Foliar Ascorbic Acid Alleviates Salt-induced Oxidative Stress in Maize
Автор: Ali Doğru, Ebru Torlak
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 1 т.17, 2021 года.
Бесплатный доступ
The effects of the foliar ascorbic acid application on major antioxidant enzymes, photosynthetic pigment content, malondialdehyde, hydrogen peroxide, free proline and the reduced ascorbate were investigated in salt-stressed (75 mM NaCl) maize genotype (ADA 9510). The results showed that salt stress significantly decreased chlorophyll a, chlorophyll b, total chlorophyll, total carotenoid and the reduced ascorbate contents and the activities of superoxide dismutase, ascorbate peroxidase and glutathione reductase. Conversely, malondialdehyde, hydrogen peroxide and free proline contents were increased by salt stress. These results showed that salinity led to the oxidative stress and destruction of photosynthetic pigments in maize leaves. The foliar ascorbic acid application, on the other hand, caused to the increased chlorophyll a and the reduced ascorbate content, elevated level of antioxidant enzymes and decreased malondialdehyde, hydrogen peroxide and free proline content. This kind of changes may indicate that the foliar ascorbic acid application activates the antioxidant defence system and counteract the oxidative stress. Thus, it may be concluded that the foliar ascorbic acid application improves salt tolerance and encourage the growth of maize plants under salt stress.
Antioxidant enzymes, foliar ascorbic acid, maize, salinity, salt tolerance
Короткий адрес: https://sciup.org/143173873
IDR: 143173873
Текст научной статьи Foliar Ascorbic Acid Alleviates Salt-induced Oxidative Stress in Maize
Salt stress is an agro-ecological problem restricting plant growth and development in arid and semi-arid region of the world. Munns and Tester (2008) have reported that 45 million hectares land have been affected by salinity worldwide, and 1.5 million hectares are taken out of cultivation each year due to overaccumulation of salts in soil. High level of salinity causes water stress, specific ion toxicity, nutritional disorders, oxidative stress, alteration of metabolic processes, membrane disorganisation, reduction of cell division and expansion and genotoxicity (Hasegawa et al. , 2000; Munns, 2002; Zhu, 2007).
The induction of salt tolerance in plants is crucial to maintain their economic yield. In recent decades, considerable improvements in salinity tolerance have been made in crop plants through conventional selection and breeding techniques (Shannon, 1998; Ashraf, 1994; Noble et al. , 1984; Ashraf, 2002). Many scientists have suggested that selection is more convenient if the plant species possesses distinctive indicators of salt tolerance at the whole plant, tissue or cellular level (Munns, 2002; Ashraf, 2002). However, there are no well-defined plant indicators for salinity tolerance that could practically be used by plant breeders to improve salt tolerance in crop plants. This is partly due to the fact that the mechanism of salt tolerance is very complex and that variation occurs not only amongst species but, in many cases, also among cultivars of the single species (Ashraf, 1994; Ashraf, 2002). As a result, despite the great deal of research on salinity tolerance of plants, the metabolic sites at which salt stress damages plants and the adaptive mechanisms utilized by plants to survive salt stress are still not well understood.
The strategies of plant breeding and genetic engineering are long-term and complex processes to develop salt tolerance that still has limited success (Ashraf et al., 2008). Alternatively, the application of non-enzymatic antioxidants, such as ascorbic acid (AsA), may be an efficient technique to cope with the adverse effects of salinity on plants (Khan et al., 2006). AsA occurs ubiquitously in plants and has been reported to play a vital role in alleviating the adverse effects of salt on plant growth and metabolism in many crop plants (Hamada, 1998). In general, effects of AsA in mitigating the adverse effects of salt stress have been ascribed to activation of some of the enzymatic reactions (Kafeli, 1981). Furthermore, such positive effects of AsA in overcoming the adverse effects of salinity were attributed to the stabilization and protection of photosynthetic pigments and photosynthetic apparatus from oxidative damage (Hamada, 1998).
The aim of the present work is to assess whether the foliar AsA application could alter antioxidant capacity and photosynthetic pigment metabolism in maize plants under salt stress.
MATERIALS AND METHODS
Plant materials, growth conditions and experimental design
Maize ( Zea mays L.) cultivars, ADA9510, were grown in growth chamber in plastic pots containing Hoagland nutrient solution. The average temperature for day/night was 25/18 C respectively, relative humidity was 40-50%, the photoperiod for the day/night cycle was 16/8 h respectively, and the maximum photosynthetically active radiation was about 200 µmol photon m-2 s-1. After 10 days of growth, the applications were done as follows: Control (0 mM NaCl), S75 (75 mM NaCl), AsA (100 ppm foliar ascorbic acid) and S75+AsA (75 mM NaCl+100 ppm AsA). Control plants were sprayed with distilled water only. The seedlings were harvested after 5 days of applications and leaves are kept at -80 C until analysis.
Photosynthetic pigment analysis
Photosynthetic pigments were extracted from leaf segments in 3 mL 100% acetone. The absorbance of the extracts was measured at 470, 644.8 and 661.6 nm using a Shimadzu mini 1240 UV visible spectrophotometer. The concentrations of chlorophyll a, chlorophyll b, total chlorophyll (a+b) and total carotenoids (x+c) were calculated according to Lichtenthaler (1987).
Malondialdehyde (MDA) and hydrogen peroxide (H2O2) analysis
MDA and H2O2 content were determined by the method of Heath and Packer (1968) and Ohkawa et al. (1979), respectively. Fresh leaf material (0.1 g) was homogenized in 6 ml of 5% TCA (4 °C) and centrifuged at 10 000g for 15 min and the supernatant was used in the subsequent determination. To 0.5 ml of the supernatant were added 0.5 ml of 0.1 M Tris-HCl (pH 7.6) and 1 ml of TCA–TBA reagent. The mixture was heated at 95 °C for 60 min and then quickly cooled in an ice bath. After centrifugation at 10 000g for 5 min to remove suspended turbidity, the absorbance of supernatant at 532 nm was recorded. Non-specific absorbance at 600 nm was measured and subtracted from the readings recorded at 532 nm. Concentration of MDA was calculated using its extinction coefficient of 155 mM-1 cm-1. For determination of hydrogen peroxide, 0.5 ml of 0.1 M Tris-HCl (pH 7.6) and 1 ml of 1 M KI were added to 0.5 ml of supernatant. After 90 min, the absorbance was measured at 390 nm. A standard curve for hydrogen peroxide was prepared to calculate hydrogen peroxide concentration in each sample.
Free proline analysis
Approximately 10 mg powdered dry leaf material was extracted in 4 mL distilled water on a hot plate at 100ºC for 10 min according to Bates et al. (1973). Extracts were filtered and the same procedure was repeated two times. The liquid phase of the homogenate was collected and centrifuged at 3500g for 10 min. Two mL of the supernatant was reacted with 2 mL of acid ninhydrin and 2 mL of glacial acetic acid at 100°C for 1 h. The reaction mixture was mixed with 4 mL toluene and vortexed for 20 s. The chromophore containing toluene was separated and the absorbance of the pink upper phase was recorded at 520 nm against toluene blank. A standard curve for proline in the range of 0.2-1 µmol mL-1 was prepared to determine free proline concentration in each sample.
Antioxidant enzyme activities
For determination of enzyme activities, 0.3 g fresh leaf material were powdered with liquid nitrogen and suspended in specific buffer with proper pH values for each enzyme. The homogenates were centrifuged at 14,000g for 20 min at 4°C and resulting supernatants were used for enzyme assay. The protein concentrations of leaf crude extracts were determined according to Bradford (1976), using BSA as a standard.
Superoxide dismutase (SOD; EC 1. 15. 1. 1) activity was determined by the method of Beyer and Fridovich (1987), based on the photo reduction of NBT (nitro blue tetrazolium). Extraction was performed in 1.5 mL homogenization buffer containing 10 mM K2HPO4 buffer (pH 7.0), 2% PVP and 1 mM Na2EDTA. The reaction mixture consisted of 100 mM K2HPO4 buffer (pH 7.8), containing 9.9x10-3 M methionine, 5.7x10-5 M NBT, %1 triton X-100 and enzyme extract. Reaction was started by the addition of 0.9 µM riboflavin and mixture was exposed to light with an intensity of 375 µmol m-2 s-1. After 15 min, reaction was stopped by switching off the light and absorbance was read at 560 nm. SOD activity was calculated by a standard graphic and expressed as unit mg-1 protein.
Ascorbate peroxidase (APX; EC 1. 11. 1. 11) activity was determined according to Wang et al. (1991) by estimating the decreasing rate of ascorbate oxidation at 290 nm. APX extraction was performed in 50 mM Tris– HCl (pH 7.2), 2% PVP, 1 mM Na 2 EDTA, and 2 mM ascorbate. The reaction mixture consisted of 50mM KH 2 PO 4 buffer (pH 6.6), 2.5 mM ascorbate, 10 mM H 2 O 2 and enzyme, containing 100 µg proteins in a final volume of 1 mL. The enzyme activity was calculated from initial rate of the reaction using the extinction coefficient of ascorbate (E = 2.8 mM cm-1 at 290 nm).
Glutathione reductase (GR; EC 1. 6. 4. 2) activity was measured with the method of Sgherri et al. (1994). Extraction was performed in 1.5 ml of suspension solution, containing 100 mM KH 2 PO 4 buffer (pH 7.0), 1 mM Na 2 EDTA, and 2% PVP. The reaction mixture (total volume of 1 mL) contained 100 mM KH 2 PO 4 buffer (pH 7.8), 2 mM Na 2 EDTA, 0.5 mM oxidised glutathione (GSSG), 0.2 mM NADPH and enzyme extract containing 100 µg protein. Decrease in absorbance at 340 nm was recorded. Correction was made for the non-enzymatic oxidation of NADPH by recording the decrease at 340 nm without adding GSSG to assay mixture. The enzyme activity was calculated from the initial rate of the reaction after subtracting the non-enzymatic oxidation using the extinction coefficient of NADPH (E = 6.2 mM cm-1 at 340 nm).
Statistical analysis
Experiments were a randomised complete block design with three independent replicates. Analysis of variance (ANOVA) was performed using SPSS 20.0 statistical software for Windows. To separate significant differences between means, Duncan test was used at *P = 0.05.
RESULTS AND DISCUSSION
Our results clearly showed that maize plants exhibited a significant decrease in chlorophyll a, chlorophyll b, total chlorophyll and total carotenoid content under salinity (Fig 1a, b, c and d). In consistent with these results, it has been reported that salt stress led to the reductions in the photosynthetic pigment content in several crop plants such as pea, wheat, rice and tomato (Ahmad and Jhon, 2005; Ashraf et al. , 2002; Anuradha and Rao, 2003; Al-Aghabary et al. , 2004). Bybordi (2012), for example, has indicated that the reduced photosynthetic pigment content in plants under salt stress may be due to inhibition of the biosynthesis of the pigments by the accumulated sodium ions in leaves. In addition, Taibi et al. (2016) has reported that the decreased photosynthetic pigment level in salt-stressed plants has been considered as a typical symptom of oxidative stress and was attributed to the activation of its degradation by the proteolytic enzyme chlorophyllase. As a result, reduction in photosynthetic pigment content may result from either slow synthesis or the accelerated degradation in salt-stressed maize plants in our study. Carotenoids, on the other hand, has been known to be protective pigments against oxidative stress and thus avoiding chlorophyll loss. In this study, the foliar AsA application did not stimulate total carotenoid content in leaves, indicating the lack of a such protective mechanism in maize plants under salt stress (Doğru and Çakırlar, 2020a).
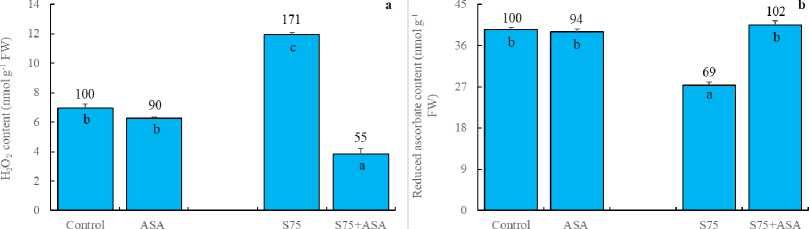
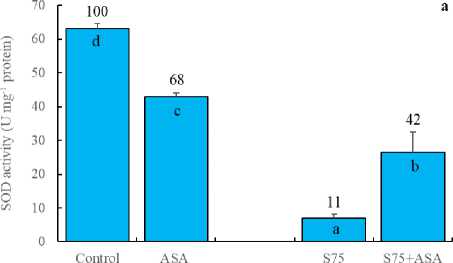
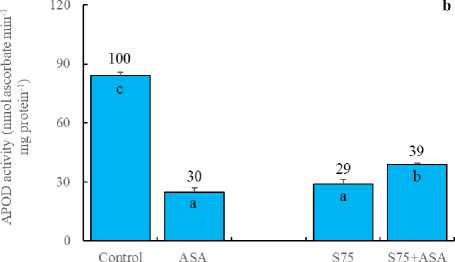
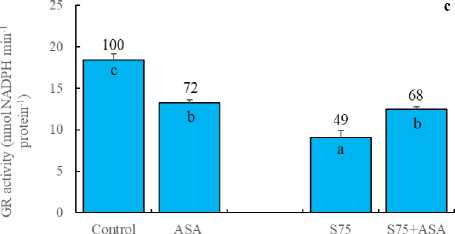
In the present study, the foliar AsA application decreased the activities of SOD, APOD and GR (Fig 3a, b and c). SOD is responsible for the dismutation of the superoxide in plants (Doğru and Çakırlar, 2020b). APX, on the other hand, reduces H 2 O 2 into H 2 O and O 2 using ascorbate as the electron donor. GR catalyses the NADPH-dependent reduction of oxidized glutathione (GSSG) to the reduced form GSH. APX and GR are associated with H 2 O 2 scavenging via ascorbateglutathione cycle (Doğru and Çakırlar, 2020a). Similarly,
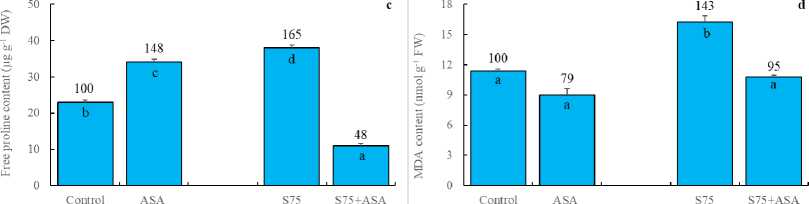
AsA has been known to be a detoxifier or neutralizer of superoxide, H 2 O 2 and singlet oxygen species. As a result, we may hypothesize that the foliar AsA application scavenged free radicals and decreased SOD, APOD and GR activities as reported by Bybordi (2012). In other words, it can be said that the decreased level of SOD, APOD and GR activities arise from the lack of the substrates consumed by exogenously applied AsA. In consistent with these results, H 2 O 2 and MDA accumulation was higher in the leaves of maize plants under salt stress (Fig 2a and d). It has been indicated that an increase in the antioxidant enzymes helps plants maintain their growth under stress and may be regarded as indicators of salt tolerance (Azevedo et al. , 2011). The foliar AsA application has been found to activate the ability of dismutation of superoxide and detoxification of H 2 O 2 in maize plants under salinity, as indicated by the increased activities of SOD, APOD and GR, respectively. In those plants, membrane integrity was better preserved as confirmed by lower level of H 2 O 2 and MDA.
Proline is a water-soluble amino acid. It has been shown that proline accumulation in plant tissues under salt stress regulates osmotic potential (Ali et al. , 1999). It has also been declared that proline is involved in free radical scavenging (Ashraf and Foolad, 2007). Munns (2005) has indicated that salinity up-regulated the enzymes involved in proline biosynthesis. In the present study, the foliar AsA application led to the increased proline content in maize leaves in comparison with control (Fig 2c), probably due to induction of proline biosynthetic enzymes. The foliar AsA treatment in maize plants under salt stress, however, caused lower level of proline. It is probable that the foliar AsA application scavenged free radicals and prevented biosynthesis of extra proline (Dolatabadian et al. , 2008). In this study, the reduced AsA content was declined by salt stress in maize leaves (Fig 2b) probably due to direct destruction of the reduced AsA by free radicals as observed earlier in pea plants (Iturbe-Ormaetxe et al. , 1998). The foliar AsA application, however, enhanced the reduced AsA content in maize leaves. It has been well known that the reduced AsA is able to scavenge free radicals such as superoxide, hydroxyl radical, singlet oxygen and H 2 O 2 .
High level of the reduced AsA is essential to maintain the antioxidant defence system (Shigeoka et al. , 2002). It is well evident that plant cells are strongly redox buffered due to large amount of AsA (Hartmann et al. ,
2003). Thus, it is possible that the increased AsA due to foliar application of AsA may have protected plant cells from salt-induced oxidative stress by controlling cellular redox state.
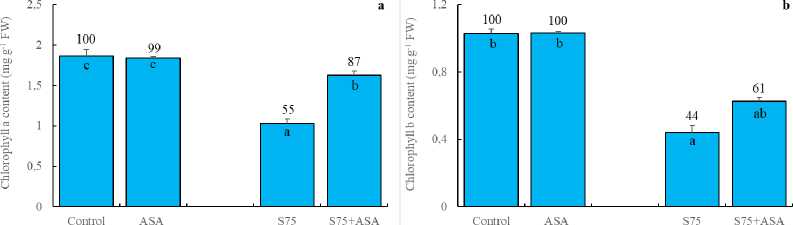
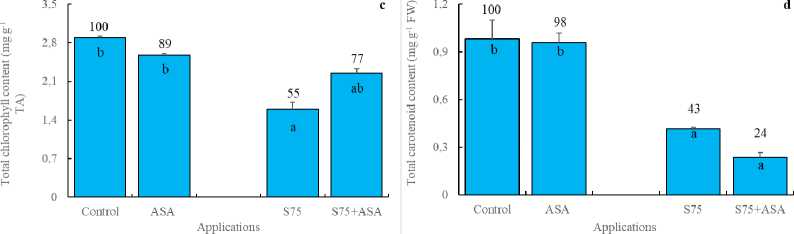
Figure 1. Effect of the foliar ascorbic acid on (a) chlorophyll a, (b) chlorophyll b, (c) total chlorophyll and (d) total carotenoid content of maize plants under salt stress. (Columns with different letters mean significant differences between the treatments according to Duncan’s multiple range test (P0.05) and numbers on the columns indicate % change relative to control, control=100)
Applications Applications |
Figure 2. Effect of the foliar ascorbic acid on (a) H 2 O 2 , (b) the reduced ascorbate, (c) free proline and (d) MDA content of maize plants under salt stress. (Columns with different letters mean significant differences between the treatments according to Duncan’s multiple range test (P0.05) and numbers on the columns indicate % change relative to control, control=100)
Applications
Figure 3. Effect of the foliar ascorbic acid on (a) SOD, (b) APOD, (c) free proline and (d) GR activity of maize plants under salt stress. (Columns with different letters mean significant differences between the treatments according to Duncan’s multiple range test (P0.05) and numbers on the columns indicate % change relative to control, control=100)
CONCLUSION
In conclusion, the foliar AsA application increased significantly salt tolerance in maize plants. This is demonstrated by the fact that the foliar AsA application increased chlorophyll a and the reduced AsA content. In addition, the foliar AsA application increased level of the reduced AsA, SOD, APOD and GR and decreased H2O2 and MDA accumulation in the leaves of maize plants under salt stress, indicating the lowered level of oxidative stress. Thus, the foliar AsA application may be involved in the activation of the antioxidant defence system and had positive effect on maize growth.
CONFLICTS OF INTEREST
The authors declare that there is no conflict of interest.
Список литературы Foliar Ascorbic Acid Alleviates Salt-induced Oxidative Stress in Maize
- Ahmad, P., Jhon R., (2005). Effect of salt stress on growth and biochemical parameters of Pisum sativum L. Archieves of Agronomy and Soil Science 51, 665-672.
- Al-Aghabary, K., Zhu, Z., Qinhua, S., (2004). Influence of silicon supply on chlorophyll content, chlorophyll fluorescence and antioxidative enzyme activities on tomato plants under salt stress. Journal of Plant Nutrition 27, 2101-2115.
- Ali, G., Srivastava, P.S., Iqbal, M., (1999). Proline accumulation, protein pattern and photosynthesis in regenerants grown under NaCl stress. Biologia Plantarum 42, 89-95.
- Anuradha, S., Rao, S.S.R., (2003). Application of brassinosteroids in rice seeds (Oryza sativa L.) reduced the impact of salt stress on growth and improved photosynthetic pigment levels and nitrate reductase activity. Plant Growth Regulation 40, 29-32.
- Ashraf, M., (1994). Breeding for salinity tolerance in plants. Critical Reviews in Plant Science 13, 17-42.
- Ashraf, M., (2002). Salt tolerance of cotton: some new advances. Critical Reviews in Plant Sciences 21, 1-30.
- Ashraf, M., Athar, H.R., Harris, P.J.C., & Kwon, T.R., (2008). Some prospective strategies for improving crop salt tolerance. Advances in Agronomy 97, 45-110.
- Ashraf, M., Foolad, M.R., (2007). Roles of glycinebetaine and proline in improving plant abiotic stress tolerance. Environmental and Experimental Botany 59, 206-216.
- Ashraf, M., Karim, F., Rasul, E., (2002). Interactive effects of gibberellic acid (GA3) and salt stress on growth, ion accumulation and photosynthetic capacity of two spring wheat (Triticum aestivum L.) cultivars differing in salt tolerance. Plant Growth Regulation 36, 49-59.
- Azevedo, R.A., Carvalho, R.F., Cia, M.C., Gratao, P.L., (2011). Sugarcane under pressure: an overview of biochemical and physiological studies of abiotic stress. Tropical Plant Biology 4, 42-51.
- Bates, L.S., Waldren, R.P., Teare, I.D., (1973). Rapid determination of free proline for water-stress studies. Plant and Soil 39, 205-207.
- Beyer, W.F., Fridovich, I., (1987). Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Analytical Biochemistry 161, 559-566.
- Bradford, M.M., (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry 72, 248-254.
- Bybordi, A., (2012). Effect of ascorbic acid and silicium on photosynthesis, antioxidant enzyme activity, and fatty acid contents in canola exposure to salt stress. Journal of Integrative Agriculture 11, 1610-1620.
- Doğru, A., Çakırlar, H., (2020a). Is leaf age a predictor for cold tolerance in winter oilseed rape plants? Functional Plant Biology 47, 250-262.
- Doğru, A., Çakırlar, H., (2020b). Effects of leaf age on chlorophyll fluorescence and antioxidant enzymes in winter rapeseeds leaves under cold acclimation conditions. Brazilian Journal of Botany 43, 11-20.
- Dolatabadian, A., Sanavy, S.A.M.M., Chashmi, N. A. (2008). The effects of foliar application of ascorbic acid (vitamin C) on antioxidant enzymes activities, lipid peroxidation and proline accumulation of canola (Brassica napus L.) under conditions of salt stress. Journal of Agronomy and Crop Science 194, 206-213.
- Hamada, A.M., (1998). Effect of exogenously added ascorbic acid, thiamine or aspirin on photosynthesis and some related activities of drought-stressed wheat plants. In: Proceedings of XIth International Photosynthesis Conference. Budapest, Hungary, August, pp. 17-22
- Hartmann, T.N., Fricker, M.D., Rennenberg, H., Meyer, A.J., (2003). Cell specific measurement of cytosolic glutathione in poplar leaves. Plant Cell and Environment 26, 965-975.
- Hasegawa, P.M., Bressan, R.A., Zhu, J.K, Bohnert, H.J., (2000). Plant cellular and molecular responses to high salinity. Annual Review of Plant Physiology and Plant Molecular Biology 51, 463-499.
- Heath, R.L., Packer, L., (1968). Photoperoxidation in isolated chloroplasts I. Kinetic and stoichiometry of fatty acid peroxidation. Archives of Biochemistry and Biophysics 125, 189-198.
- Iturbe-Ormaetxe, I., Escudero, P.R., Arrese-Igor, C., Becana, M., (1998). Oxidative damage in pea plants exposed to water deficit or paraquat. Plant Physiology 116, 173-181.
- Kafeli, V.I., (1981). Vitamins and some other representatives of non-hormonal plant growth regulators. Prible Biochemistry and Microbiology 17, 5-15.
- Khan, A., Ahmad, M.S.A., Athar, R.E., Ashraf, M., (2006). Interactive effect of foliarly applied ascorbic acid and salt stress on wheat (Triticum aestivum L.) at the seedling state. Pakistan Journal of Botany 38, 1407-1414.
- Lichtenthaler, H.K., (1987). Chlorophylls and carotenoids: pigments of photosynthetic membranes. Methods in Enzymology 148, 350-382.
- Munns, R., (2002). Comparative physiology of salt and water stress. Plant Cell and Environment 33, 453-467.
- Munns, R., (2005). Genes and salt tolerance: bringing them together. New Phytologists 167, 645-663.
- Munns, R., Tester, M., (2008). Mechanisms of salinity tolerance. Annual Review of Plant Biology 59, 651-681.
- Noble, C.L., Halloran, G.M., West, D.W., (1984). Identification and selection for salt tolerance in lucerne (Medicado sativa L.). Australian Journal of Agricultural Research 35, 239-252.
- Ohkawa, H., Ohishi, N., Yagi, N.Y., (1979). Assay of lipid peroxides in animal tissue by thiobarbituric acid reaction. Analytical Biochemistry 95, 351-358.
- Sanchez-Romero, C., Garcia-Gomez, M.L., Priego-Alfaro, F., Heredis, A., (1993). Peroxidase activities, isoenzyme profiles associated with development of avocado (Persea americana M.) leaves at different ontogenetic stages. Journal of Plant Growth Regulation 12, 95-100.
- Sgherri, C.L.M., Loggini, B., Puliga, S., Navari-Izzo, F., (1994). Antioxidant system in Sporobolus stapfianus: changes in response to desiccation and rehydration. Phytochemistry 35, 561-565.
- Shannon, M.C., (1998). Adaptation of plants to salinity. Advances in Agronomy 60, 75-119.
- Shigeoka, S., Ishikawa, T., Tamoi, M., Miyagawa, Y., Takeda, T., Yabuta, Y., Yoshimura, K., (2002). Regulation and function of ascorbate peroxidase isoenzymes. Journal of Experimental Botany 53, 1305-1319.
- Taibi, K., Taibi, F., Abderrahim, L.A., Annejah, A., Belkhodja, M., Mulet, J.M., (2016). Effect of salt stress on growth, chlorophyll content, lipid peroxidation and antioxidant defence system in Phaseolus vulgaris L. South African Journal of Botany 105, 306-312.
- Wang, S.Y., Jiao, H., Faust, M., (1991). Changes in ascorbate, glutathione and related enzyme activity during thidiazuron-induced bud break of apple. Plant Physiology 82, 231-236.


