Фармакологические аспекты лечения острой гликозидной интоксикации
Автор: Шперлинг М.И., Кручинин Е.Г., Гоголевский А.С.
Журнал: Вестник медицинского института "РЕАВИЗ": реабилитация, врач и здоровье @vestnik-reaviz
Рубрика: Клиническая медицина
Статья в выпуске: 4 (52), 2021 года.
Бесплатный доступ
Несмотря на активное внедрение хирургических методов лечения сердечной патологии и новых синтетических кардиотонических средств, сердечные гликозиды (СГ) остаются важной составляющей фармакологической терапии заболеваний сердца. Более того, интерес к данной группе препаратов только возрастает с учетом открытия новых фармакологических эффектов, таких как противоопухолевый и противовирусный. Однако, несмотря на многолетний опыт применения, вопрос ведения пациентов с симптомами как острой, так и хронической интоксикации СГ остается одним из ведущих. В обзоре литературы приводится краткий исторический очерк отравлений сердечными гликозидами, описываются особенности фармакокинетики и фармакодинамики данной группы препаратов. Рассмотрена клиническая картина отравлений СГ. Проанализированы современные представления и ограничения антидотной терапии, а также особенности патогенетического и симптоматического лечения пациентов с острой гликозидной интоксикацией.
Сердечные гликозиды, гликозидная интоксикация, fab-фрагменты антител к дигоксину
Короткий адрес: https://sciup.org/143177458
IDR: 143177458 | УДК: 615.065 | DOI: 10.20340/vmi-rvz.2021.4.CLIN.1
Текст научной статьи Фармакологические аспекты лечения острой гликозидной интоксикации
УДК 615.065
Сердечные гликозиды (СГ) – это вещества растительного происхождения, оказывающие кардиотоническое действие. На протяжении более 200 лет их применяют для лечения сердечной недостаточности. Впервые лечебные эффекты были описаны в 1785 году британским ученым Уильямом Уитерингом [1]. Также недавно установлено, что СГ оказывают противоопухолевое [2] и противовирусное [3] действие. Серьезным недостатком СГ является их высокая токсичность и небольшая широта терапевтического действия.
Результаты исследования и их обсуждение
На сегодняшний день препараты из группы СГ доказано применяются при лечении хронической сердечной недостаточности (ХСН) и фибрилляции предсердий (ФП) [4, 5]. Несмотря на ограниченное применение СГ в кардиологической практике, доля отравлений ими в группе Т 46 по МКБ-10 (отравление препаратами, действующими преимущественно на сердечно-сосудистую систему) по-прежнему высока и составляет 5,8–6,25 % от общего количества отравлений [6]. Смертность от острых отравлений СГ достигает 6,4 %, от хронических отравлений – 13,4 % [7]. Основными причинами острой интоксикации СГ являются суицидальные действия, ошибочное дозирование препаратов и употребление частей гликозидоносных растений. Среди СГ одним из наиболее токсичных является церберин, содержащийся в цербере одолламской (Cerbera odollam). В Индии и других странах Южной Азии ее называют «деревом самоубийц» (suicide tree) [8]. Также токсическое действие оказывают фитотоксины из группы СГ. К примеру, отравление теветинами – гликозидами желтого олеандра, произрастающего на Шри-Ланке, ежегодно реги- стрируется у 400 человек, смертность при этом достигает 10 % [9].
Особый интерес вызывают исторические случаи использования СГ в качестве криминогенных ядов. Так, «жабьим ядом», содержащим буфадиенолиды, отравили английского короля Иоанна Безземельного, яд был подмешан в «заздравную чашу». В 1864 г. широкий общественный резонанс вызвало дело доктора Кути де ля Поммрэ, который отравил наперстянкой (дигиталисом) свою любовницу. Некоторые современники утверждают, что наперстянкой отравился Винсент Ван Гог. Его известное пристрастие к оттенкам желтого цвета и душевное заболевание можно объяснить хроническим отравлением дигиталисом. В период с 1990 по 2003 гг. сотрудник домов престарелых в американских штатах Нью-Джерси и Пенсильвания Чарльз Каллен отравил дигоксином 40 человек. Наперстянку использовали в мошеннических целях в 1930-х гг., когда врачи и юристы договорились применять это растение, чтобы спровоцировать на короткое время симптомы заболевания сердца и затем потребовать возмещение от страховых компаний [10].
По состоянию на апрель 2021 г. в Государственный реестр лекарственных средств (ГРЛС), разрешенных к медицинскому использованию в Российской Федерации, помимо различных лекарственных форм дигоксина, внесены ланатозид Ц, ландыша листьев гликозид и уабаин [11].
Основные источники СГ в Российской Федерации – наперстянка пурпуровая, наперстянка шерстистая, наперстянка крупноцветковая, горицвет весенний, ландыш майский, за рубежом – различные виды наперстянки, строфанта, олеандр желтый [12, 13].
Химическое строение СГ установлено в 30-е годы ХХ века в работах американских ученых W.A. Jacobs, R. Tschesche. Молекула СГ состоит из двух частей: сахаристой (гликона) и несахаристой (агликона или ге-нина) [14]. Гликоны влияют на фармакокинетику СГ: растворимость и проникновение в ткани. Агликоны имеют стероидное строение и определяют биологическую активность и частично фармакокинетику. К стероидному ядру СГ присоединено лактонное кольцо [15]. СГ с пятичленным лактонным кольцом получили название карденолиды, СГ с шестичленным лактонным кольцом – буфадиенолиды. Карденолидами являются большинство сердечных гликозидов. Буфа-диенолиды обнаружены в морском луке, морознике и секрете кожных желез лягушек и жаб (Bufo – род жаб) [16].
Фармако- и токсикодинамика
Сердечные гликозиды связываются лактонным кольцом (агликоновой частью) с сульфгидрильными группами α-субъединицы фосфорилированной формы Na+, K+-зависимой АТФазы на внешней поверхности сарколеммы кардиомиоцитов и обратимо ингибируют фермент [17]. Примечательно, что высокое содержание ионов калия в плазме усиливает дефосфорилирование Na+, K+-зависимой АТФазы и препятствует полноценному связыванию СГ с ферментом. Этим объясняется усиление токсичности СГ при исходно низком уровне калия в плазме [18, 19]. При ингибировании Na+, K+-зависимой АТФазы ионы натрия во время реполяризации полностью не выводятся из кардиомиоцитов. Вследствие этого уменьшается электрохимический градиент для входа ионов натрия и нарушается функционирование Na+/Ca2+-обменника. Результатом является задержка выхода кальция и его накопление внутри клетки. Высокая концентрация ионов кальция в цитоплазме способствует усилению образования актомиозинового комплекса, что приводит к ускорению и усилению сокращения миофибрилл (положительный инотропный эффект). Кроме того, ионы кальция с помощью транспортера SERCA2 де- понируются в саркоплазматическом ретикулуме (СПР) [20]. Активность SERCA2 регулируется специфическим белком – фос-фоламбаном. В дефосфорилированном состоянии он связан с транспортером и инактивирует его. При фосфорилировании связь с SERCA2 отсутствует, что усиливает утилизацию кальция. Это приводит как к большему диастолическому расслаблению (положительный лузитропный эффект), так и к усиленному сокращению в систолу за счет мобилизации большего количества кальция из СПР (положительный инотропный эффект) [21]. Фосфоламбан фосфорилируется преимущественно через активированную протеинкиназу А (при стимуляции бета-адренорецепторов) и через кальций-кальмодуллин зависимую протеинкиназу (CaMKII) (за счет повышения концентрации кальция в цитозоле). Посредством последней активируется SERCA2 при действии СГ (уабаин приводит к усилению концентрации цитоплазматического кальция – активация СаМКII – фосфорилирование фосфоламба-на – повышение активности переносчика). Эта особенность описана как концепция так называемой «плазмеросомы» (PlasmERosome), которая представляет собой пространство местно повышенной концентрации натрия и кальция между плазма-леммой и СПР [20]. Чрезмерный рост кальция здесь (при нарушении работы натрийкальциевого переносчика (СГ)) приводит приводит к активации SERCA2. В дальнейшем посредством активации инозитол-3-фосфатного рецептора (IP3R) при воздействии любого агониста рецепторов, сопряженных с Gq-белком (аргинин-вазопрессин, эндотелин-1, эпинефрин, ангиотензин-2) стимулируется открытие рианодиновых кальциевых каналов (RYR2) СПР (по механизму кальций-индуцированного высвобождения кальция [22]), что приводит к дополнительному выбросу кальция из СПР и повышению его концентрации в цитозоле. Также СГ реализуют свой механизм с другой стороны: cвязавшись с Na+, K+-зависимой АТФазой, молекула СГ приводит к конфор- мационным изменениям N-концевого фрагмента α-субъединицы данного фермента, что приводит к его связыванию с фосфолипазой С (ФЛ-С) [23]. Данная фосфолипаза способствует, во-первых, активации IP3R, а во-вторых, образованию диа-цилглицерола (ДАГ) и активации протеин-киназы С, которая, в свою очередь, способна фосфорилировать и активировать трансмембранные Ca2+ каналы L-типа, что также увеличивает свободную внутриклеточную концентрацию кальция [24] (рис. 1).
Кроме усиления сократительной способности миокарда, СГ оказывают влияние и на другие кардиотропные эффекты, реализуемые посредством как прямого, так и опосредованного действия. Прямое действие заключается в непосредственном снижении симпатического тонуса (сниже- ние выброса катехоламинов), в то время как опосредованное за счет гиперактивации парасимпатической нервной системы из-за блокады Na+, K+-зависимой АТФазы на мембране нейронов, повышения выработки ацетилхолина в нервных терминалях и повышения чувствительности мускариновых рецепторов к ним. Помимо этого, увеличенный ударный объем крови стимулирует барорецепторы дуги аорты и каротидного синуса, что дополнительно рефлекторно повышает тонус блуждающего нерва. Перечисленные эффекты в совокупности реализуются в виде снижения ЧСС (отрицательный хронотропный эффект), возбудимости (отрицательный батмотропный эффект) и проводимости (отрицательный дро-мотропный эффект) [18, 25].
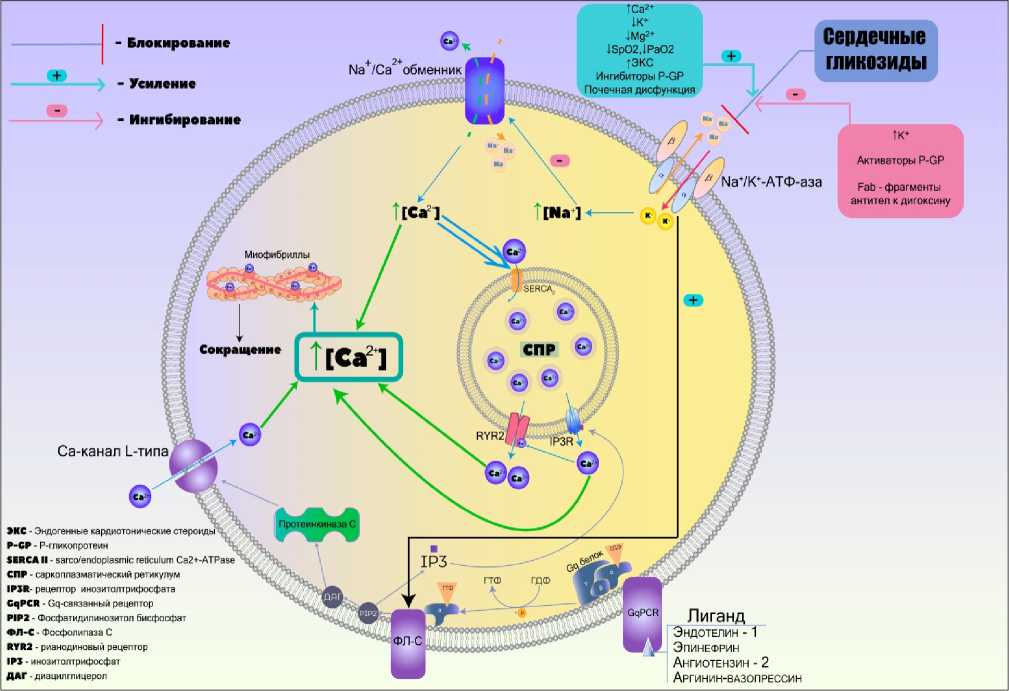
Рис. 1. Клеточные особенности механизма действия сердечных гликозидов
Fig. 1. Comprehensive cellular features of the cardiac glycosides action
Токсическое действие СГ обусловлено чрезмерным ингибированием Na+, K+-зависимой АТФазы. При остром отравлении это приводит к нарушениям сразу в нескольких направлениях. Чрезмерное повышение внутриклеточной концентрации ионов натрия и гипокалигистия в миокарде могут вызывать жизнеугрожающие желудочковые аритмии [26]. Снижение активности синоатриального узла и замедление проведения по АВ-узлу создают предпосылки для развития предсердных триггерных (эктопических) и реципрокных («re-entry») тахиаритмий [25].
Высокий риск интоксикации СГ связан с малой широтой их терапевтического действия, длительным периодом полуэлиминации, значительным влиянием экстракар-диальных факторов. Интоксикация может развиваться даже при нормальной концентрации СГ в плазме [27]. Чувствительность миокарда к токсическому действию СГ повышают гиперкальциемия, гипокалиемия, гипомагниемия, почечная недостаточность, гипоксемия, нарушение кислотно-основного состояния плазмы, высокое содержание эндогенных кардиотонических стероидов [28, 29]. Необходимо также учитывать взаимодействие СГ с совместно назначенными лекарственными средствами.
Фармако- и токсикокинетика
Несмотря на близость химической структуры СГ отличаются по параметрам фармакокинетики. Это объясняется степенью полярности молекул, которая зависит от наличия гидроксильных групп в агликоне: полярные СГ (строфантин) хорошо растворимы в воде, плохо всасываются из ЖКТ и почти не связываются с белками плазмы, в то время как неполярные (дигитоксин) – наоборот. В настоящее время наиболее изученным с точки зрения отравлений считается дигоксин, молекула которого относительно полярна. Дигоксин на 70–80 % всасывается из проксимального отдела тонкой кишки. Биодоступность его при приеме внутрь варьируется в пределах от 50 % до 90 %, но при приеме в форме желатинизированных капсул достигает 100 %. Около 75 % дозы гликозида элиминируется с мочой в неизмененном виде. В малой степени дигоксин метаболизируется печенью и кишечной микрофлорой (с образованием соответственно неактивных ди-гоксигенина и дигидродигоксина). Система цитохромов Р450 не играет значительной роли в кинетике дигоксина, что исключает значительное влияние активаторов и ингибиторов данного фермента на кинетику препарата [18]. Однако при этом он является субстратом гликопротеина Р энтероци-тов и нефроцитов [30]. Индукторы гликопротеина Р (рифампицин) ускоряют элиминацию дигоксина, ингибиторы (кларитромицин) – замедляют и усиливают действие [31]. У людей с сохранненной функцией почек период полувыведения дигоксина составляет 36–48 ч, дигитоксина – 8 дней, уабаина – 11 ч, олеандрина – 2,5 ч.
Особенности биораспределения СГ можно рассмотреть на примере дигоксина. При пероральном поступлении дигоксин следует двухфазной (двухкомпартментной) модели распределения [32]. В начальную фазу препарат накапливается в центральном компартменте (малый объем), состоящем в основном из плазмы и тканей с высокой перфузией, таких как печень. Вскоре наступает вторая, более медленная фаза распределения, которая перемещает лекарственное средство из плазмы в периферический глубокий тканевой компарт-мент (большой объем), который включает в себя сердце (рис. 2). Следовательно, выраженность и время начала клинических (кардиальных) проявлений интоксикации зависит от накопления дигоксина во втором компартменте. Поэтому концентрация препарата в плазме не может отражать истинную концентрацию лекарственного средства в точке приложения дигоксина (миокард) до достижения равновесия концентрации препарата между компартмен-тами (не менее 6 часов от момента отравления). Доказано, что концентрация дигок- сина в органах и тканях до установления равновесия выше, чем таковая в плазме, что также может привести к неверной интерпретации употребленной дозы. Этим также объясняется поздний пик клинических проявлений интоксикации (чаще не раньше 6 часов), несмотря на более раннее достижение максимальной концентрации дигоксина в плазме (1–2 часа) [33].
Клиническая картина острой гликозидной интоксикации
Картина острой интоксикации в целом сходна и не зависит от вида СГ. Выделяют кардиальные и экстракардиальные симптомы интоксикации. Кардиальные симптомы включают различные виды нарушений ритма и проводимости сердца: пароксизмальную предсердную тахикардию, тахикардию из атриовентрикулярного узла, двунаправленную желудочковую тахикардию или синусовую брадикардию. Наиболее частым проявлением нарушения проводимости является развитие атриовентрикулярной блокады, нередко сочетаемое с идиовентрикулярным ритмом, что при полной блокаде и ФП называют синдромом Фредерика [34]. Именно тяжесть кардиальных нарушений определяет исход интоксикации. Основной причиной смерти является фибрилляция желудочков и возникающая вследствие этого острая сердечнососудистая недостаточность [25]. Экстра-кардиальные проявления при остром отравлении СГ представлены чаще всего психоневрологическими (головокружение, спутанность сознания, нарушение зрения, слабость, дезориентация) и желудочнокишечными (анорексия, тошнота, рвота, диарея) симптомами [18].
Диагностика острой гликозидной интоксикации Стратификация риска смерти при острой гликозидной интоксикации в настоящее время не разработана. Это связано c рядом причин. Во-первых, низкая корреляция между концентрацией дигоксина в плазме и тяжестью клинических проявлений интоксикации, что связано с особенностями распределения. Во-вторых, наличие эндогенных дигиталис-подобных факторов в организме может в той или иной степени влиять на значение концентрации СГ [18]. В-третьих, индивидуальный профиль пациентов и сопутствующие состояния, прием некоторых препаратов делают границу интоксикации весьма условной. Тем не менее, общепринятым значением, при котором риск острой интоксикации возрастает, считается концентрация дигоксина в плазме 2,0 нг/мл [18, 25]. Для верификации диагноза необходимо учитывать анамнез и клинические симптомы с целью уточнения источника отравления и примерной дозы СГ [18, 35]. Риски, связанные с интоксикацией, уменьшаются, если на ЭКГ отсутствуют признаки аритмии, концентрация ионов калия в плазме определяется в пределах референсных значений и концентрация дигоксина ниже 2,3 нмоль/л [36]. При отравлении СГ чаще всего повышается концентрация ионов калия в плазме (особенно при нарушенной почечной функции), но возможны нормо- и гипокалиемия [18]. По данным Bismuth C. et al. границей неблагоприятного прогноза при острой интоксикации СГ необходимо считать уровень калия в плазме в диапазоне 5,0–5,5 ммоль/л [37].
В настоящее время обязательным является проведение токсикологического исследования. Для лабораторной диагностики СГ наиболее часто используют поляризационный флуоресцентный иммуноанализ (ПФИА) [35, 38]. СГ обладают свойством перекрестной иммунореактивности, что позволяет оценивать при помощи антител к дигоксину не только концентрацию дигоксина, но и других СГ. Вместе с тем неспе-цифичность может быть и недостатком. Реже могут быть применены хемолюминесцентный и турбидиметрический иммуно-ферментный анализ [38]. Широкое применение нашла также масс-спектрометрия в сочетании с высокоэффективной или со сверхбыстрой жидкостной хроматографией
-
[39] . В качестве экспресс-диагностики разработаны и внедряются в практику иммуно-хроматографические тест-полоски [40].
Кроме вышеперечисленных методов диагностики особое место занимает электрокардиография. Отравление СГ зачастую характеризуется проявлением типичных для данного состояния ЭКГ-паттернов: корытообразное смещение сегмента RS – Т ниже изолинии, наличие двухфазного или отрицательного зубца Т, а также нарушения ритма и проводимости, о которых было сказано выше.
Лечение острой гликозидной интоксикации
Лечение острого отравления сердечными гликозидами должно быть комплексным и включать детоксикационную, антидотную, патогенетическую и симптоматическую терапию (рис. 2).
Ускорение элиминациии СГ из организма и детоксикация
Долгое время терапия острого отравления СГ ограничивалась лишь мероприятиями, направленными на ограничение всасывания вещества из желудочно-кишечного тракта, и поддерживающей терапией. Ведущим подходом в лечении считалось применение энтеросорбентов в виде активированного угля. Из других сорбентов ряд авторов обнаружили положительный эффект от применения холестирамина и колести-пола, прием которых сопровождался выделением дигитоксина с калом посредством усиления выведения препарата из энтеро-гепатической циркуляции и снижения всасывания в ЖКТ [41, 42]. Во второй половине ХХ века были разработаны методы, целью которых являлась скорейшая элиминация СГ из организма. В 1978 г. были предложены методы гемоперфузии и гемодиализа через угольные фильтры для выведения дигоксина и дигитоксина [43]. Кли- ренс препарата повышался при использовании гемофильтрации, однако состояние пострадавших улучшалось незначительно. Причиной неэффективности является большой объем распределения дигоксина в тканях, а также большая масса молекулы. В настоящее время однозначно определено, что эффективность любых экстракорпоральных методов детоксикации при отравлении СГ не доказана, и к применению они не рекомендованы [44].
В 2006 г. был опубликован систематический обзор 24 исследований (1966–2006 гг.), посвященных характеристике антидотов и средств патогенетической и симптоматической терапии при острой гликозидной интоксикации [45]. Обсуждалось использование атропина, изопреналина, фруктозо-1,6-дифосфата, глюкагона, натрия гидрокарбоната, солей магния, фенитоина, Fab-фрагментов антител к дигоксину и многократное применение активированного угля. Впоследствии из этого списка были исключены 22 обзора, так как в них рассматривались результаты нерандомизированных клинических испытаний.
В одном из исследований данного метаанализа для ускорения элиминации СГ 201 пострадавший принимал внутрь активированный уголь по 50 г каждые 6 ч на протяжении 3 дней [46]. При многократном применении активированного угля смертность уменьшилась на 5 % (16 случаев против 3; ОР = 0,31, 95 % ДИ 0,12–0,83), число случаев тяжелых аритмий – на 5 % (14 случаев против 3; ОР = 0,21, 95 % ДИ 0,06–0,71), потребность в кардиостимуляции – на 5,5 % (11 случаев против 1; ОР = 0,09, 95 % ДИ 0,01–0,70). В результате вышеприведенного и ряда других исследований было доказано, что оптимально принимать активированный уголь в дозе 50 г в течение 2 ч после отравления с последующим приемом в дозе 0,5–1,0 г/кг массы тела пациента каждые 2–4 ч [47].
|
Острая гликозидная интоксикация |
Замедление i__J промывание абсорбции ™Ka Нагрузочная: 50 мг per os. Затем 0.5 -1.0 Активированный ,-----г мг/кг каждые 2-4 ч в _____Уголь_______ течение суток ________ Детоксикация — __________ —► Другие сорбенты а----—Холестирамин, ________________ Коле стипол |
Жизнеугрожающие аритмии
Разовая летальная доза (> 10 мг у здоровых взрослых; > 4 мг (> 0.1 мг/кг) у здоровых детей)
Признаки полиорганной недостаточности
Гиперкалиемия (> S.S мЭкв/л у взрослых;
-
> 6 мЭкв/л у детей)
Концентрация сердечных гликозидов в плазме более 10 нмоль/л
Лечение аритмий
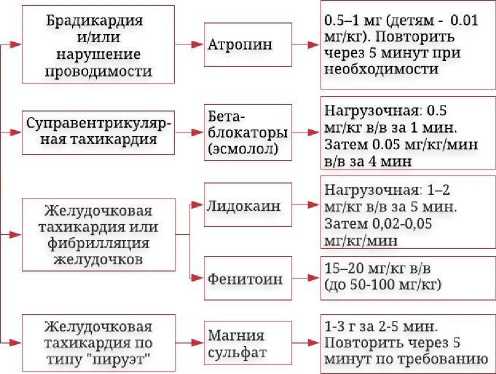
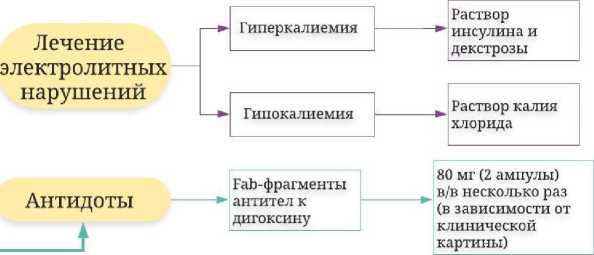
Рис. 2. Лечение острой гликозидной интоксикации
Fig. 2. Management of acute cardiac glycoside poisoning
Антидотная терапия
В другом исследовании вышеуказанного систематического обзора для лечения острой интоксикации желтым олеандром использовали Fab-фрагменты антител к дигоксину [48]. В исследовании приняли участие 34 пациента с острой гликозидной интоксикацией желтым олеандром, которые получали антитела к дигоксину. Было установлено, что спустя 2 ч после введения Fab-фрагментов аритмии прекратились у 15 пациентов, в контрольной группе – у 2 пациентов (p < 0,001). Также определено, что при введении Fab-фрагментов значительно увеличилась частота сердечных сокращений у пациентов с брадиаритмиями (с 49,1 до 66,8 уд./мин.), в плазме через 2 ч снизился уровень ионов калия. В контрольной группе эти показатели практически не изменились.
В другом когортном исследовании 150 пациентов с тяжелой острой гликозидной интоксикацией получали Fab-фрагменты антител к дигоксину. У 80 % пострадавших через 19 мин. симптомы отравления проходили [49].
Fab-фрагменты антител к дигоксину не обладают видовой специфичностью и, ввиду своей перекрестной иммунореактивности, связывают другие СГ [50]. Отсутствие
Fc-фрагмента приводит к снижению возникновения аллергических реакций (в 1 % случаев), а также к уменьшению молекулы, что приводит к значительному увеличению объема распределения [51]. Fab-фрагменты антител связывают молекулу СГ в неактивный комплекс, и, обладая высоким аффинитетом к Na+, K+-зависимой АТФазе, препятствуют ее ингибированию гликозидами. При введении Fab-фрагментов концентрация СГ в плазме в течение 30 мин снижается до следовых значений. Комплекс СГ с Fab-фрагментом при нормальной функции почек элиминируется с мочой за 15–20 ч [52]. Необходимо отметить, что анализаторы не различают свободные СГ от связанных [53].
На сегодняшний день в США зарегистрировано 2 препарата Fab-фрагментов антител к дигоксину – DigiFab и Digibind (ампулы, содержащие 40 мг и 38 мг Fab-фрагментов овечьих антител соответственно). Содержимое ампулы вводится внутривенно после разведения в 4 мл воды для инъекций. Препараты имеют высокую стоимость, поэтому должны быть определены точные показания к его применению и оптимальный режим дозирования. Управлением по санитарному надзору за качеством пищевых продуктов и медикаментов (Food and drug administration, FDA) одобрены следующие показания к назначению Fab-фрагментов к дигоксину при острой интоксикации [54]:
-
1. Любая передозировка дигоксином, приведшая к жизнеугрожающей аритмии (желудочковой тахикардии или фибрилляции желудочков, асистолии, атриовентрикулярной блокаде 2 степени (Мобитц II) или полной атриовентрикулярной блокаде, брадикардии).
-
2. Разовое употребление смертельной дозы (> 10 мг у здоровых взрослых; > 4 мг (> 0,1 мг/кг) у здоровых детей).
-
3. Признаки органной недостаточности (почечной недостаточности, нарушении сознания).
-
4. Гиперкалиемия (> 5,5 мг-экв/л у взрослых; > 6 мг-экв/л у детей).
-
5. Концентрация СГ в плазме более 10 нмоль/л.
Согласно FDA, введение антидота должно осуществляться единовременно и в больших дозах исходя из концентрации СГ в плазме или поглощенной дозы (при неизвестном количестве яда – 20 доз (800 мг), при известном – из расчета, что одна ампула инактивирует примерно 0,5 мг СГ). Однако оптимальный режим дозирования до сих пор не установлен. Многие исследователи считают, что антидот вводят в неоправданно больших дозах, ориентируясь лишь на концентрацию СГ в плазме и количество яда без учета особенностей распределения. Ряд авторов полагают, что оптимально использовать Fab-фрагменты антител в стартовой дозе 80 мг, затем вводить повторно в этой же дозе [33, 55], исходя из двухкомпартментной модели распределения. Более детально данный подход к терапии удалось подтвердить методом физиологического фармакокинетического моделирования (Physiologically based pharmacokinetic model – PBPM). В исследовании Bracken L.M. et al. фармакокинетику дигоксина изучали в организме, разделенном на 10 компартментов: плазму, сердце, скелетные мышцы, жировую ткань, головной мозг, кости, кожу, печень, почки и остаточный объем распределения. В исследовании принимали участие пациенты с клинической картиной острой интоксикации дигоксином, у которых оценивали концентрацию дигоксина в плазме в разные периоды до и после лечения Fab-фрагментами антител. Аналогичные показатели были получены при помощи моделирования. Между реальными и моделированными данными регистрировалась сильная корреляция (r > 0,7; p < 0,005 во всех случаях). Это подтверждает валидность модели. По моделированным концентрациям дигоксина в компартментах в различных временных точках было установлено, что болюсное введение антидота в большой концентрации менее эффективно, чем дробное введение в намного более низких концентрациях. Это заключение было подтверждено и в клинической части того же исследования. Дробное введение Fab-фрагментов в дозе 80 мг четырехкратно приводило к значительно более низкой концентрации дигоксина на всех контрольных точках по сравнению с введением 800 мг однократно [56].
При невозможности введения Fab-фрагментов антител к дигоксину проводят инфузионную терапию. При этом необходимо учитывать содержание электролитов в плазме и кислотно-основное равновесие. В Российской Федерации в качестве неспецифического антидота используют ди-меркаптопропансульфонат натрия (уни-тиол). За счет двух сульфгидрильных групп через лактонное кольцо унитиол связывает циркулирующие СГ, и тем самым реактивирует Na+-К+-АТФазу. Препарат вводят внутримышечно в виде 5 % водного раствора из расчета 1 мл на 10 кг массы тела пациента 2–3 раза в сутки в течение 3–4 сут. Допустимо введение в однократной дозе 250–500 мг (5–10 мл 5 % раствора). Суточная доза составляет 3 г и более (15–20 мг/кг).
Лечение аритмий
Для устранения брадикардии и атриовентрикулярной блокады целесообразно введение м-холиноблокатора атропина в дозе 0,5–1 мг (детям – в дозе 0,01 мг/кг массы тела) и повторное введение через 5 мин при необходимости. При частоте сердечных сокращений менее 40 ударов в минуту атропин вводят в дозе 2–3 мг [36]. В больших дозах он может вызывать тахиаритмии. При наджелудочковых тахиаритмиях применяют β -адреноблокаторы, преимущественно короткодействующий эсмо-лол, так как он меньше нарушает атриовентрикулярную проводимость. При желудочковой тахикардии и фибрилляции желудочков внутривенно вводят лидокаин в дозе 1,0–1,5 мг/кг (в дальнейшем в дозе
1–4 мг/мин) или фенитоин (в нагрузочной дозе 15–20 мг/кг), слабо влияющие на атриовентрикулярную проводимость [12]. В случае возникновения полиморфной желудочковой тахикардии по типу «пируэт» требуется внутривенное введение раствора сернокислой магнезии в дозе 1–3 г за 2–5 минут. Снижение концентрации СГ при использовании Fab-фрагментов антител к дигоксину также способствует устранению аритмий [12, 36, 45, 57]. Кроме этого, ряд авторов отмечают высокий антиаритмоген-ный эффект при использовании таурина в случае интоксикации СГ [58, 59], что связано с модулирующим действием таурина на концентрацию ионов кальция и натрия в цитозоле за счет реактивации Na+/Ca2+-переносчика и предотвращения кальций-индуцированной гибели кардиомиоцитов [60]. Применение электрической кардиоверсии не рекомендуется из-за высокого риска развития фибрилляции желудочков [61]. Запрещено использование недигидро-пиридиновых блокаторов кальциевых каналов (верапамила), α- , β -адреноблокаторов (карведилола), амиодарона, хинидина, про-пафенона. Эти лекарственные средства ингибируют гликопротеин Р и снижают почечный клиренс дигоксина [57, 62].
Лечение электролитных нарушений
При острой гликозидной интоксикации достаточно часто возникает жизнеугрожающая гиперкалиемия, требующая неотложной коррекции. Необходимо помнить об опасности внутривенного введения кальция хлорида с целью устранения гиперкалиемии, вызванной токсическим действием СГ, так как ионы кальция способны усиливать проаритмогенное действие СГ [63]. Однако ряд авторов в клинических [64, 65] и экспериментальных [66] исследованиях отметили, что введение кальция хлорида далеко не всегда ассоциируется с повышением смертности от интоксикации СГ.
Доказана эффективность внутривенного введения инсулина в растворе декстрозы для снижения уровня калия в плазме.
Так, в эксперименте на крысах с моделированной интоксикацией дигоксином, такое введение приводило к снижению уровня ионов калия в плазме с 7,0 до 4,5 ммоль/л [67]. Устранению гиперкалиемии способствует также применение Fab-фрагментов антител к дигоксину [33, 48, 51].
Заключение
Несмотря на многолетнюю историю изучения острой гликозидной интоксикации, многие вопросы оптимального ведения пациентов остаются нерешенными. Частота отравлений и летальность от применения сердечных гликозидов остается слишком высокой. Особенности фармакологии главного представителя из группы СГ – дигоксина, а также его узкое терапевтическое окно являются главными факторами, лежащими в основе развития тяжелой интоксикации. Особенно трудна диагностика и прогнозирование тяжести интоксикации ввиду множества факторов, влияющих на интерпретацию результатов лабораторных исследований. Картина отравлений СГ широко представлена различными патологи- ческими изменениями, главными из которых являются жизнеугрожающие аритмии, электролитные нарушения и неврологические расстройства. Основными направлениями лечения являются детоксикационная антидотная терапия, а также коррекция развившихся осложнений. Доказана терапевтическая эффективность Fab-фрагментов антител к дигоксину. Главным ограничением для использования данного метода являются отсутствие препарата в Российской Федерации, а также его высокая стоимость, ввиду чего наиболее эффективным может являться применение унитиола. Лечебный эффект достигается также при многократном применении активированного угля. Применение в качестве антидотов других средств требует более глубокого изучения и на сегодняшний день не является однозначно доказанным. Основа патогенетической терапии заключается главным образом в борьбе с жизнеугрожающими аритмиями, а также в проведении детоксикационных мероприятий и коррекции электролитных нарушений.
Список литературы Фармакологические аспекты лечения острой гликозидной интоксикации
- Withering W. An account on the foxglove, and some of its medical uses with practical remark on dropsy and other diseases. G. G. J. & J. Robinson, London.1785:207. https://doi.org/10.5962/bhl.title.3869
- Calderón-Montaño J.M., Burgos-Morón E., Orta M.L., Maldonado-Navas D., García-Domínguez I., López-Lázaro M. Evaluating the cancer therapeutic potential of cardiac glycosides. Biomed. Res. Int. 2014:794930. https://doi.org/10.1155/2014/793930
- Amarelle L, Lecuona E. The Antiviral Effects of Na,K-ATPase Inhibition: A Minireview. Int J Mol Sci. 2018;19 (8):2154. https://doi.org/10.3390/ijms19082154
- Ponikowski P., Voors A.A., Anker S.D., Bueno H., Cleland J.G.F., Coats A.J.S. et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. European Heart Journal. 2016;37(27): 2129-2200. https://doi.org/10.1093/eurheartj/ehw128
- Kirchhof P., Benussi S., Kotecha D., Ahlsson A., Atar D., Casadei B. et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS, European Heart Journal. 2016;37(38):2893-2962. https://doi.org/10.1093/eurheartj/ehw210
- Suvorov A.V., Kaurov Ya.V., Suvorov M.A. Features of cardiac arrhythmias and conduction in acute poisoning with cardiotoxic substances. Bulletin of RUDN university, Medicine. 2014;(3):26-30 (in Russ).
- Supervia Caparros A., Salgado Garcia E., Calpe Perarnau X., Galicia Paredes M., Garcia Gibert L., Cordoba Ruiz F., Clemente Rodríguez C., Nogué Xarau S. Immediate and 30 days mortality in digoxin poisoning cases attended in the Hospital Emergency Services of Catalonia, Spain. Emergencias Rev la Soc Esp Med Emergencias. 2019;31(1):39-42.
- Gaillard Y., Krishnamoorthy A., Bevalot F. Cerebra odollam: a 'suicide tree' and cause of death in the state of Kerala. JEthnopharmacol. 2004;95(2-3):123-126. https://doi.org/10.1016/j.jep.2004.08.004
- Eddleston M, Ariaratnam C.A., Meyer W.P., Perera G., Kularatne A.M., Attapattu S., Sheriff M.H., Warrell D.A. Epidemic of self-poisoning with seeds of the yellow oleander tree (Thevetia peruviana) in northern Sri Lanka. Trop Med Int Health. 1999;4(4):266-273. https://doi.org/10.1046/j.1365-3156.1999.00397.x
- Burchell H.B. Digitalis poisoning: historical and forensic aspects. J Am Coll Cardiol. 1983;1(1):506-516. https://doi.org/10.1016/s0735-1097(83)80080-1
- Gosudarstvennyj reestr lekarstvennyh sredstv. URL: https://grls.rosminzdrav.ru (in Russ).
- Kanji S., MacLean R.D. Cardiac glycoside toxicity: more than 200 years and counting. Crit Care Clin. 2012;28(4):527-535. https://doi.org/10.1016Zj.ccc.2012.07.005
- Morsy N. Cardiac Glycosides in Medicinal Plants. Aromatic and Medicinal Plants - Back to Nature. 2017;(2):29-45. https://doi.org/10.5772/65963
- Schonfeld W., Weiland J., Lindig C., Masnyk M., Kabat M.M., Kurek A., Wicha J., Repke K.R.H. The lead structure in cardiac glycosides is 5p,14p-androstane-3p14-diol. Naunyn Schmiedebergs Arch. Pharmacol. 1985;329:414-426. https://doi.org/10.1007/BF00496377
- Brown L., Thomas R., Watson T. Cardiac glycosides with non-rotting steroid to sugar linkages: tools for the study of digitalis structure-activity relationships. Arch. Pharmacol. 1986;332(1):98-102. https://doi.org/10.1007/bf00633205
- Sousa L.Q., Machado K.D., Oliveira S.F., et al. Bufadienolides from amphibians: A promising source of anticancer prototypes for radical innovation, apoptosis triggering and Na+/K+-ATPase inhibition. Toxicon. 2017;127:63-76. https://doi.org/10.1016/j.toxicon.2017.01.004
- Ogawa H., Shinoda T., Cornelius F., Toyoshima C. Crystal structure of the sodium-potassium pump (Na+,K+-ATPase) with bound potassium and ouabain. Proc Natl Acad Sci USA. 2009; 106(33): 13742-13747. https://doi.org/10.1073/pnas.0907054106
- FDA Approved Drug Products: Lanoxin (digoxin) oral tablets, 2015 (revised in 2019). https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/020405s015lbl.pdf
- Dipolo R., Beauge L. Sodium/calcium exchanger: Influence of metabolic regulation on ion carrier interactions. Physiological Reviews journal. 2006;86(1):155-203. https://doi.org/10.1152/physrev.00018.2005
- Schoner W., Scheiner-Bobis G. Endogenous and exogenous cardiac glycosides and their mechanisms of action. Am J Cardiovasc Drugs. 2007;7(3):173-189. https://doi.org/10.2165/00129784-200707030-00004
- Gonano L.A., Sepulveda M., Rico Y., Kaetzel M., Valverde C.A., Dedman J., Mattiazzi A., Vila Petroff M. Cal-cium-calmodulin kinase II mediates digitalis-induced arrhythmias. Circ Arrhythm Electrophysiol. 2011;4(6):947-957. https://doi.org/10.1161/CIRCEP.111.964908
- Fabiato A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am J Physiol. 1983;245(1):C1-C14. https://doi.org/10.1152/ajpcell.1983.245.1.C1
- Yuan Z., Cai T., Tian J., Ivanov A.V., Giovannucci D.R., Xie Z. Na/K-ATPase tethers phospholipase C and IP3 receptor into a calcium-regulatory complex. Mol Biol Cell. 2005;16(9):4034-4045. https://doi.org/10.1091/mbc.e05-04-0295
- Kamp T.J., Hell J.W. Regulation of cardiac L-type calcium channels by protein kinase A and protein kinase C. Circ Res. 2000;87(12):1095-1102. https://doi.org/10.1161/01.res.87.12.1095
- Kushakovskij M.S., Grishkin Yu.N. Arrhythmias of the heart. Heart rhythm and conduction disorder: a guide for doctors. 4th edition. St. Petersburg: Foliant, 2017:720. (in Russ).
- Levi A.J., Dalton G.R., Hancox J.C., Mitcheson J.S., Issberner J., Bates J.A. Evans S.J., Howarth F.C., Hobai I.A., Jones J.V. Role of intracellular sodium overload in the genesis of cardiac arrhythmias. J Cardiovasc Electrophysiol. 1997;8(6):700-721. https://doi.org/10.1111/j.1540-8167.1997.tb01834.x
- Rathore S.S., Curtis J.P., Wang Y., Bristow M.R., Krumholz H.M. Association of serum digoxin concentration and outcomes in patients with heart failure. JAMA. 2003;289(7):871-878. https://doi.org/10.1001/jama. 289.7.871
- Bismuth C., Gaultier M., Conso F., Efthymiou M.L. Hyperkalemia in acute digitalis poisoning: prognostic significance and therapeutic implications. Clin Toxicol. 1973;6(2): 153-162. https://doi.org/10.3109/15563657308990513
- Bagrov A.Y., Shapiro J. I., Fedorova O.V. Endogenous cardiotonic steroids: physiology, pharmacology, and novel therapeutic targets. Pharmacological reviews. 2009;61(1):9-38. https://doi.org/10.1124/pr.108.000711
- Yang E.H., Shah S., Criley J.M. Digitalis toxicity: a fading but crucial complication to recognize. Am J Med. 2012;125(4):337-343. https://doi.org/10.1016/j.amjmed.2011.09.019
- Ito S., Woodland C., Harper P.A., Koren G. P-glycoprotein-mediated renal tubular secretion of digoxin: the toxicological significance of the urine-blood barrier model. Life Sci. 1993;53(2):PL25-PL31. https://doi.org/ 10.1016/0024-3205(93)90667-R
- Hanke N., Frechen S., Moj D., Britz H., Eissing T., Wendl T., Lehr T. PBPK models for CYP3A4 and P-gp DDI prediction: a modeling network of rifampicin, itraconazole, clarithromycin, midazolam, alfentanil, and digoxin. CPTPharmacometrics Syst Pharmacol. 2018;7(10):647-659. https://doi.org/10.1002/psp4.12343
- Roberts D.M., Buckley N.A. Pharmacokinetic considerations in clinical toxicology: clinical applications. Clin Pharmacokinet. 2007;46(11):897-939. https://doi.org/10.2165/00003088-200746110-00001
- Chan B.S., Buckley N.A. Digoxin-specific antibody fragments in the treatment of digoxin toxicity. Clin Toxicol (Phila). 2014;52(8):824-836. https://doi.org/10.3109/15563650.2014.943907
- Vodoevich V.P., Maksimov A.I., Pashkovskij A.R., Snitko V.N. Two cases of complete drug-induced atrioventricular block. Medical business: scientific and practical therapeutic journal. 2017;3(55):68-70 (in Russ).
- Bandara V., Weinstein S. A., White J., Eddleston M. A review of the natural history, toxinology, diagnosis and clinical management of Nerium oleander (common oleander) and Thevetia peruviana (yellow oleander) poisoning. Toxicon. 2010;56(3):273-281. https://doi.org/10.1016Zj.toxicon.2010.03.026
- Roberts D.M., Gallapatthy G., Dunuwille A., Chan B.S. Pharmacological treatment of cardiac glycoside poisoning. Br J Clin Pharmacol. 2016;81(3):488-495. https://doi.org/10.1111/bcp.12814
- Bismuth C., Gaultier M., Conso F., Efthymiou M.L. Hyperkalemia in acute digitalis poisoning: prognostic significance and therapeutic implications. Clin Toxicol. 1973;6(2):153-162. https://doi.org/10.3109/15563657308990513
- Dasgupta A., Datta P. Rapid detection of oleander poisoning using digoxin immunoassays: comparison of five assays. Ther Drug Monit. 2004;26(6):658-663. https://doi.org/10.1097/00007691-200412000-00012
- Min J.S., Kim J., Kim J.H., Kim D., Zheng Y.F., Park J.E., Lee W., Bae S.K. Quantitative determination of arenobufagin in rat plasma by ultra fast liquid chromatography-tandem mass spectrometry and its application in a pharmacokinetic study. J Pharm Biomed Anal. 2013;15(939):86-91. https://doi.org/10.1016/j.jpba.2017.08.048
- Omidfar K., Kia S., Kashanian S., Paknejad M., Besharatie A., Kashanian S., Larijani B. Colloidal nanogold-based immunochromatographic strip test for the detection of digoxin toxicity. Applied Biochemistry and Biotechnology. 2010;160(3):843-855. https://doi.org/10.1007/s12010-009-8535-x
- Payne V.W., Secter R.A., Noback R.K. Use of colestipol in a patient with digoxin intoxication. Drug Intell Clin Pharm. 1981;15(11):902-903. https://doi.org/10.1177/106002808101501109
- Caldwell J.H., Greenberger N.J. Interruption of the enterohepatic circulation of digitoxin by cholestyramine. I. Protection against lethal digitoxin intoxication. J Clin Invest. 1971;50(12):2626-2637. https://doi.org/10.1172/JCI106763
- Gilfrich H.J., Okonek S., Manns M., Schuster C.J. Digoxin and digitoxin elimination in man by charcoal hemoperfusion. Klin Wochenschr. 1978;56 (23):1179-1183. https://doi.org/10.1007/bf01476862
- Mowry J.B, Burdmann A.E., Anseeuw K., Ayoub P., Ghannoum M., Hoffman R.S., Lavergne V., Nolin T.D., Gosselin S., the EXTRIP Workgroup. Extracorporeal treatment for digoxin poisoning: systematic review and recommendations from the EXTRIP Workgroup, Clinical Toxicology. 2016;54(2):103-114. https://doi.org/10.3109/15563650.2015.1118488
- Roberts D.M., Buckley N. Antidotes for acute cardenolide (cardiac glycoside) poisoning. Cochrane Database of Systematic Reviews. 2006; 4 Art. No.: CD005490. https://doi.org/10.1002/14651858.CD005490.pub2
- de Silva H.A., Fonseka M.M., Pathmeswaran A., Alahakone D.G., Ratnatilake G.A., Gunatilake S.B., Rana-sinha C.D., Lalloo D.G., Aronson J.K., de Silva H.J. Multiple-dose activated charcoal for treatment of yellow oleander poisoning: a single-blind, randomised, placebo-controlled trial. Lancet. 2003;361(9373):1935-1938. https://doi.org/10.1016/s0140-6736(03)13581-7
- Eddleston M., Juszczak E., Buckley N.A., Senarathna L., Mohamed F., Sheriff M.H.S., Warrell D.A. Randomised controlled trial of routine single or multiple dose superactivated charcoal for self-poisoning in a region with high mortality. Clinical Toxicology 2005;43:442-443.
- Eddleston M., Rajapakse S., Rajakanthan S., Sjöström L., Santharaj W., Thenabadu P.N., Sheriff M.H.R., Warrell D.A. Anti-digoxin Fab fragments in cardiotoxicity induced by ingestion of yellow oleander: a randomised controlled trial. Lancet. 2000;355(9208):967-972. https://doi.org/10.1016/s0140-6736(00)90014-x
- Antman E.M., Wenger T.L., Butler V.P. Jr., Haber E., Smith T.W. Treatment of 150 cases of lifethreatening digitalis intoxication with digoxin-specific Fab antibody fragments. Final report of a multicenter study. Circulation. 1990;81 (6):1744-1752. https://doi.org/10.1161/01.cir.81.6.1744
- Felicilda-Reynaldo R.F. Cardiac glycosides, digoxin toxicity, and the antidote. Medsurg Nurs. 2013;22(4): 258-261.
- Hickey A.R., Wenger T.L., Carpenter V.P., Tilson H.H., Hlatky M.A., Furberg C.D., Kirkpatrick C.H., Strauss H.C., Smith T.W. Digoxin Immune Fab therapy in the management of digitalis intoxication: safety and efficacy results of an observational surveillance study. Journal of the American College of Cardiology. 1991;17(3): 590-598. https://doi.org/10.1016/s0735-1097(10)80170-6
- Ujhelyi M.R., Robert S. Pharmacokinetic aspects of digoxin-specific fab therapy in the management of digitalis toxicity. Clin Pharmacokinet. 1995;28(6):483-493. https://doi.org/10.2165/00003088-199528060-00006
- Hursting M.J., Raisys V.A., Opheim K.E., Bell J.L., Trobaugh G.B., Smith T.W. Determination of free digoxin concentrations in serum for monitoring Fab treatment of digoxin overdose. Clin Chem. 1987;33(9):1652-1655.
- FDA-approved labeling information: Digoxin Immune Fab (Ovine) (DigiFab), 2001 (revised in 2018). https://www.fda.gov/media/74693/
- Hoffman R.S., Howland M., Levin N.A., Nelson L.S., Goldfrank L.R. Goldfrank's Toxicologic Emergencies, 10th edition. New York: McGraw-Hill Education, 2014:1904. ISSN 978-0071801843
- Bracken L.M., Chan B.S.H., Buckley N.A. Physiologically based pharmacokinetic modelling of acute digoxin toxicity and the effect of digoxinspecific antibody fragments. Clinical Toxicology. 2019;57(2):117-124. https://doi.org/10.1080/15563650.2018.1503288
- Wessler D.J., Grip L.T., Mendell J., Giugliano R.P. The P-Glycoprotein Transport System and Cardiovascular Drugs. JACC. 2013;61(25):2495-2502. https://doi.org/10.1016/j.jacc.2013.02.058
- Schaffer S., Kim H.W. Effects and Mechanisms of Taurine as a Therapeutic Agent. Biomol Ther (Seoul). 2018;26(3):225-241. https://doi.org/10.4062/biomolther.2017.251
- Ma H., Jiang, J., Zhang J., Zhou J., Ding A., Lv G., Duan, J. Protective effect of taurine on cardiotoxicity of the bufadienolides derived from toad (Bufo bufo gargarizans Canto) venom in guinea-pigs in vivo and in vitro. Toxicology Mechanisms and Methods. 2011:22(1):1-8. https://doi.org/10.3109/15376516.2011.583295
- Satoh H. Cardiac actions of taurine as a modulator of the ion channels. Adv Exp Med Biol. 1998;442:121-128. https://doi.org/10.1007/978-1-4899-0117-0_16
- Pincus M. Management of digoxin toxicity. Aust Prescr. 2016;39(1):18-20. https://doi.org/10.18773/austprescr.2016.006
- Shulkin A.V., Yakusheva E.N., Popova N.M. The role of P-glycoprotein in rational pharmacotherapy in cardiology. Ration Pharmacother Cardiol. 2013;9(6):701-707. https://doi.org/10.20996/1819-6446-2013-9-6-701-707
- Nola G.T., Pope S., Harrison D.C. Assessment of the synergistic relationship between serum calcium and digitalis. Am Heart J. 1970;79(4):499-507. https://doi.org/10.1016/0002-8703(70)90255-3
- Levine M., Nikkanen H., Pallin D.J. The effects of intravenous calcium in patients with digoxin toxicity. J Emerg Med. 2011;40(1):41-46. https://doi.org/10.1016/j.jemermed.2008.09.027
- Van Deusen S.K., Birkhahn R.H., Gaeta T.J. Treatment of hyperkalemia in a patient with unrecognized digitalis toxicity. J Clin Toxicol. 2003;41 (4):377-379. https://doi.org/10.1081/clt-120022006
- Hack J.B., Woody J.H., Lewis D.E., Brewer K., Meggs W.J. The effect of calcium chloride in treating hyperkalemia due to acute digoxin toxicity in a porcine model. J Toxicol Clin Toxicol. 2004;42(4):337-342. https://doi.org/10.1081/clt-120039538
- Oubaassine R., Bilbault P., Roegel J.C., Alexandre E., Sigrist S., Lavaux T., Jaeger A., Pinget M., Kessler L. Cardio protective effect of glucose-insulin infusion on acute digoxin toxicity in rat. Toxicology. 2006;224(3): 238-243. https://doi.org/10.1016Zj.tox.2006.04.035


