linked to fruit fly circadian rhythms, according to studies by (Toh et al., 2001; Xu et al., 2005). The functions of homologs of CLK, CYC (also referred to as BMAL1 in humans), and PER in mammals are similar to those in Drosophila melanogaster (Partch et al., 2014). Though, as mammals' The clock receives light input as supplied not belonging to a cell through Renal ganglion cells that are inherently photosensitive (Güler et al., 2008), the closest mammalian homolog of CRY seems to be acting like TIM, working with PER to suppress its own transcription, rather than acting as a photoreceptor. It appears that some insects, like butterflies and honeybees, possess a CRY similar to that of mammals, which can function as an antagonistic transcriptional regulator. This suggests that the mammalian CRY is a remnant of the evolutionary past, even though it appears to have disappeared in Drosophila melanogaster (Rubin et al., 2006). An additional transcriptional feedback loop in mammals is comparable within the fly PDP1/VRI loop (Partch et al., 2014).
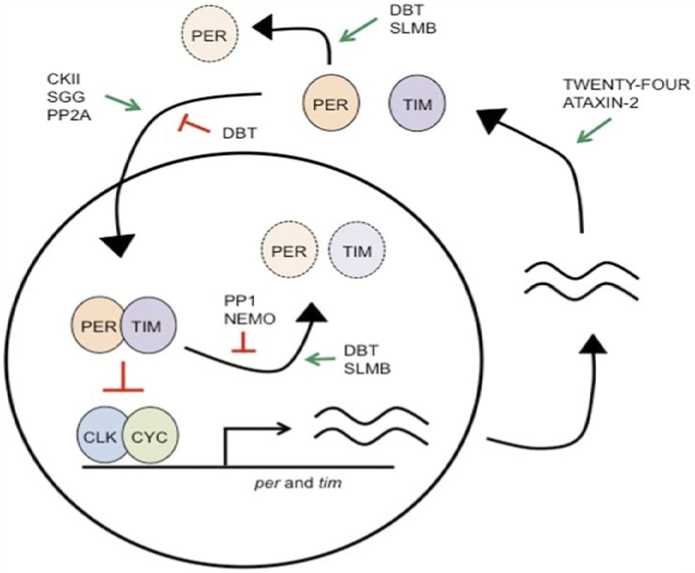
Table 1: Expression of period and timeless genes and its molecular feedback loop
Courtesy:
Regulatory mechanism of circadian gene expression
Higher eukaryotes' circadian clock is thought as a preserved self-regulatory feedback mechanism for transcription and translation that is regulated by rhythmic transcriptional activation and repression. The circadian clocks of mammals and some groups are based on interlocking transcriptional feedback loops that activate transcription by using fundamental helix-loop-helix and Per-Arnt-Sim heterodimers are important transcription factors (Partch et al., 2014). A heterodimer made up of CYCLE (CYC) and CLOCK (CLK) functions as the main transcription factor in the fruit fly Drosophila melanogaster's clock circuit (Allada et al., 1998b; Rutila et al., 1998). When CLK/CYC associates It binds DNA using a conventional Sequences that resemble the Ebox CACGTG (Darlington et al., 2000; Hao et al., 1997; McDonald et al., 2001). In particular, circadian biology is interested in the identification of transcripts that are regulated by CLK/CYC. However, since the sequence pattern is found across the fly genome, e-boxes are not very good markers of potential clock-regulated genes. Conversely, the atoms that make up surrounding the either the E-box or a pattern multiple similar themes spaced near together likely increase synchronize transcriptional process, Particularity of TFs, and improved bonding propensity (Kyriacou & Rosato, 2000; Muñoz et al., 2002). Circadian transcriptional control in Drosophila melanogaster requires Ebox-dependent enhancers, according to investigations on the areas where two clock genes are promoted that are known to be present: period (per) and timeless (tim). As far as circadian enhancer motifs go, the per promoter has probably been explored the most. An enhancer, which stimulates the production of genes related to circadian rhythmsis a 69-base pair tract located beyond the start point of transcribing (Hao et al., 1997). The production of substantial amplitudes and tissue-specific expression by this enhancer is dependent on three sequences near the E-box, while transcriptional activation is mediated by an E-box motif (Hao et al., 1997; Lyons et al., 2000). A 18bp region with an E-box that can recapitulate per spatial and temporal expression.(Darlington et al., 2000). According to (McDonald et al. 2001), the TIM promoter is outfitted with closely spaced E- and TER boxes, a version of the widely used E-box sequence. The two non- original E-boxes, TER1 and TER2, are required for this time enhancer to significantly start the cycle and activate gene expression. Standard E-box included with the accentuater does not seem to work well enough by itself, suggesting that the TER boxes are required to get it to work. More resources for identifying the components in charge of transcriptional control have become available as a result of growing understanding complete cDNAs that have been cloned and annotated, transcription initiation points and locations where transcription factors bind of the Drosophila melanogaster genome (Adams et al., 2000; Stapleton et al., 2002). In a previous investigation, it was discovered that the 69 bp per enhancer contained a similar motif made up of two closely spaced E-box-like components and other CYC/CLK-regulated genes. In response to our earlier findings, a second distinct and separate theme of 29 bps. As the current paper describes the latter motif, also referred to as "CATAC," which stands for Clock-Associated Transcriptional Activation Cassette, and the facts about expression in space and time it provided (Paquet et al., 2008).
Effect of high-fat diet on lifespan, metabolism, fertility and senescence behaviour in Drosophila
Consumption a high-fat diet to increase your chances of becoming obese, which in turn increases your chances of developing diabetes, cancer, heart disease, as well as additional metabolic illnesses. (Bray & Popkin, 1998; Dietrich et al ., 2013; Musselman & Kühnlein, 2018) . There is a connection between obesity and a higher risk of cognitive impairment (Hwang et al ., 2010; Liu et al ., 2015) . Since it has a short life span and is easily raised in big numbers, the fly with genetic tractability A fascinating organism for studying how nutrition affects metabolism, behavior, aging, and lifespan is the Drosophila melanogaster (Heier & Kühnlein, 2018a; Owusu-Ansah & Perrimon, 2014a). Because approximately 65% of the genes linked to human disease have functional orthologs in flies Moreover, Drosophila melanogaster has been used to model a number of human diseases (Ugur et al ., 2016) .
Recent research has shown how the HFD affects the physiology and overall health of Drosophila melanogaster (von Frieling et al ., 2020) . While some of this research looked at the impact of HFD on lifespan, the majority of these studies focused on the consequences of very brief exposure to HFD, lasting one to three weeks (Rivera et al ., 2019) . Previous research has demonstrated that a excessive fat intake substantially shortens the life expectancy of flies, both male and female, (Woodcock et al ., 2015) and negatively impacts their climbing behavior, short-term phototaxis memory, and behavioral reactions to smell (Rivera et al ., 2019) .
Insulin signaling, glucose homeostasis, negative geotaxis, cardiac function, and gut physiology are all negatively impacted by even a brief exposure to a low-fat diet (HFD), lasting only one week (Birse et al., 2010a). It just takes five days of high-fat diet (HFD) exposure for flies to become more susceptible to famine, resulting in an increase in the hyperactivity caused by fasting (Huang et al., 2020). Moreover, it has been demonstrated that the HFD alters the transcription of genes related to cell signaling, memory, metabolism, olfaction, mitosis, and motor function (Rivera et al., 2019). It is unclear how a high-fat diet affects Drosophila melanogaster physiology and longevity negatively. Lipids are necessary for the manufacture of signaling molecules, the assembly of cellular membranes, energy metabolism, and, in adult female flies, oogenesis in particular (Toprak et al., 2020). Since TAG is scarce in the Drosophila melanogaster natural diet, carbohydrates are a primary dietary source of stored lipids. The stomach and fat body subsequently convert these carbs into TAG (Heier & Kühnlein, 2018a). Nevertheless, many lab diets contain lipids, and Drosophila melanogaster is equipped with taste receptors that can identify free fatty acids, (Ahn et al., 2017). When FFA levels are low, these receptors mediate attraction;
Therefore, in order to reduce the negative consequences of increasing dietary FFA intake, flies must mount a reaction. In examining the immediate and long-term impacts of high-fat diet (HFD) on Drosophila melanogaster physiology and longevity, the female flies, whether virgin or mated, exposed to HFD have shorter lifespans and have a faster aging-related loss in their capacity to climb. Three weeks on a high-fat diet (HFD) causes a reduction in fecundity in virgin females, and sleep fragmentation is higher in these females than in controls. A storage organ connected to the foregut, the crop, grows larger after prolonged exposure to a high-fat diet. Based on Nile Red staining, it appears that the distended crops retain fats.
It astonished us that there were no consequences of a high-fat diet over time on body mass triacylglycerids (TAG), glycogen, or glucose levels. Conversely, mated flies that were fed a high-fat diet for a week showed increased TAG and decreased body mass. The mobilization of stored lipids for energy during exercise or fasting is regulated by adipokinetic hormone (AKH), which functions similarly to glucagon in insects (Grönke et al ., 2007; Heier & Kühnlein, 2018a; Toprak et al ., 2020). On the other hand, a number of Drosophila melanogaster insulin-like peptides regulate lipogenesis and the storage of fat after feeding (Heier & Kühnlein, 2018a; Toprak et al ., 2020) . Thus, at the transcript levels of Akh and dilp in flies fed a high-fat diet (HFD) and found that whereas dilp transcripts remained unchanged, Akh transcript levels increased . Lastly, since HFD-fed flies had higher levels of Akh transcripts, AKH may control the impact of HFD on fertility and lifespan. When compared to control flies, akh mutant flies exhibit longer lifespans; nevertheless, both mutants and controls have shorter lifespans when on HFD. Even Nevertheless, both short- and long-term HFD lowers fecundity in both mutants and controls. Additionally, fertility is affected in Akh mutant flies.
Taken together, our findings show that a high-fat diet (HFD) decreases enhances AKH signaling, lowers the amounts of the synthesising enzyme tyrosine hydroxylase in dopaminergic neurons, and negatively impacts longevity, sleep, negative geotaxis, and fertility. Additionally, it appears that the crop may significantly affect the body's response to a diet rich in fat.
Impact of high sugar diet in Drosophila Hypoglycemia in Drosophila melanogaster caused by a high-sugar diet
A high-calorie meal with elevated protein, fat content, or sugar content to wild-type Canton-S Drosophila , while maintaining the same amounts of all other ingredients. Comparable to a banana in terms of proportion, According to (Carsten et al ., 2005) 86.4% of the calories in the high-sugar diet were from carbs. Invertebrates on the elevated glucose diet consumed fewer calories than the larvae on the high-fat and -protein diets, but in comparison to other high-calorie diets, they acquired greater blood glucose levels or more severe hyperglycemia. Trehalose is a glucose disaccharide that is created from intracellular glucose in fat body cells and released into the hemolymph. Chow raised their blood sugar levels to get similar results. According to (Rulifson et al ., 2002b; Song et al ., 2010), Trehalose levels increased in a manner reminiscent of that seen in Drosophila melanogaster with low or no insulin. Conversely, there was a decrease in glycogen levels in larvae fed an excessive sugar intake. Insulin pathway mutants and glycogen levels decreased in a comparable manner (Böhni et al ., 1999; Shingleton et al ., 2005)
Insulin resistance was the outcome of high-sugar diet
UAS-DILP2-FLAG larvae to measure the amounts of circulating DILP. When compared to larvae raised on control diet, the hemolymph of larvae raised on high sugar had higher amounts of FLAG-tagged DILP2. These findings suggested that peripheral insulin resistance was a fundamental problem upon high-sugar feeding, and they also showed that the hemolymph of larvae raised on high sugar was not insulin deficient. Evaluating the efficacy of exogenous insulin to induce Akt phosphorylation at position Ser505 in order to measure insulin resistance. This residue functions as a marker of the activity of the insulin system and is analogous to Ser473 in mammals. When exposed to Human insulin recombinant at 0.5 μM, wandering L3 larvae fed a control diet showed an average 2.8-fold increase in phospho-Akt. In comparison to controls, larvae fed a high-sugar diet showed a markedly reduced sensitivity to insulin. When combined, the traits of insulin-defective growth phenotypes, hyperglycemia, and insulin resistance are indicative of a type 2 diabetes model in larvae fed high sugar.
Impact of high alcohol diet in Drosphila
Measuring alcohol sensitivity in Drosophila
Fermenting plants, or those with three percent or higher alcohol content, are part of Drosophila's natural habitat. Fruit flies may thus effectively metabolize alcohol to use it as a starting material for the synthesis of lipids or as an energy source, making them immune to the harmful effects of alcohol (Geer et al ., 1993) Alcohol is introduced by researchers to the fly culture medium are fed in order to determine whether a specific Drosophila melanogaster strain is immune to the harmful effects of alcohol (Geer et al ., 1993). According to these findings, there are differences in the alcohol resistance of Drosophila melanogaster strains that have been obtained from the wild (Kamping & van Delden, 1978). Moreover, in a laboratory context, researchers found that they could quickly and dramatically increase the alcohol tolerance of a Drosophila melanogaster population. By carefully breeding flies that were resistant to the effects of alcohol vapour (Cohan & Hoffmann, 1986) or that survived exposure to high alcohol levels in their diet (Chambers, 1991), for example, resistant strains were created.
When exposed to vaporized alcohol, adult Drosophila melanogaster display a number of symptoms, including as diminished motor control, that are comparable to acute intoxication in mammals. Scientists placed flies in a small area with grid lines to measure the effect of alcohol on flight. To measure locomotor behavior, one can look at the number of grid lines crossed over time (Singh & Heberlein, 2000). After being exposed, the flies exhibit drowsy and uncoordinated behavior, followed by hyperactivity and confusion in a matter of minutes. When exposed for roughly 20 minutes, they grow motionless;
Using an inebriometer to measure the incapacity of Drosophila melanogaster to stand following alcohol consumption is another method of assessing the effects of alcohol on the species. This device, which was initially created to grow flies immune to alcohol selectively (Weber & Diggins, 1990) consists of a 125 cm vertical tube with several sloping mesh baffles that allow flies to perch. The flies are then made drunk by passing alcohol vapor through the tube. The flies thus lose their ability to maintain their posture and start to fall through the tube. The amount of time it takes them to emerge from the bottom of the tube at a specific alcohol concentration indicates how sensitive they are to alcohol consumption. Therefore, flies that are more susceptible to the loss of postural control caused by alcohol come out of the tube sooner than flies that is more resilient to the effects of alcohol.
Impact of high protein diet in Drosophila
Protein restriction diets (PRDs) are dietary interventions that lower protein intake without putting a person at risk for malnutrition (Green et al ., 2022). Previous researches have documented the advantages of PRD, including enhanced lifespan, treatment of chronic disorders, and betterment of overall organism welfare (Ferraz-Bannitz et al ., 2022). Dietary patterns associated with longevity, including the Guangxi and Okinawan patterns (which consist of 85% carbohydrates and 9% protein), have been linked to Parkinson's disease (PRD) (Han et al ., 2022; Kitada et al ., 2018). According to the throwaway soma idea, PRD's life span benefits result from the transfer of scarce energy sources, spanning from sexual reproduction to bodily upkeep (Shanley & Kirkwood, 2000). Living and procreating in the context of PRD, however, appear to be influenced by the harmony of the macronutrients in food rather than the total calorie the diet's composition, according to recent research (Zanco et al ., 2021).
Furthermore, research indicates that deficiencies in particular amino acids, consisting of branch chain amino acids, tryptophan, and methionine, may have an indirect impact on the restorative and beneficial effects of PRD (Grandison et al., 2009; Orentreich et al., 1993; Segall & Timiras, 1976). Disease and shortened life spans can result from these protein building blocks ' interactions adding additional amino acids or byproducts, which can impair the molecular and physiological homeostatic activities of several organs, whole organisms, and/or organisms (Grandison et al., 2009). It is expensive to maintain mammalian models to simulate metabolic conditions;
The "thrifty gene" theory states those 30,000 years ago, individuals survived better during times of hunger and starvation due to the accumulation of energy during prosperous times. This is how obesity initially emerged (Haslam, 2007; NEEL, 1962). The idea of the "Obesity paradox" (Park et al ., 2014) stems from the observation that whereas a BMI of > 30 kg/m 2 is associated with a rise in all-cause mortality (Flegal et al ., 2013), while a BMI of 25 < 30 kg/m 2 is connected to a fall in deaths from all causes (Di Angelantonio et al ., 2016) and a better prognosis for disease. The preventive functions of obesity considering the fact that elevated adiposity exists independently of the known harmful consequences of obesity, like a rise in co-morbidities and mortality associated with obesity, are also being revealed by obesity research employing Drosophila melanogaster (Lushchak et al ., 2011) . On the other hand, these have been linked to ectopic lipid accumulation, reduced rate of fat turnover, glucotoxicity, and build-up of lipid autotoxins during fly development (Wen et al ., 2018). Lipid accumulation caused by autotoxins, ectopic fat growth, low fat turnover, and glucotoxicity interact with several biological processes, including those that control gene expression, energy homeostasis, cellular stress regulation, pro-inflammatory cytokine regulation, and Protein kinase II reliant on calcium and phosphoinositol-3-protein kinase/Akt pathways; these processes include the Jun-N-terminal kinase pathway and the Adenosine Monophosphate Protein Kinase pathway (Dias et al ., 2018; Wen et al ., 2018). This in turn disturbs regular multi-organ cellular homeostatic processes because of elevated mitochondrial and endoplasmic reticulum stressors. These results in a host of detrimental effects linked to obesity, such as the formation of foam cells, increased production of cytokines, insulin resistance, and hypoxia. These can be clinically manifested as dyslipidaemia, polycystic ovarian syndrome, type II diabetes, osteoarthritis, some malignancies, coronary artery disease, stroke, hypertension, liver disease, and psychiatric problems (Pillon et al ., 2021).
Despite the fact that Drosophila melanogaster is a very good model for obesity (Trinh & Boulianne, 2013), there are few comparative studies on obese phenotypes produced by food. Prior research indicates that Drosophila melanogaster living under a PRD live longer (Regan et al ., 2016). Moreover, PRD has been linked to higher locomotor activity in Drosophila , lower stress resistance, and increased expression of antioxidant genes (Sun et al ., 2012). PRD correlated with increased fat reserves in Drosophila melanogaster larvae in adult flies because of elevated gene expression associated with fat mobilization and droplets of lipid storage in adult flies (Rehman & Varghese, 2021). There are still several difficulties in utilizing Drosophila melanogaster to represent obesity diseaseDespite the fact that Drosophila melanogaster is a highly accurate model of obesity, (Trinh & Boulianne, 2013), there are few comparative studies on obese phenotypes produced by food. There are still several difficulties in utilizing Drosophila melanogasterto represent obesity diseaseThe adoption of the High Sugar Diet to mimic obesity has been related to hyperosmolarity and glucotoxicity in flies (Na et al ., 2015; Rovenko et al ., 2015). Yet, the use of a high-fat diet has been connected to lipotoxicity, which triggers inflammatory processes that endanger signaling networks within cells (Hong et al ., 2016). This effort aimed to make a diet-induced fly obesity model more reliable and healthier by reducing its number of variables. Thus, the purpose of this study was to examine the effects of three distinct diets on the survival, mass, total protein, sterol and triglyceride content, total protein, and catalase activity of obese Drosophila melanogaster larvae and adults. The diets included high fat, high sugar, and protein restriction.
Impact of high stravation diet in Drosophila melanogaster
Starved Drosophila melanogaster larvae exhibit carnivorous behaviour
Recent research has demonstrated that when the melanogaster Drosophila melanogaster larvae are under nutritional stress, they can successfully metamorphose into predators and subsist consuming larger conspecifics as food in a cannibalistic manner (Vijendravarma et al., 2013). Our objective was to assess the degree of starvation-induced carnivorous behavior in because Drosophila melanogaster larvae do not eat carnivorous foods; they are generally classified as herbivores. To track the larvae's propensity for cannibalism, they were raised only on whole, conspecific adult carcasses. Drosophila melanogaster red eye pigment accumulated in the larval intestine as a result of consuming adult corpses. This data aligns nicely with previous research that indicated Drosophila hydei larvae can consume adult carcasses of the same species (Gregg et al., 1990). Following the discovery of cannibalism caused by malnutrition in Drosophila melanogaster larvae, further investigated the possibility of starving larvae consuming carcasses belonging to different taxonomic groups. For the purpose of this study, Larvae were cultivated exclusively on decontaminated carcasses of Araneae sp., Musca domestica, and Apis mellifera. Larvae gathered the adult carcasses in each case and ate them. It was possible to evaluate the preferred diet of well-fed larvae by presenting the Musca domestica carcass and cornmeal medium simultaneously. After 30 minutes of exposure to the carcass and cornmeal medium, there was a noticeable amount of larvae aggregation around the medium as opposed to the carcass.
Starved larvae scavenge conspecific larval carcasses
It has been observed that younger Drosophila melanogaster larvae consume live wandering-stage conspecific larva (Vijendravarma et al ., 2013). Stain-treated larval carcasses were given to the hungry larvae in order to assess their capacity for scavenging conspecific larval carcasses. The accumulation of green dye in the larval stomach indicates that, similar to how they absorbed dyed eggs, starving larvae also consumed stained conspecific larval carcasses. Larval carcasses were stained with two distinct dye colors (red and green), and then they were offered to starving larvae beside a piece of agar with a separate color. On the same petri plate, one hundred starving larvae were given the option of red-colored larval carcasses or green agar. After switching the colors, another batch of 100
starving larvae was given the identical option. Significant larval aggregation was seen for each trial after 30 minutes, suggesting that the nutritional content of the dead conspecifics was devoured by famished larvae and that the color of the dye had no effect on the larvae's intake of their corpses.
Adult Drosophila melanogaster also display cannibalistic behaviour when they are starved
The adult flies could also feed on the carcasses of Drosophila melanogaster larvae when they were starving because of the cannibalistic behavior of Drosophila melanogaster larvae towards dead larvae. In order to ascertain the existence of this type of cannibalism in adult Drosophila , entire, sterilized third-instar larval carcasses were fed to famished flies. Interestingly, these famished adult flies perished in less than a day without eating the corpses of their conspecific larvae. This finding is consistent with a prior study in which adult carcasses were provided to famished flies, but no signs of cannibalism were noted (HUEY et al ., 2004). This may be because adult Drosophila melanogaster do not have the necessary physical features to puncture conspecifics' cuticles (Spencer, 1953). During the same experiment, it's important to note that female adult flies were shown to oviposit primarily on and around the dead carcasses of conspecific third instar larvae. To further understand this behaviour, two sets of larval carcasses coated with Para film or left nude were shown by flies. Compared to the carcasses wrapped with Para film, a notably higher quantity of eggs was laid around the nude larvae. It is commonly known that Drosophila melanogaster egg laying is directly correlated with food availability (Terashima & Bownes, 2004), and that females prefer to lay their eggs close to nutritional substrates (Miller et al ., 2011). Our findings indicate that adult flies favourably deposited their eggs on and around bare larval remains, despite the fact that they are incapable of consuming complete larval carcasses.
Molecular mechanism of aging signaling pathway and circadian clock dependent metabolic pathway
Nutrient signalling in yeast enables colonies physiology and co-ordinate cell division in response to external stimuli. When nutrients are few, 90–99% of the colony dies of natural causes (Fabrizio et al., 2004), leaving the remains of the colony to endure on the limited nutrients. This contributes to the accomplishment of a clonal population's main goal, which is to maximize future replication of its shared genome, by ensuring that a small number of individuals will survive the harsh environmental conditions. A group of tissues in multicellular creatures have unique nutrition sensing abilities. For instance, in C. elegans, the gonad, the colon, and neurons are the main sites of nutrition signalling. Insulin/insulin-like growth factor (IIS) and target of rapamycin (TOR), which are both known to modify longevity, are the key nutritional signalling pathways, just like in higher animals (Lapierre & Hansen, 2012; Panowski & Dillin, 2009). In neurons, the insulin receptor binds insulin-like peptides that are produced in response to nutritional sensing. By communicating with the colon and gonads, loss of DAF-2, the worm's sole insulin/IGF-1-like receptor, lengthens worm life (Libina et al., 2003). Longevity is also aided by germline loss, which enhances autophagy and releases stored fat in the gut (Lapierre et al., 2011; Wang et al., 2008). Lastly, prolonging life and simulating the effects of calorie restriction are achieved by decreasing TOR activity (Kapahi et al., 2010). Combined, these findings demonstrate that C. elegans aging and lifespan are influenced by changes in nutrition signalling during development and reproduction. Coordinating growth and reproduction with the environment through long-range signals, mostly insulin, allows the body to store and use energy efficiently. Similar to C. elegans, IIS signalling is integrated to the TOR pathway in Drosophila melanogaster and mammals via AKT/PI3K to facilitate growth and proliferation (Laplante & Sabatini, 2009; Niccoli & Partridge, 2012; Partridge et al., 2011). Delays in development and smaller, less fertile flies with longer lifespans are caused by inhibition of the TOR or IIS pathways in Drosophila melanogaster (Kapahi et al., 2010; Partridge et al., 2011). Aging-related traits do not arise as quickly in adult flies when similar treatments are applied (Kapahi et al., 2010; Partridge et al., 2011). Moreover, animals deficient in ribosomal S6 protein kinase 1 (S6K1), a downstream element of the TOR system, boast an extended lifetime and demonstrate resilience against age-related illnesses (Bjedov & Partridge, 2011; Shima, 1998; Um et al., 2004; Zhang et al., 2011). Giving rapamycin to worms, flies, or mice prolongs their lives by decreasing TOR signalling (Bjedov & Partridge, 2011). Mice that have reduced levels of the anabolic hormones insulin, growth hormone or IGF-1 live longer and are less susceptible to age-related disease (Brown-Borg & Bartke, 2012; Vinciguerra et al., 2012). Reduced plasma IGF-1-producing mutant mice exhibit a shorter lifespan (Panici et al., 2010)and a lower stature. Similarly, animals lacking the insulin receptor substrate 1 are long-lived but exhibit reduced insulin sensitivity (Selman et al., 2008). A hallmark of longer lifespans in humans is greater insulin sensitivity, which is demonstrated by lower levels of plasma insulin, IGF-1, and glucose (Barzilai et al., 2012). In fact, those lacking the GH receptor and suffering from Laron syndrome exhibit a notable decrease in pro-aging signaling, cancer, and diabetes (Guevara-Aguirre et al., 2011).
Mechanisms of the circadian clock and nutrition metabolism
The majority of species are subject to periodicity due to the yearly cycle around the Sun and the every day the Earth rotates around its axis. Molecular circadian clocks may have developed to best time activities like feeding, mating, and general patterns of activity by co-ordinating internal metabolic rhythms to dependable environmental cycles 38. Furthermore, an increasing amount of data points implies a mutual connection between the circadian rhythm and the nutrition sensing pathway (Peek et al ., 2012).
Circadian signaling in worms
Compared to flies and mammals, C. elegans appears to employ a distinct mechanism to create daily cycles (Banerjee et al ., 2005; Temmerman et al ., 2011;
van der Linden et al ., 2010). Despite the fact that the majority of fundamental circadian genes are preserved in C. elegans (Hasegawa et al ., 2005; Temmerman et al ., 2011) these genes are not known to express periodically in maturity (van der Linden et al ., 2010) and instead often serve to time the periodic moults that occur during nematode larval development (Banerjee et al ., 2005; Tennessen et al ., 2006). These species do, however, exhibit observable regular cycles of circadian that are tied down by warm/cool or light or dark cycles. In terms of traits like motility (Saigusa et al ., 2002; Simonetta et al ., 2009) stress-resistance (Kippert et al ., 2002; Simonetta et al ., 2008), pathogen-resistance (Romanowski et al ., 2011), food and oxygen consumption (Migliori et al ., 2011), and gene expression (van der Linden et al ., 2010), this rhythmicity can be measured in both larvae and adults. Moreover, the circadian process in C. elegans involves conserved signaling components. First, research has shown that C. elegans contributes to circadian rhythmicity during movement (Janssen et al ., 2009). This is due to the action of pigment dispersion factor (PDF) and its receptor, which in insects, similarly to mammalian VIP (vasoactive intestinal peptide), translates circadian oscillations to downstream activities (Duvall & Taghert, 2012; Loh et al ., 2011). Second, although its functional characterization is still pending, it has been recently discovered that melatonin and its synthase undergo circadian cycling (Migliori et al ., 2012). Profiling genes that are rhythmically expressed during and after a 12-hour light cycle is carried out further downstream. Warm or cool cycles, in particular, revealed GO keywords associated with a wide range of biological processes, primarily metabolic processes, that have a higher concentration of genes that are responsive to changes in temperature or can be activated (van der Linden et al ., 2010) . Notably, this study discovered that, in contrast to Drosophila , for instance, cycling of light and temperature entrained unique groups of genes. The discovery suggests that there could be multiple distinct clock systems or distinct gene tagging effects resulting from external stimuli.
Circadian signalling in flies
Moreover, peripheral clocks are present in the majority of fly cells and include cells that oscillate in the excretory system, glia, fat, and sensory neurons are distinct to a certain tissue and not dependent on the central nervous system (Allada & Chung, 2010). Among them rhythmic clock output genes in both peripheral and central clock cells; examples include the enzymes that are involved in the production of glutathione (Beaver et al ., 2012).
CONCLUSION
This review accomplishes that dietary stress induces substantial alterations in Drosophila melanogaster circadian gene expression. High fat and high sugar diets disrupt metabolic pathways and circadian rhythms, contributing to metabolic disorders and obesity. High alcohol diets impair liver function and metabolic regulation, while high protein diets and starvation impact energy balance and aging processes. The findings emphasize the complex interplay between diet, circadian gene expression, and metabolic health. Understanding these interactions can inform strategies to mitigate the adverse effects of dietary stress on circadian rhythms and metabolic health, potentially leading to interventions that promote healthier aging. The review highlights the need for further research to elucidate the detailed molecular mechanisms underlying these effects and to explore potential therapeutic approaches for maintaining circadian and metabolic homeostasis under dietary stress conditions.
CONFLICTS OF INTEREST
The authors declare that they have no potential conflicts of interest.
Список литературы Expression of circadian genes in dietary stress induced Drosophila melanogaster
- Arif Y.K., Singh P., Siddiqui H., Bajguz A. and Hayat S. (2020). Salinity induced physiological and biochemical changes in plants: An omic approach towards salt stress tolerance. Plant Physiol. Biochem., 156, 64-77.
- Baskaran P., Rajeswari B.R. and Jayabalan N. (2006). Development of an In Vitro Regeneration System in Sorghum [Sorghum bicolor (L.) Moench] using Root Transverse Thin Cell Layers. Turkish J. Botany. 30, 1-9.
- Belide S., Vanhercke T., Petrie J.R. and Singh S.P. (2017). Robust genetic transformation of sorghum (Sorghum bicolor L.) using differentiating embryogenic callus induced from immature embryos. Plant Methods,13,109.
- Bhaskaran S. and Smith R.H. (2006). Control of morphogenesis in sorghum by 2,4-D and cytokinins. Ann Bot., 64.
- Brar, D. S., Rambold, S., Gamborg, O., & Constabel, F. (1979). Tissue culture of corn and sorghum. Zeitschrift für Pflanzenphysiologie, 95(5), 377-388.
- Ceasar S.A. and Ignacimuthu S. (2008). Efficient somatic embryogenesis and plant regeneration from shoot apex explants of different Indian genotypes of finger millet (Eleusine coracana (L.) Gaertn.). In Vitro Cell Dev Biol-Plant, 44, 427-435.
- Chen X., Li O., Shi L., Wu X., Xia B. and Pei Z. (2015). To establish the regeneration system of sweet sorghum immature embryos. Adv. Appl. Biotechnol., 333,83–91.
- Chraibi K.M., Latche A., Roustan J.P. and Fallot J. (1991). Stimulation of shoot regeneration from cotyledons shoot regeneration from cotyledons of Helianthus annus by ethylene inhibitors; silver and cobalt. Plant Cell Rep., 10, 204-207.
- Do P.T., Lee H., Mookkan M., Folk W.R. and Zhang Z.J. (2016). Rapid and efficient Agrobacterium-mediated transformation of sorghum (Sorghum bicolor) employing standard binary vectors and bar gene as a selectable marker. Plant Cell Rep., 35, 2065–2076.
- Doganlar Z.B.P. and Yurekli F. (2009). Interactions between cadmium and phytochelatin accumulation in two different sunflower cultivars. Fresenius Environ Bull., 18, 304–310.
- Drouhot S., Raoul F., Crini N., et al., (2014). Responses of wild small mammals to arsenic pollution at a partially remediated mining site in Southern France. Sci. Total Environ., 470–471, 1012–1022. https://doi.org/10.1016/j.scitotenv.2013.10.053
- Elkonin L.A., Lopushanskaya R.F. and Pakhomova N.V. (1996). Embryogenic callus of Sorghum (Sorghum bicolor (L.) Moench) by amino acids. Maydica. 40, 153-157.
- Epelde L., Mijangos I., Becerril J.M. and Garbisu C. (2009). Soil microbial community as bioindicator of the recovery of soil functioning derived from metal phytoextraction with sorghum. Soil Biol. Biochem., 41, 1788–1794.
- Espinoza-Sánchez E. A., Sánchez-Peña Y.A., Torres-Castillo J.A., García-Zambrano E.A., Ramírez J.T., Zavala-García F. and Sinagawa-García S.R. (2018). Somatic embryogenesis induction from immature embryos of Sorghum bicolor L. (Moench). Phyton-Int. J. Exp. Bot., 87, 105–112.
- Fawzy E.M. (2008). Soil remediation using in situ immobilization techniques. Chemistry and Ecology, 24(2), 147-156.
- George L. and Eapen S. (1989). Callus growth and plant regeneration in some Indian cultivates of Sorghum. Current Science, 58, 308-310.
- Gismera M.J., Lacal J., daSilver P., Garcia R., Sevilla M.T. and Procopio J.R. (2004). Study of metal fractionation in river sediments. A comparison between kinetic and sequential extraction procedures. Environ. Pollut., 127, 175-182.
- Gnansounou E., Dauriat A. and Wyman C.E. (2005). Refining sweet sorghum to ethanol and sugar: Economic trade-offs in the context of North China. Bioresour. Technology, 96, 985-1002.
- Grootboom A.W., Mkhonza N.L., O’Kennedy M.M., Chakauya E., Kunert K. and Chikwamba R.K. (2008). In vitro culture and plant regeneration of sorghum genotypes using immature zygotic embryos as plant source. Int. J. Bot., 4, 450–455.
- Gupta S., Khanna V.K., Rameshwar S. and Garg G.K. (2006). Strategies for overcoming genotypic limitations of in vitro regeneration and determination of genetic components of variability of plant regeneration traits in sorghum. PCTOC. 86:376-388.
- Gurel S, Gurel E, Miller TI, Lemaux PG (2012) Agrobacterium-mediated transformation of Sorghum bicolor using immature embryos. Methods Mol. Biol., 847, 109–122.
- Hadebe S.T., Modi A.T. and Mabhaudhi T. (2017). Drought tolerance and water use of cereal crops: a focus on sorghum as a food security crop in sub-Saharan Africa. Journal of Agronomy and Crop Science, 203, 177–191.
- Harshavardhan D., Rani T.S., Ulaganathan S. and Seetharama N. (2002). An improved protocol for regeneration of Sorghum bicolor from Isolated Shoot Apices. Plant Biotechnol., 19(3),163-171.
- Hernandez L.E. and Cooke D.T. (1997). Modifications of root plasma membrane lipid composition of cadmium treated Pisum sativum. J. Exp. Bot., 48, 1375–1381.
- Hossain M.A., Hasanuzzaman M. and Fujita M. (2010). Upregulation of antioxidant and glyoxalase systems by exogenous glycine betaine and proline in mung bean confer tolerance to cadmium stress. PMBP, 16(3), 259-272.
- Huang R. (2018). Research progress on plant tolerance to soil salinity and alkalinity in sorghum. Journal of Integrative Agriculture, 17, 739–746.
- Jha P., Yadav C.B., Anjaiah V. and Bhat V. (2009). In vitro plant regeneration through somatic embryogenesis and direct shoot organogenesis in Pennisetum glaucum (L.) R, Br. In vitro Cell Dev. Biol. Plant, 45, 145-154.
- Jogeswar G., Ranadheer D., Anjaiah V. and Kishor P.B.K. (2007). High frequency somatic embryogenesis and regeneration in different genotypes of Sorghum bicolor (L.) Moench from immature inflorescence explants. In Vitro Cell Development Biology Plant, 43,159-166.
- Kingsley A.P. and Ignacimuthu S. (2014). Enhanced plant regeneration involving somatic embryogenesis from shoot tip explants of Sorghum bicolor (L.) Moench. Asian J. Plant Sci. Res., 4, 26-34.
- Kishore S.N., Visarada K.B.R.S., Lakshmi A.Y., Pashupatinath E., Rao S.V. and Seetharama N. (2006). In vitro culture methods in Sorghum with shoot tips the explant Material. Plant Cell Reports, 25, 174–182.
- Kuriakose S.V. and Prasad M.N.V. (2008). Cadmium stress affects seed germination and seedling growth in Sorghum bicolor (L.) Moench by changing the activities of hydrolyzing enzymes. Plant Growth Regul., 54, 143–156.
- Kuruvinashetti M.S., Patil V.M., Sumangala B. and Maheshwar H. (1998). High frequency plant regeneration from embryogenic callus cultures in genus Sorghum. IJAS, 68(1), 27-28.
- Liu D., Cao X., Zhang H., Liu Z., Yang J. and Wang K. (2014). Effects of Pb. Cd, stress on the growth and Pb, Cd uptake of forage sorghum. Acta AgrestiaSinica, 22(4), 776-782.
- Marchiol L., Fellet G., Perosa D. and Zerbi G. (2007). Removal of trace metals by Sorghum bicolor and Helianthus annuus in a site polluted by industrial wastes: a field experience. Plant Physiol.Biochem., 45, 379–387.
- McKinnon C., Gunderson G. and Nabors M.W. (1986). Plant regeneration by somatic embryogenesis from callus cultures of sweet sorghum. Plant Cell Rep., 5, 349-51.
- Michel-López C.Y., Espadasy G.F., Fuentes O.G., Santamaría J.M., González-Mendoza D., Ceceña-Duran C. and Grimaldo J.O. (2016). Bioaccumulation and effect of cadmium in the photosynthetic apparatus of Prosopis juliflora. Chem. Speciat. Bioavailab., 28, 1–6.
- Mishra A. and Khurana P. (2003). Genotype dependent somatic embryogenesis and regeneration from leaf base cultures of Sorghum bicolor. JPBB, 12, 53-56.
- Murashige T. and Skoog F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant, 15, 473–497.
- Muratova A., Lyubun Y., German K. and Turkovskaya O. (2015). Effect of cadmium stress and inoculation with a heavy-metal-resistant bacterium on the growth and enzyme activity of Sorghum bicolor. Environ. Sci. Pollut. Res., Mythili P., Madhavi A., Reddy V.D. and Seetharam N. (2001). Efficient regeneration of pearl millet Pennisetum glaucum (L.) from shoot tip cultures. Indian J. Exp. Biol., 39, 1274–1279.
- Nayak P. and Sen S.K. (1989). Plant regeneration through somatic embryogenesis from suspension cultures of a minor millet, Paspalum scrobiculatum L. Plant Cell Rep., 8, 296–299.
- Nguyen T., Thu T.T., Claeys M. and Angenon G. (2007). Agrobacterium- mediated transformation of sorghum (Sorghum bicolor (L.) Moench) using an improved in vitro regeneration system. PCTOC, 91,155-164.
- Nirwan R.S. and Kothari S.L. (2004). High frequency shoot organogenesis in Sorghum bicolor (L.) Moench. JPBB, 13, 149-152.
- Okem A., Moyo M., Stirk W., Finnie J. and Van S.J. (2016). Investigating the effect of Cd and aluminium on growth and stress-induced responses in the micropropagated medicinal plant Hypoxishemero callidea. Plant Biol., 18, 805–815.
- Omer R.A., Suliman S. and Beshi M.M. (2021). Regeneration of Sorghum through Tissue Culture Techniques. Int. J. Genetic Engineering, 9(1), 16-20.
- Patnaik D., Mahalakshmi A. and Khurana P. (2005). Effect of water stress and heavy metals on induction of somatic embryogenesis in wheat leaf base cultures. Ind. J. Exp. Bio., 43, 740-745.
- Pola S.R. and Mani S.N. (2006). Somatic embryogenesis and plantlet regeneration in Sorghum bicolor (L.) Moench, from leaf segments. JCMB, 5(2), 99-107.
- Prunhauser L. and Gyulai G. (1993). Effect of copper on shoot and root regeneration in wheat, triticale, rape and tobacco tissue cultures. PCTOC, 35, 131-139.
- Radchuk V., Radchuk R., Pirko Y., Vankova R. and Gaudinova A. (2012). A somaclonal line SE7 of finger millet (Eleusine coracana) exhibits modified cytokinin homeostasis and increased grain yield. JXB, 63, 5497–5506.
- Rao A.M., Sree K.P. and Kishor P.B.K. (1995). Enhanced plant regeneration in grain and sweet sorghum by asparagine, proline and cefotaxime. PCR. 15:72-75.
- Rascio N. and Navari-Izzo F. (2011). Heavy metal hyper accumulating plants: how and why do they do it? And what makes them so interesting? Plant Science, 180(2), 169-181.
- Rooney W.L., Blumenthal J., Bean B. and Mullet J.E. (2007). Designing sorghum as a dedicated bioenergy feedstock. Biofuels, Bioproducts and Biorefining, 1, 147-157.
- Roustan J.P., Latche A. and Fallot J. (1989). Stimulation of Daucus carrota somatic embryogenesis by inhibitors of ethylene synthesis: Cobalt and Nickel. Plant Cell Rep., 8,182.
- Rout G,R,, Samantaray S. and Das P. (1998). The role of nickel on somatic embryogenesis in Setaria italic L., in vitro. Euphytica, 101, 319-324.
- Seetharama N., Sairam R.V. and Rani T.S. (2000). Regeneration of Sorghum bicolor (L.) Moench from shoot tip cultures and field performance of the progeny. PCTOC, 61,169–173.
- Soudek P., Petrová Š., Vaňková R., Song J. and Vaněk T. (2014). Accumulation of heavy metals using Sorghum sp. Chemosphere, 104, 15–24.
- Srivastav S. and Kothari S.L. (2002). Embryonic callus induction and high frequency plant regeneration in pearl millet. Cer. Res. Commun., 30, 69–74.
- Thomas T.D. and Maseena E.A. (2006). Callus induction and plant regeneration in Cardiospermum halicacabum (L.) an important medicinal plant. Scientia Horticulturae, 108,332-336.
- Tiecoura K., Ledoux L. and Dinant M. (2003). Tissue culture of pearl millet. Agronomie Africaine, 15 (3), 105-121.
- Tran T.A. and Popova L.P. (2013). Functions and toxicity of cadmium in plants: recent advances and future prospects. Turk. J. Bot., 37,1–13.
- Varoquaux N., Cole B., Gao C., et al., (2019). Transcriptomic analysis of field droughted sorghum from seedling to maturity reveals biotic and metabolic responses. Proceedings of the National Academy of Sciences, USA 116, 27124–27132.
- Vijayarengan P. (2005). Nitrogen and potassium status of green gram (Vigna radiata) cultivars under nickel stress. Nature Environmental Pollution and Technology, 4(1), 65-69.
- Vijendra PD, Huchappa KM, Lingappa R, Basappa G, Jayanna SG, Kumar V (2016) Physiological and Biochemical Changes in Moth Bean (Vigna aconitifolia L.) under Cadmium Stress. J. Bot., http://dx.doi.org/10.1155/2016/6403938
- Vikrant (2015). Induction of Somatic Embryos from Mature Embryo Culture under Abiotic Stress and Estimation of Proline Status in a Millet Crop, Paspalum scrobiculatum L. IJABR, 6 (1), 96-109.
- Visarada K.B.R.S., SaiKishore N., Balakrishna D. and Rao S.V. (2003). Transient gus expression studies in Sorghum to develop a simple protocol for Agrobacterium mediated genetic transformation. Journal of Genetics and Breeding, 57, 147–154.
- Wuana R.A. and Okieimen F.E. (2011). Heavy metals in contaminated soils: A review of sources, chemistry, risks and best available strategies for remediation. ISRN Ecology, 2011,1-20.
- Xu Z., Wang D., Yang L. and Wei Z. (1984). Somatic embryogenesis and plant regeneration in callus cultured immature inflorescence of Setaria italica. Plant Cell Rep., 3, 144–150.
- Zhuang P., Wensheng S., Zhian L., Bin L., Jintian L. and Jingsong S. (2009). Removal of metals by sorghum plants from contaminated land. J. Environ. Sci., 21,1432–1437.


