Formation of nanomaterials based on non-ferrous and noble metals in autoclaves
Автор: Belousov Oleg V., Sirotina Anastasia V., Belousova Nataliya V., Fesik Elena V., Borisov Roman V., Malchikov Gennadiy D.
Журнал: Журнал Сибирского федерального университета. Серия: Техника и технологии @technologies-sfu
Статья в выпуске: 2 т.7, 2014 года.
Бесплатный доступ
The physicochemical laws of formation of multicomponent phases containing noble metals in hydrothermal conditions were investigated. Approaches to a purposeful variation of structure and composition of mono-, bi- and polymetallic phases based on non-ferrous and noble metals were realized. Structural, phase and chemical stability of polymetallic nanostructures in some carrier materials was investigated.
Nanomaterials, non-ferrous metals, noble metals, autoclaves, phase formation
Короткий адрес: https://sciup.org/146114828
IDR: 146114828 | УДК: 542.941:541.123.24
Текст научной статьи Formation of nanomaterials based on non-ferrous and noble metals in autoclaves
Fine-dispersed powders have a unique set of thermodynamic, mechanical, electric and other properties differing from those of bulk materials, and find more and more extending practical application [1]. In some cases bi- and polymetallic particles of noble metals are more effective then single metallic nanoparticles. Catalysts based on such particles are suitable for solving problems of air purification, decreasing of automotive gases burning-out temperature and hydrogen purification for the fuel elements [2].
Chemical resistance of noble metals predetermines the unique opportunities of their use in nanotechnology, especially for materials contacting with water medium. However by going from micro-to nanostate, physical, thermodynamic and chemical properties may change significantly, therefore both the determination of stability thresholds of polymetallic nanoparticles and nanostructures, and the study of specific mechanisms of their transformation are obviously very important.
Nowadays the approaches to the methods of chemical synthesis of noble metal nanocrystal powders are multiform and versatile: from the physical dispersion to the steam condensation. One of the most commonly used, effective and economically expedient methods of the synthesis of bimetallic nanoparticles is the reduction of noble metals from water solutions of their chlorocomplexes [3].
The analysis of the modern state of researches in the field of obtaining of powders, including bimetallic, with given properties, the investigations of their structure and various physicochemical regularities [4-12] show that the correct choice of the synthesis method and the control of main experiment parameters predetermine the production of high-quality materials based on the noble metal nanoparticles with the required dispersity, composition and structure.
One of the ways to achieve this purpose is to carry out the processes under hydrothermal conditions with the use of autoclave technologies which were well proved, for example, in thermolysis of ammoniac complexes of noble metals [13], in the reactions of contact reduction [14-17]. The application of autoclave technologies for obtaining and studying the properties of noble metal compounds, which differ by their inactivity, is rather attractively for some reasons, namely, ecological safety of processes, effective use of reagents, relative simplicity of standardization of experiment conditions [18]. Despite obvious advantages, autoclave processes haven’t found wide application over methodological difficulties.
The purpose of the present work was to study the physicochemical laws of formation of nanophases based on non-ferrous and noble metals.
Experimental Section
Fine-dispersed powders of noble metals, complex salts of some noble and non-ferrous metals; N 2 H 4 ∙2HCl; NaBH 4 were used in the present work. Solutions were prepared in 0,05М КОН and 1М НСl purified by isothermal distillation. The purity of each reagent was at least of CP grade.
Experiments were conducted in air thermostat in quartz autoclaves in accordance with a procedure published elsewhere [17] and in fluoroplastic autoclaves [18].
X-ray diffraction (XRD) data were collected on a PANalytical X’Pert PRO MPD powder diffractometer. The microstructure characteristics of the materials were determined using the fullprofile analysis of XRD patterns by the Rietveld method. The full-profile refinement was performed by the derivative difference minimization. The peak shape was approximated by the TCH pseudoVoigt function taking into account the instrumental, microstrain and crystal size contributions. XRD pattern of a coarsely crystalline corundum sample was used for the instrumental broadening determination.
The adsorption measurements were conducted using nitrogen adsorption method (ASAP 2420, Micromeritics Inc.). The specific surface area was calculated from the adsorption isotherms according to the BET method.
The chemical composition of solid phase was found by the dissolution of powders accompanied by the atomic absorption analysis with the use of AAnalyst-400 spectrometer (PerkinElmer).
The transmission electron microscopy analyses (TEM) were performed using a JEOL JEM-2100 microscope equipped with an Oxford Instruments Inca TEM 250 energy dispersion spectrometer; the accelerating voltage was 200 kV.
The investigation of complex salts thermolysis was conducted using thermal analysis (NETZSCH STA 449 C/4/G Jupiter).
Concentrations of ions of the metals in solution were found by atomic absorption (A Analyst-400, Perkin Elmer) and spectrophotometric analysis (Shimadzu UV-300). The amount of solid phase was determined by gravimetric analysis.
Results and discussion
Chlorocomplexes of platinum (II) in muriatic solutions under standard conditions are stable, as because of kinetic difficulties, reaction
2 [PtCl 4 ] 2- = [PtCl 6 ] 2- + 2Cl – + Pt (1)
doesn’t proceed in spite of the thermodynamic possibility. The situation changes by carrying out the process under the hydrothermal conditions, and at 180°С the rates of direct and reverse reactions become appreciable. This allows controlling purposefully the structural characteristics of metallic platinum in a wide range by changing the ratio of Pt (II) and Pt (IV) concentrations, and thus by changing the direction of reaction (1).
Fine-dispersed palladium, reduced from the muriatic solutions of palladium (II) chloride by sodium formate or hydrazine chloride, has a monoblock structure and represents the chain-aggregated crystallites with average size of 10 nm. The main parameters of purposeful variation of nanodispersed palladium size in hydrothermal conditions were established earlier [15] and the mechanism of adequate explanation of occurring processes was suggested.
The application of autoclave technologies was found to be rather successful not only for the smooth variation of fine-dispersed palladium structural characteristics, but also for the development of the unified techniques of synthesis of nanocrystal powders of platinum metals. The cases of kinetically inactive complexes, in particular rhodium and iridium, deserve a special attention.
The experimental data show that in autoclave rhodium is reduced quantitatively for 20 minutes at 60°С, and iridium – within 3 hours at 120°С.
The comparison of processes of iridium and rhodium reduction under the action of microwave irradiation and thermal heating shows that the direction of reactions and visually observed effects are similar, but the temperature and the time of reactions are essentially different. The use of microwave heating allows decreasing time and temperature of reduction process, especially for iridium: the total time of reduction process is decreased in three times and the average temperature is decreased from 120 ºС to 100 ºС.
According to the X-ray diffraction method, the crystallite sizes of iridium and rhodium blacks amount to 9,4 and 6 nm, respectively. The size of rhodium particles determined by transmission electron microscopy is about 15 nm that allows suggesting that the structure of Rh black is close to monoblock, unlike earlier used techniques of synthesis in open systems, when the rhodium black has polyblock structure.
The coarsening processes of fine-dispersed rhodium powders were studied at 130 ºС and 180 ºС. It was found that the rate of rhodium growth in muriatic solutions was very slow and it depended essentially on temperature. Thus, at 180 ºС the rhodium black in 1M muriatic solutions of rhodium (III) chloride grew up to 11,6 nm, and at at 150 ºС – up to 8 nm.
The research of behavior of fine-dispersed platinum metals in hydrothermal conditions shows the significant role of chemisorbed oxygen in the dissolution processes of fine-dispersed Pt, Pd и Rh in – 140 –
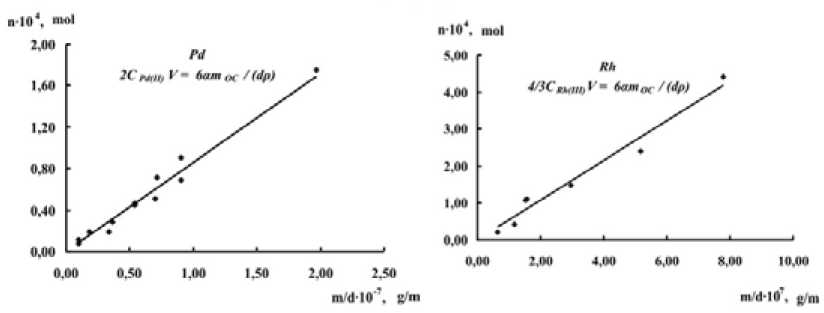
Fig. 1. The influence of chemisorbed oxygen on the solubility of palladium (2С Pd(II) V = 6αm 0 /dρ), rhodium (4/3С Rh(III) V = 6αm 0 /dρ)
muriatic solutions. The quantity of dissolved metal (Pd, Rh) was shown to be in good agreement with specific surface area of corresponding metals (Fig. 1).
The researches of reduction of noble metals from muriatic solutions of their complexes by platinum metals with smaller standard redox potential showed the possibility of production of bimetallic particles with various structures (mechanical mixes of individual metals, solid solutions, isolation of reducing metal by cementing agent).
The reason of such difference in solid phase structure and was studied by us in [15]. The mechanism of formation of substitution solid solutions as a result of the reduction of chlorocomplexes of platinum and iridium by fine-dispersed palladium (equations (2) – (3)) was proposed:
[PtCl4]2- + Pd = Pt + [PdCl4]2-,(2)
2[IrCl6]3- + 3Pd = 2Ir + 3[PdCl4]2-.(3)
The reduction of palladium and platinum
3Pd2+ + 2Rh = 3Pd + Rh3+,(4)
3Pt2+ + 2Rh = 3Pt + Rh3+(5)
by rhodium obtained according to the classical technique and having polyblock structure leads to the formation of not solid solutions, but of a mechanical mix of individual metals [15, 19]. The main reason is the ratio between the rates of coarsening and reduction processes. Actually there is a small growth of particles of Rh black having polyblock structure and the rate of this process is much slower then the rates of reactions (4, 5).
In case of gold reduction by palladium which quickly coarsens in muriatic solutions, Au- Pd substitution solid solution is obtained, while in case of contact reduction of gold (III) chlorocomplexes by platinum, which growth rate is insignificant, a mechanical mix is formed.
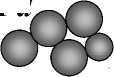
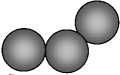
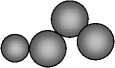
The scheme of the coarsening process and the formation of solid phase at the contact reduction was shown in Fig. 2.
Pd
Т, [PdCl 4 ]2-
Coarsening of particles
Pd
Pd
Т, + [AuCl 4 ]-
- [PdCl 4 ]2-
Pd-Au
Formation of solid solution
Pt
Т, + [AuCl 4 ]-
Pt, Au

- [PtCl 4 ]2-
Isolating of Pt particles by gold layer
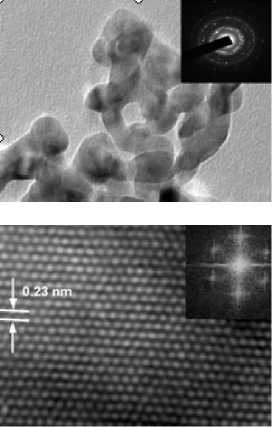
Fig. 2. The coarsening and phase formation model at the cementation
Really, the use of rhodium black obtained in autoclave conditions and having monoblock structure for the reduction of palladium from muriatic solutions of its chlorocomplexes (reaction (4)) leads to the formation of Pd-Rh substitution solid solution.
Let’s consider the phase formation in alkali solutions of halogen-ammoniac complexes under the autoclave conditions. The main reaction (6) is the irreversible reduction of metal by the intraspheric ammonia:
[М(NH 3 ) 4-i Cl i ]2-i + 2OH- → М + 1/ 3 N 2 + (10/ 3 -i)NH 3 + iCl- + 2H 2 O, (6)
where М – metals: Pt, Cu, Au, Ag, Ir, Ga, Bi, Ni, Co, etc.
The state of solid metal phase (powder, sponge, suspended matter in a solution, noncoherent or dense coating on substrates) depends on the composition and the structure of the initial complexes, the composition and pH of the solutions, temperature, time, agitation, etc. The conditions in the system predetermine primary routes leading to the metal phase. The irreversible reduction of ammoniac complexes was realized for platinum, palladium, rhodium, ruthenium, cobalt, nickel, etc. [20-22]. The same processes of autoclave thermolysis proceed in aminocomplexes of platinum, where amine is ethylenediamine, pyridine, glycine and others.
The performance of the experiments in a dispersion medium for the metallic phase precipitating in structurally constrained conditions, which can control a particle size, is of basic interest. In autoclave conditions the aminocomplexes of platinum metals and also of cobalt, nickel, copper and gold in alkali solutions are irreversibly reduced by the intraspheric ammonia to the metallic state. Representing mechanisms of metal deposition on substrates, many researchers place special emphasis on hydroxoforms. On the other hand, methods of sol-gel technology widely used for the synthesis of hydroxides and oxides of amphoteric metals which are intermediate products of oxide carriers or surface layers on dense sorption-inert materials of carriers (metals, ceramics) are known. Autoclaves conditions allow controlling the crystalline state of hydroxides and oxides. A combination of sol-gel technology, – 142 – autoclave methods for producing metal dispersions in gel-like medium of hydroxides allows widening preparation possibilities for new catalyst systems, combining all sequential technological operations. For example, boehmite is formed in autoclave conditions. Under the same conditions ammoniac complexes of platinum metals are reduced to the metal state. Combining these two processes, one can produce a suspension or a coating containing boehmite and platinum metals. Drying and calcination will lead to the desired combination of metal on γ-Al2O3. Such method of the synthesis is likely to give the uniform distribution of metal in hydroxide and then in aluminum oxide. Besides, the reduction of metal and the crystallization of the metal phase proceed in a “microreactor” of gel, which contributes to the high dispersity of metal particles. Preliminary experiments yielded positive results on platinum and ruthenium systems.
Investigated samples differed in quantity of the deposited metal. It was established that the increasing quantity of deposited metal results not only in increasing surface of ruthenium coating but also in the uniformity of its distribution over the surface.
Based on the fact that at autoclave thermolysis in alkali solutions there is the reduction (6) of the ammoniac complexes of metals, having at standard conditions negative electrode potentials (Ni, Co), by the intraspheric ammonia, we proposed that at high temperature ammonia «at the moment of gas emission» can play a role of the reducing agent for the metals forming oxygen-containing anions.
The stoichiometry of reaction (7)
3[Pt(NH 3 ) 4 ]Cl 2 + 6NH 4 ReO 4 + 6KOH =
= 3Pt + 6Re +8N 2 ↑ + 2NH 3 ↑+ 6KCl+ +30H 2 O (7)
was studied and proved [23].
The deposition was a solid solution or a mixture of metallic phases of platinum and rhenium. Preliminary researches shown that reactions with participation of chromate and molybdate proceed similarly:
[Pt(NH 3 ) 4 ]Cl 2 ∙H 2 O + (NH 4 ) 2 CrO 4 + 6KOH =
= 3Pt + Сr + 2N 2 ↑ + 10NH 3 ↑+ +6KCl + 10H 2 O, (8)
12[Pt(NH 3 ) 4 ]Cl 2 ∙H 2 O + (NH 4 ) 6 Mo 7 O 24 ∙4H 2 O + 24KOH =
= 12Pt + 7Mo + 11N 2 ↑ + 32NH 3 ↑+ 24KCl + 64H 2 O. (9)
The obtained results give one more variant of autoclave synthesis of bimetallic dispersed materials. The experimental facts listed in the present work illustrate the possibilities of autoclave technologies for synthesis of non-ferrous and noble metals in the nanostate.
This work was supported by the Integration Project № 29.


