Fusarium blight of common buckwheat in Primorsky krai
Автор: Matsishina Nathalie V., Borovaya Svetlana A.
Журнал: Овощи России @vegetables
Рубрика: Защита растений
Статья в выпуске: 2 (64), 2022 года.
Бесплатный доступ
Relevance. Common buckwheat is one of the most important cereal and melliferous crops being in demand both in Russia and overseas. Despite the fact that buckwheat has lower susceptibility to infectious diseases in comparisons with other grain and cereal crops, research on its pathogens is a topical issue considering a high disease rate for this crop in Primorsky krai. Materials and methods. The study on pathogenic composition was conducted in selective crop rotations of FSBSI “FSC of Agricultural Biotechnology of the Far East named after A.K. Chaiki” at the territory of Ussuriysky district in Primorsky krai. Indeterminate (Izumrud, Pri 7, Bashkirskaya krasnostebel'naya) and determinate (Dikul) buckwheat varieties were used for the study. Fungi were isolated from rhizoplane using water washing technique, from soil and rhizosphere via the dilution method, from leaves and root systems by accumulation in a moist chamber with subsequent transfer of culture onto selective medium. Fungal isolates were cultivated on toxigenic medium (Myro) to determine their phytotoxicity. Phytopathogenic activity of living cultures was evaluated on potato sucrose agar according to the modified method of Chelkowski and Manka . All experiments were conducted in accordance with established methods. Results. Culture filtrates of F. avenaceum and F. graminearum isolates, obtained from different anatomical parts of common buckwheat, were characterized by low toxicity, and culture filtrates of F. oxysporum isolate were highly toxic. Phytotoxicity of pathogens manifested itself not only in decrease in laboratory germination ability of buckwheat seeds, but also in inhibition of buckwheat sprout development. It proved that metabolites exerted a prolonged effect on sensitive plants. F.avenaceum, F. oxysporum and F. graminearum have pronounced phytopathogenic and aggressive properties in relation to buckwheat and test-plants in the laboratory conditions. The sum total of their studied phytotoxic properties is convincing enough to consider them potentially hazardous to buckwheat for wilt disease development.
Buckwheat, fusarium blight, culture filtrates, crops, phytotoxicity, primorsky krai
Короткий адрес: https://sciup.org/140293841
IDR: 140293841 | УДК: 632.488:633.12(571.63)
Текст научной статьи Fusarium blight of common buckwheat in Primorsky krai
Forcitations:Matsishina N.V., Borovaya S.A. Fusarium blight of common buckwheat in Primorsky krai. Vegetable crops of Russia. 2022;(2):65-71.
ommon buckwheat is one of the most important cereal crops used for food in Russia. Caloric and nutritional value of buckwheat along with its high cooking quality makes it one of the best groats [1, 2]. Buckwheat proteins have a greater nutrient value than proteins of other grain crops and are easier to digest. Biological value of buckwheat proteins is significantly higher than the one of other cereals, rice and soybean [3-8]. Set of amino acids in buckwheat is the most complete among other groats, and in respect of the lipid content it loses only to oat and millet groats. A small amount of prolamines and lack of α-gliadin make buckwheat grains a suitable source for gluten-free food products [10, 11]. In addition, buckwheat grain is characterized by high content of unsaturated fatty acids, vitamin E, niacin (vitamin PP), riboflavin (vitamin B2), folic acid, vitamin B6 and thiamine [12-14]. Buckwheat groats are rich in essential macro- and microelements, flavonoids, including rutin, phytosterols and phytoestrogens [1519].
Buckwheat is an excellent melliferous plant and reserve for useful entomofauna. More than 100 insect species (90 % of them are useful) inhabit buckwheat fields [20, 21]. It is possible to collect up to 5 kg/day of buckwheat honey from one bee-family in adequate moisture conditions. Melliferous capacity of one hectare amounts to 150-200 kg [22-24].
Buckwheat is less susceptible to diseases and pest infestation in comparison with grain crops. Among the most common diseases of buckwheat are late blight, downy mildew, noble rot, Ascochyta spot, Cercospora blight, bacteriosis and viral diseases [25]. The degree of damage caused by diseases can reach the limit of economic harm in certain years with unfavorable conditions [26]. Diseases and pests of buckwheat in the Far East and Primorsky krai are not thoroughly studied yet. A.V. K uznetsova, who studied biology and ecology of buckwheat weevil, made some attempts in this regard [27-29]. But they were unsystematic and didn’t apply to phytopathogens. Meanwhile, Primorsky krai is situated in the area of risk agriculture where diseases in combination with biotic and abiotic factors inflict significant damage on buckwheat crops resulting in yield decrease. Furthermore, microscopic fungi are presently noted for playing an important role in human pathologies. Moreover, there is a clear tendency of increase in the number of illnesses caused by their toxins [30]. Due to the fact that buckwheat is a valuable cereal crop used as dietetic food, research on its pathogen composition is crucial. All these reasons determined the aim of the work.
Materials and methods
The research was conducted in selective crop rotations of FSBSI “FSC of Agricultural Biotechnology of the Far East named after A.K. Chaiki” at the territory of Ussuriysky district in Primorsky krai. The plots formed a part of the southern taiga agricultural area. The objects of the research were rhizosphere soil and leaves of buckwheat, also isolates of the genus Fusarium fungi, obtained from rhizosphere and roots of seedlings. Corn seeds of Slavyanka variety and seeds of buckwheat (varieties Izumrud, Pri 7, Dikul and Bashkirskaya krasnostebel’naya) were used as the test-objects for evaluation of phytotoxicity and phytopathogenic activity. Indeterminate buckwheat varieties Izumrud and Pri 7, which were bred in FSC of Agricultural Biotechnology of the Far East named after A.K. Chaiki, are attributed to the group of valuable crops with a high grain quality in the Russian Federation. Izumrud Variety is characterized by strong resistance to lodging and grain shedding, also by a high content of protein in grains (13.6%). Pri 7 Variety displays good technological and cereal qualities and has the protein content of 13-16%. Indeterminate buckwheat variety Bashkirskaya krasnostebel’naya, which was bread in Bashkisrsky SRIA, is valued for its grain quality and has a high content of flavonoids in the above ground biomass, the protein content of 15.4-17.1 %, and considerable resistance to lodging and grain shedding. Determinate variety Dikul (bred in FSBSI “FSC of Legumes and Groat Crops”) is included in the list of valuable high-quality varieties and noted for its productivity, an enhanced content of rutin and essential amino acids, resistance to lodging and grain shedding, drought, high temperatures, and for the cereal yield of 72.9%. Plants were cultivated in controlled conditions of a culture room with a 16-hour photoperiod, temperature of 23°С and light intensity of 4 kcal.
The following nutrient media were used for isolation of the genus Fusarium fungi from natural objects, preservation of pure culture, identification and experimental research [31-36]:
-
1. Potato sucrose agar (PSA for isolation, identification and study on phytopathogenic activity), g/l: agar – 20; purified potato – 200; sucrose – 30.4.
-
2. Starch-and-ammonia agar (SAA for evaluation of growth rate), g/l: soluble starch - 10; (NH 4 ) 2 P0 4 - 2; MgSO 4 7H 2 O - 1; NaCl – 1; СаСО 3 – 3; К 2 НРО 4 – 1; agar – 20.5.
-
3. Basic culture media (BCM for study on vegetative reactions), g/l: sucrose – 30; NaNO 3 – 2; KH 2 PO 4 – 1; MgSO 4 · 7H 2 O – 0.5; KCl – 0.5; FeSO 4 · 7H 2 O – 0.01; agar – 20; solution of micronutrients – 0.2. Solution of micronutrients, g/95 ml: ZnSO 4 · 7H 2 O – 5; Fe(NH 4 )2(SO 4 )2 · 6H 2 O – 1; CuSO 4 · 5H 2 O – 0.25; MnSO 4 · H 2 O – 0.05; H 3 BO 4 – 0.05; citric acid – 5.
-
4. Soil extract agar (SEA for induction of chlamydospore formation), g/l: dry soil – 500; agar – 15.
-
5. Selective agar (SA for isolation from plant tissues), g/l: dextrose – 20.0; yeast extract – 1.0; NaNO 3 – 2.0; MgSO 4 ·7H 2 O – 0.5; FeSO 4 ·7H 2 O (1 % solution) – 1 ml; agar – 20.0.
-
6. Czapek's Agar with sucrose (for isolation, preservation and evaluation of growth rate), g/l: sucrose – 30; NaNO 3 – 3; KH 2 PO 4 – 1; MgSO 4 ·H 2 O – 0.5; KCl – 0.5; FeSO 4 ·7H 2 O – 0.01; agar – 20.
-
7. Czapek's Agar with glucose (for isolation, preservation and evaluation of growth rate), g/l: glucose – 30.0, NaNO 3 – 3, KH 2 PO 4 – 1, MgSO 4 ·H 2 O – 0.5, KCl – 0.5, FeSO 4 ·7H 2 O – 0.01, agar – 20.
-
8. Myro medium (for study on phytotoxicity), g/l: glycerine – 10; sucrose – 20; KH 2 PO 4 – 1; NaCl – 0.5; NaNO 3 – 3; MgSO 4 ·H 2 O – 0.5.
-
9. SNA Nirenberg medium (for identification), g/l: KNO 3 – 1; KH 2 PO 4 – 1; MgSO 4 ·7H 2 O – 0.5; KCl – 0.5; glucose – 0.2; sucrose – 0.2; agar – 15. Pieces of sterile filter paper (1-2 cm) are placed on the surface of sterile nutrient medium in a Petri dish to stimulate sporulation.
-
10. Carnation leaf agar (CLA for identification), g/l: agar – 15–20; Dianthus leaves – 5-6 leaves (fresh leaves are cut into pieces, each 5-10 mm long, sterilized in 70% lab alcohol, dried with sterile filter paper and placed on the surface of cooled medium in Petri dishes).
Fungi were isolated from rhizoplane with the use of the water washing technique, from soil and rhizosphere via the dilution method, from leaves and root systems by accumulation in a moist chamber with subsequent transfer of culture onto selective medium [31, 34-36]. Correct identification of species is possible only in the experiments with monosporous culture. In order to obtain such a culture, spore suspension was prepared in sterile water with the use of either sporodochia and pionnots or a mixture of hyphae and conidia. The selected technique allowed to achieve spore quantity that was enough to have 1-10 conidia in the field of vision of the microscope when the image was magnified 100 times. The suspension was applied with a spreader on the surface of thin-layer water agar (2 %). The Petri dishes were examined through the microscope with transmitted light illumination after 24 hours to find germinated separate conidia of fungi. They were subsequently transferred in sterile conditions onto PDA to promote growth [36, 37]. «Fusarium species: an illustrated manual for identification» [34] and «Fusarium laboratory manual» [36] were used as the main guide for identification of the genus Fusarium fungi. Both micro- and macromorphological traits were evaluated. Morphological traits of mycelium and reproductive structure (forms and structure of macroconidia; structure of conidiophores; presence, forms and distribution of microconidia and chlamydospores; presence and structural peculiarities of mesoconidium) were studied after inoculation onto CLA, SNA Nirenberg medium and soil extract agar. Typical abundant sporulation occurs within 7-14 days on CLA and SNA media. The conidia have standard proportions with low variability. Presence, quantity and distribution pattern of chlamydospores were evaluated on soil extract agar. The Petri dishes with cultures were incubated for 7-28 days with a 12-hour photoperiod at 25-27 °С. Microstructures and peculiarities of conidiogenesis were studied in preparations for vital staining and in microchambers [31] with the help of microscopes Levenhuk D740T (5.3 MP) and ZOE (BioRad), and benchtop scanning electron microscope JCM-7000. Chromogenesis was studied on PSA after 10-14 days of cultivation with a 12-hour photoperiod with evaluation of colony reverse color. Radial growth rate of colonies was determined on the media which contained various sources of carbon nutrition (glucose, sucrose, starch, cellulose) and at different temperatures (8,18,28 ± 1°С) till agar plates were fully encrusted. Mycelial height and diameter were measured in millimeters, density of colonies was evaluated according to 3-point scale (1 point – low, 2 points – average, 3 points – high) [31]. Growth index (GI) was calculated with the use of the formula:
GI = Dhg , t where D – diameter, mm; h – height of mycelium, mm; g – density of colony, point; t – age of colony, day.
Radial growth rate (RGR) was calculated with the use of the formula:
RGR = (R 2 -R 1 ) , (t 2 -t 1 )
where R 2 – radius of colony, mm; R 1 – radius of inoculation spot, mm; t 1 , t 2 – start and end time of cultivation, day.
Fungal isolates were cultivated on toxigenic medium (Myro) where sporulation was partially repressed and formation of toxins occurred [38]. B uckwheat seeds were soaked in the filtrate of culture fluid for 24 hours at 25 °С. Seeds soaked in sterile water and nutrient medium were used as the control samples. Buckwheat seeds were placed into moist chambers in batches of 20 with fivefold repetition and then left to sprout for 7-14 days at 24–26 °С. Presence of phytotoxic substances in culture fluid was determined by growth effects. Percentage of seed germination, length of sprouts and roots were evaluated in comparison with the control samples. Cultures which repressed germination rate of seeds by 50-100 % were considered highly toxic, the ones which repressed it by 30-50 % were considered moderately toxic, and the cultures which repressed germination rate by 1-30 % were thought to have low toxicity with a statistically significant difference from the control samples (confidence interval is 0.95). Phytotoxic activity of metabo- lites was determined on corn sprouts (Zea mays) of variety Slavyanka with the help of the bioprobe technique. The choice of the research object is explained by its fast germination rate and formation of sprouts with a relatively equal size. Apical growing points and meristems of forty-eight-hour corn sprouts were placed in the filtrate of culture fluid for one hour. The control sprouts were placed onto sterile nutrient medium. After that the spouts were placed into a moist chamber for 24 hours at 26 °С. Length of the roots of the experimental plants and control samples were measured. [39]. Phytotoxic activity was calculated with the use of the formula:
A ph = 100 – ( L——х -—L—i ) x 100% , L r - L i
Where A ph – phytotoxic activity of root growth inhibition, %; L x – mean length of sprout roots of experimental variant after 24 hours, mm; L r – mean length of roots of the control sample after 24 hours, mm; L i – initial length of sprout roots, mm.
Phytopathogenic activity of living cultures was evaluated on potato sucrose agar according to the modified method of Chelkowski and Manka [40]. The studied strains were inoculated onto PSA in Petri dishes and cultivated for 5 days at 24±2 оС in the darkness. Buckwheat seeds were sterilized over the surface with 70 % ethanol for 3 minutes and then soaked in sterile water for 24 hours at 24±2 °С to promote swelling. Swollen seeds were placed on the surface of grown fungal culture in batches of 10 with threefold repetition. Seeds, which were spread over sterile PSA, were used as the control samples. The dishes with fungal cultures and seeds were kept in the darkness at 24±2 °С for 7 days. After that, the length of the germinated sprouts was measured (mm) and the degree of damage was evaluated according to 4-point scale: 0 points – healthy sprout, 1 point – focal necrosis, 2 points – necrotic tissue amounts to 50%, 3 points – death. The soil substrate was sterilized with 0.5% solution of KMnO 4 before inoculation. The control seeds were placed into seed boxes while experimental seeds were soaked for 24 hours in the culture fluid of the isolate which was cultivated on toxigenic medium for 14 days at 26±2 °С. The plants grew under artificial light at 25±2 °С. Germinative energy was evaluated on the fourth day, field germination was estimated on the seventh day after sowing. Length of the above ground biomass and root system of plants, also number of leaves and leaf blade area were measured during the studied period of vegetation. Root systems of all survived plants were analyzed in moist chambers to check for inner infections.
Results and discussion
Pathological effect exerted on plants by the species of the genus Fusarium is determined by presence and the degree of manifestation of phytopathogenic factors, which vary significantly among different species and populations. Intraspecific polymorphism of phytopathogenic fungi and influence of edaphoclimatic conditions facilitate natural selection of ecologically flexible and highly pathogenic populations which occupy dominant position within phytopathogenic complex structure and drive out other species with a lower occurrence and weaker pathogenic properties. Fungi of the genus Fusarium , having a wide range of adaptive reactions, are able to switch from saprotrophic nutrition to feeding on plant tissues and organs through synthesis of a broad spectrum of bioactive substances, including mycotoxins and phytohormones. The study on intraspecific variability of phytopathogenic properties of natural populations presents an opportunity to evaluate pathogen load of the most common toxicogenic species, forecast Fusatium blight epiphytotics and select the most effective
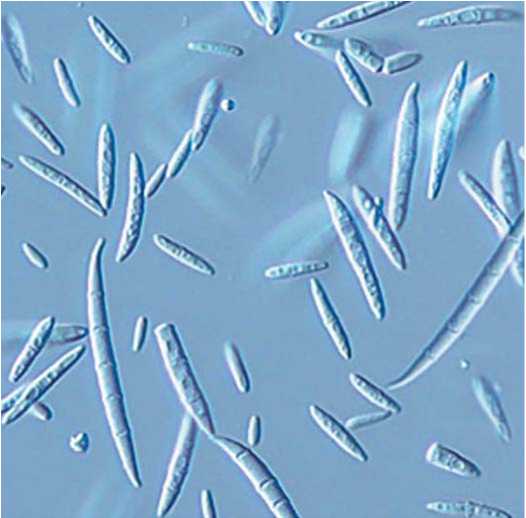
Fig.1. Macroconidia F.avenaceum. 400x , ZOE (BioRad)
Рис.1.Макроконидии F.avenaceum.400x , ZOE (BioRad)
fungicides against toxicogenic and aggressive native strains of phytopathogens.
We obtained 150 isolates on different selective media, which were later identified and attributed to 3 species. Fusarium oxyspo-rum Schltdl., 1824 and Fusarium avenaceum (Fr.) Sacc., 1886 were isolated from leaves, plant residues and fallen seeds. Fusarium graminearum Schwabe, 1839 was found predominantly in root systems and rhizosphere soil. That allows to consider these sources to be theirnatural infectious reservoir. These results do not reveal information totally unacknowledged before. It is well-known that fungi of the genus Fusarium show a high level of specialization, including phylogenetic and organotropic ones [41]. According to our data confirmed by Yu.A. Litovka [42], representatives of the species F. avenaceum form multiple macronidia with the number of septa from 0 to 3, rarely up to 9 (fig. 1). Macronidia are oval or egg-shaped with zero or 1 internal wall. Chlamichlamydospores are scarce in mycelium and conidia of an aging culture. Conidiophores are unbranched or branched poly-and monophialides. Colonies grow fast on PDA and form dense aerial white mycelium whose color changes from light to red-brown. While aging the culture forms orange sporodochia. The color of the colony reverse varies from carmine-red to dark-brown.
Fusarium oxysporum forms multiple and predominantly unicellular macroconidia of oval and budlike shape in false heads. Macroconidia are large, faintly sickle, with thin walls. Conidiophores are unbranched or branched monophialides (fig. 2). The growth is fast on PDA. Aerial mycelium is procumbent and white, but changes its color to purple in time. The colony reverse is also purple.
Fusarium graminearum forms few unicellular micronidia of oval or budlike shape. Macroconidia are formed in great quantity, they are large, cylinder-shaped, with thick walls and parallel dorsal and ventral sides along the length of conidia (fig. 3). Conidiophores are unbranched and branched monophialides. The growth is fast on PDA. Aerial mycelium is diminutive, rarely abundant. In time, multiple sporodochia develop and the surface of colony becomes cream-colored, the colony reverse turns colorless.
During the first stage of the research, we evaluated the influence of metabolites, which were synthesized on toxigenic medi-

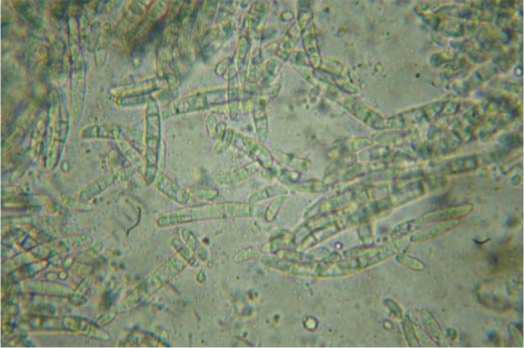
Fig.2.Electronmicrograph (х2000)
ofmicroconidia (1) infalse heads (JCM-7000)and macroconidia (2) (Levenhuk D740T) ofFusarium oxysporum.
Рис.2. Электронная микрофотография (х2000) микрокони дий (1) в ложных головках (JCM-7000)
и макроконидий (2) (LevenhukD740T) Fusarium oxysporum.
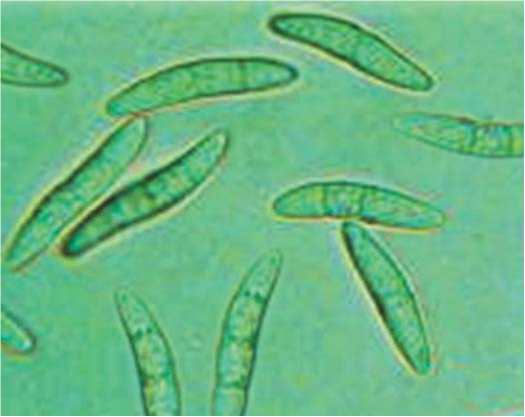
Fig.3. Macroconidia ofFusarium graminearum, 400x , ZOE (BioRad)
Рис.3.Макроконидии Fusarium graminearum, 400x , ZOE (BioRad)
Table 1. Laboratory germination of buckwheat seeds under the influence of isolates of the genus Fusarium
Таблица 1. Лабораторная всхожесть семян гречихи под действием метаболитов изолятов рода Fusarium (наименьшая существенная разница 0,5)
|
Isolate |
Laboratory germination of seeds, % of the control sample rate |
|||
|
Bashkirskaya krasnostebel’naya |
Dikul |
Izumrud |
Pri 7 |
|
|
Fusarium avenaceum |
39.8±2.27 |
24.2±1.63 |
32.4±2.37 |
41.4±2.27 |
|
Fusarium oxysporum |
12.2±2.80 |
12.4±2.27 |
12.5±1.63 |
22.5±2.21 |
|
Fusarium graminearum |
42.2±1.79 |
39.8±2.91 |
41.4±2.80 |
72.3±2.41 |
Note: differences are statistically significant at p < 0.05; p < 0.01 and p < 0.001, respectively, sample mean differences were assessed at a confidence level of 0.95.
um, exerted on seeds, sprouts and vegetative plants. The study on laboratory germination of buckwheat seeds showed that the level of phytotoxicity of fungal culture fluid varies significantly among varieties (table 1). Germination rate of the control seed samples was considered to be 100 % in the calculations.
According to the classification of Yu.A. Litovka [42] culture filtrates of F. avenaceum and F. graminearum isolates should be considered to have low toxicity and F. oxysporum should be regarded as highly toxic. Phytotoxicity of pathogens manifested itself not only in decrease in laboratory germination ability of buckwheat seeds, but also in inhibition of buckwheat sprout development. It proved that metabolites exerted a prolonged effect on sensitive plants. It is concluded that in some cases culture filtrates, obtained from isolates, influenced biometrics of sprouts and hampered the development of above and underground parts (fig. 4, 5).
It can be concluded that metabolites of most isolates produce a noticeable phytotoxic effect on seeds and sprouts of majority of studied buckwheat varieties and inhibit germination process and subsequent development of plants in laboratory conditions. Filtrates, obtained after 49-day cultivation of isolates, were used for quantitative evaluation of phytotoxic activity. On toxigenic medium (Myro) where sporulation was partially repressed and synthesis of toxin complex occurred [43]. The study was conducted on forty-eight-hour corn sprouts for the reason that this test-object is
Bashkirskaya krasnostebel'naya 11 Dikul ■ Izumrud Pri 7
Fig.4. Influence exerted by culturefiltrates offungalisolates of the genus Fusarium on morphometric parameters ofbuckwheat sprout roots
Рис.4.Влияние культуральных фильтратов изолятов грибов рода Fusarium на морфометрические показатели корня проростков гречихи
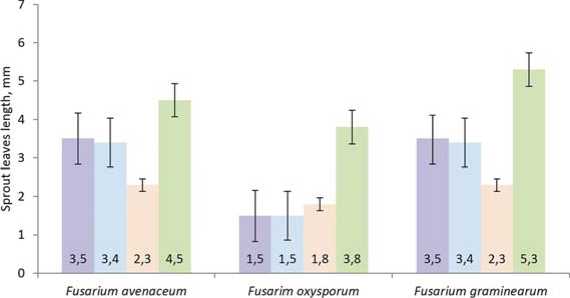
Bashkirskaya krasnostebel'naya Dikul Izumrud Pri 7
Fig.5. Influence exerted by culturefiltrates offungalisolates ofthegenus Fusarium on morphometric parameters ofbuckwheat sprout leaves
Рис.5.Влияние культуральных фильтратов изолятов грибов рода Fusarium на морфометрические показатели листа проростков гречихи
Table 2. Phytotoxic activity of culture filtrates (PACF) of Fusarium fungal isolates demonstrated on corn sprouts, % Таблица 2. Фитотоксическая активность культуральных фильтратов (ФАКФ) изолятов грибов рода Fusarium на проростки кукурузы, % , (наименьшая существенная разница 0,5)
|
Culture |
Parameters |
Culture filtrate |
Control |
||
|
Fusarium avenaceum |
Fusarium oxysporum |
Fusarium graminearum |
|||
|
Zea mays |
Mean root length, mm |
20.5±0.18 |
- |
- |
25±0.18 |
|
PACF on inhibition of roots, % |
18.0±1.12 |
100 |
100 |
- |
|
Note: differences are statistically significant at p < 0.05; p < 0.01 and p < 0.001, respectively, sample mean differences were assessed at a confidence level of 0.95
the most convenient in terms of both sensitivity to the influence of metabolites and rate of sprouting. It is concluded that all isolates are characterized by high phytotoxic activity (table 2).
It should be noted that similar results have been obtained from the study on phytopathogenic properties of Fusarium fungi conducted on buckwheat sprouts. All studied isolates inhibited the development of buckwheat sprouts in comparison with the control sample. The maximum degree of necrotic process observed in tissues of sprouts infected by F.oxysporum amounted to 1.6 points. 44 % of sprouts had the degree of necrosis equal to 0.9-1.6 points according to 3-point scale. In general, the conducted study showed that F.avenaceum, F. oxysporum and F. graminearum had pronounced phytopathogenic and aggressive properties in relation to buckwheat and test-plants in the laboratory condition. It means that they can pose a threat of infectious focus development and epiphytotics in unfavorable conditions. The total sum of their studied phytotoxic properties is convincing enough to consider them potentially hazardous to buckwheat for wilt disease development because species of the genus Fusarium are characterized by a wide range of adaptive reactions. It is known that representatives of the genus Fusarium can synthesize a wide spectrum of enzymes and are active mineralization agents of organic compounds and thereby being able to colonize various substrates [44]. They show a high level of phylogenetic specialization, are not strictly limited by the geographic range of theirhost-plant and have different environmental needs [45]. The conducted research is preliminary to the study on living cultures and metabolites of some isolates of the genus Fusarium fungi for creation of resistant buckwheat varieties with the cell selection method.
Об авторах:
Conclusions
-
1. Isolates of Fusarium oxysporum Schltdl., 1824 and Fusarium avenaceum (Fr.), Sacc., 1886 and Fusarium gramin-earum Schwabe, 1839 were obtained from buckwheat leaves, seeds and rhizosphere in the course of our research. Their morphological traits corresponded with the ones described in literature.
-
2. Culture filtrates of F. avenaceum and F. graminearum isolates should be considered to have low toxicity, and filtrates of F. oxysporum isolate should be regarded as highly toxic. Phytotoxicity of pathogens manifested itself not only in decrease of laboratory germination ability of buckwheat seeds, but also in inhibition of buckwheat sprout development. It proved that metabolites exerted a prolonged effect on sensitive plants.
-
3. All studied isolates inhibited the development of buckwheat sprouts in comparison with the control sample. The maximum degree of necrotic process observed in tissues of sprouts infected by F.oxysporum amounted to 1.6 points. 44 % of sprouts had the degree of necrosis equal to 0.9-1.6 points according to 3-point scale.
-
4. The conducted study showed that F.avenaceum, F. oxys-porum and F. graminearum had pronounced phytopathogenic and aggressive properties in relation to buckwheat and testplants in the laboratory condition. It means that they can pose a threat of infectious focus development and epiphytotics in favorable for them conditions. The total sum of their studied phytotoxic properties is convincing enough to consider them potentially hazardous to buckwheat for wilt disease development.
About theauthors:
Nathalia V. Matsishina – Cand. Sci. (Biology), Senior Researcher, the laboratory of breeding and genetic research of field crops, Correspondence Author, ,
Svetlana A. Borovaya – Postgraduate Student, Researcher at the laboratory of breeding and genetic research of field crops, ,
-
• References (In Russ.)
-
10. Petrova N.A., Ivanchenko O.B. Non-traditional low-gluten raw material in technologies of speciality beers Beer and beverages. 2006;(6):38-41. (In Russ.)
-
13. Pavlovskaya N.E., Gor'kova I.V., Gagarina I.N., Gneusheva I.A, Gagarina A.YU. Technology for development of biologically active food supplements for animal agriculture. Vestnik OrelGAU. 2011;(6):29-32. (In Russ.)
-
20. Kulikov N.I., Naumkin V.P. Insects on buckwheat flowers. Sbornik nauchnykh trudov po pchelovodstvu. Orel. 1999;(3):63-67. (In Russ.)
-
21. Parakhin N.V. Buckwheat: biological capabilities and ways of their implementation. Vestnik OrelGAU. 2010;4(25):4-8. (In Russ.)
-
23. Martynov A.I. Harvest components. Pchelovodstvo. 1984;(6):9-10. (In Russ.)
-
24. Martynov S.P. Assessment of the environmental plasticity of crop cultivars. Agricultural Вiology. 1989;(3):124-128. (In Russ.)
-
25. Chudakov N. Вuckwheat: increasing yields with a minimum of costs. Agrarnoe obozrenie. 2016;3(55):56-59. (In Russ.)
-
26. Afonin A.N., Green S.L., Dzyubenko N.I., Frolov A.N. Agroecological atlas of Russia and neighboring countries: economically significant plants, their pests, diseases and weeds. Available from: http://www.agroatlas.ru [Accessed 20 September 2021]
-
27. Potemkina V.I., Kuznetsova A.V., Lelei A.S., Korotyaev Yu.A., Timoshinov R.V. Buckwheat weevil (Rhinoncus Sibiricus Faust) – dangerous pest of buckwheat in Primorsky krai. Plant protection and quarantine. 2008;(6):38. (In Russ.)]
-
28. Kuznetsova A.V., Klykov A.G. Assessment of weevil damage degree of buckwheat varieties in Primorsky krai. Grain economy of Russia. 2015;(3):37-40. (In Russ.)
-
29. Kuznetsova A.V., Klykov A.G. Biology of buckwheat weevil (Rhinoncus Sibiricusfaust, 1893) in Primorsky krai. Belgorod State University Scientific Bulletin. Natural sciences. 2014;26(3(174):58-61. (In Russ.)
-
30. Khairullin R.M., Kutluberdina D.R. Occurrence of the genus Fusarium fungi in summer wheat grain in the southern forest-steppe of the Republic of Bashkortostan. Vestnik of the Orenburg State University. 2008;(12):32-36. (In Russ.)
-
31. Bilai V.I. (ed.) Methods of experimental mycology. Kyiv: Naukova dumka; 1982 (In Russ.)
-
32. Zvyagintsev D.G. (ed.) Methods of soil microbiology and biochemistry. Moscow: Izdatel'stvo MGU; 1991 (In Russ.)
-
33. Netrusov A.I., Egorova M.A., Zakharchuk L.M. Practical course on microbiology. Moscow: Akademiya; 2005 (In Russ.)
-
37. Gagkaeva T.YU., Gannibal F.B., Gavrilova O.P. Infestation of wheat grain with fungi of the genus Fusarium and genus Alternaria in southern Russia in 2010. Plant protection and quarantine. 2012;(1):37-41. (In Russ.)
-
39. Bilai V.I. Fusarium fungi. Kyiv: Naukova dumka; 1977. (In Russ.)
-
40. Sokolova G.D. Clonal variation of toxigenicity and vegetative compatibility of Fusarium graminnearum. Mycology and Phytopathology. 2000;34(2):63-66. (In Russ.)
-
41. Gagkaeva T.YU., Gavrilova O.P., Levitin M.M., Novozhilov K.V. Fusarium blight of grain crops. Plant protection and quarantine. Suppl. 2011;(5):70-112. (In Russ.)
-
42. Litovka Yu.A. Species composition and representative occurrence of the genus Fusarium fungi on grain crops (wheat and barley) cultivated in the conditions of Central Siberia. Bulletin of KSAU . 2017;6(129):140-149. (In Russ.)
-
44. Ivashchenko V.G., Shipilova N.P., Nefedova L.I. Bioecological and phytosanitary aspects of research on Fusarium head blight. Mycology and Phytopathology. 1997;31(2):58-63. (In Russ.)
-
45. Levitin M.M., Ivashchenko V.G., Shipilova N.P., Nesterov A.N., Gagkaeva T.YU., Potorochina I.G., Afanas'eva O.B. Fusarium head blight pathogens of grain crops and manifestations of the disease in northwestern Russia. Mycology and Phytopathology. 1994;28(3):58-64. (In Russ.)
Список литературы Fusarium blight of common buckwheat in Primorsky krai
- Varlakhova L., Bobkov S., Zotikov V., Mikhailova I. Grain qualities of Russian buckwheat (Fagopyrum esculentum Moench) varieties. The Proceedings of Papers. 12th International Symposium on Buckwheat, August 21-25. Laško. 2013:181-182.
- Kreft I. Comparison of buckwheat bread products with the bread from other alternative sources. Fagopyrum. 2010;(27):41-46.
- Dziedzic K., Gorecka D., Kobus-Cisowska J., Jessika M. Możliwości wykorzystania gryki w produkcji żywności funkcjonalnej. Nauka Przyroda Technologia. 2010;4(2):1-7.
- Zhang L., Li Z. Functional characteristics of traditional buckwheat product. Chinese cereals and oils. 2009;24(3):53-57.
- Bonafaccia G., Marocchini M., Kreft I. Composition and technological properties of the flour and bran in common and Tartary buckwheat. Food Chem. 2003;80(1):9-15. https://doi.org/10.1016/S0308-8146(02)00228-5
- Li S., Zhang G.H. Advances in the development of functional foods from buckwheat. Crit. Rev. Food Sci. Nutr. 2001;41(6):451-464. https://doi.org/10.1080/20014091091887
- Podolska G. The effect of habitat conditions and agrotechnical factors on the nutritional value of buckwheat. Zhou M., Kreft I., Woo S.-H., Chrungoo N., Wieslander G. (eds.) Molecular breeding and nutritional aspects of buckwheat. 2016;22:283-297. https://doi.org/10.1016/B978-0-12-803692-1.00022-5
- Lee J., Jiang W., Qiao Y., Cho Y.I., Woo M.O., Chin J.H., Kwon S.W., Hong S.S., Choi I.Y., Koh H.J. Shotgun proteomic analysis for detecting differentially expressed proteins in the reduced culm number rice. Proteomics. 2011;11(3):455-468. https://doi.org/10.1002/pmic.201000077
- Cepkova P., Dvoracek V. Seed protein polymorphism of four common buckwheat varieties registered in the Czech Republic. Fagopyrum. 2006;23:17-22.
- Петрова Н.А., Иванченко О.Б. Нетрадиционное низкоглютеновое сырье в технологиях специальных сортов пива. Пиво и напитки. 2008;6:38-41.
- Wijngaard H.H., Arendt E.K. Optimization of a mashing program for 100% malted buckwheat. J. institute of Brewing. 2006;112(1):57-65. https://doi.org/10.1002/j.2050-0416.2006.tb00708.x
- Steadman K.J., Burgoon M.S., Lewis B.A., Edwardson S.E., Obendorf R.L. Buckwheat seed milling fraction: description, macronutrient composition and dietary fiber. J. Cereal Sci. 2001;33(3):271-278. https://doi.org/10.1006/jcrs.2001.0366
- Павловская Н.Е., Горькова И.В., Гагарина И.Н., Гнеушева И.А, Гагарина А.Ю. Технология создания биологически активных добавок для животноводства. Вестник ОрелГАУ. 2011;(6):29-32.
- Guo X., Ma Y., Parry J., Gao J., Yu L., Wang M. Phenolics сontent and аntioxidant аctivity of buckwheat from different locations. Molecules. 2011;(16):9850-9867. https://doi.org/10.3390 / molecules 16129850
- Ikeda S., Kreft I., Asami Y., Mochida N., Ikeda K. Nutrition educational aspects of the utilization of some buckwheat foods. Fagopyrum. 2008;(25):57-64.
- Ikeda S., Yamashita Y., Tomura K., Kreft I. Nutritional comparison in mineral characteristics between buckwheat and cereals. Fagopyrum. 2006;(23):61-65.
- Campbell C.G. Buckwheat. Fagopyrum esculentum Moench. RomeGatersleben: IPGRI. 1997.
- Kreft S., Strukelj В., Gaberscik A., Kreft I. Rutin in buckwheat herbs grown at different UV-B radiation levels: comparison of two UV spectrophotometric and an HPLC method. J. Exp. Bot. 2002;53(375):18011804. https://doi.org/10.1093/jxb/erf032
- Zhang L., Li Z. Functional characteristics of traditional buckwheat product. Chinese cereals and oils. 2009;24(3):53-57.
- Куликов Н.И., Наумкин В.П. Насекомые на цветках гречихи. Сборник научных трудов по пчеловодству. Орел, 1999;(3):63-67
- Парахин Н.В. Гречиха: биологические возможности и пути их реализации. Вестник ОрелГАУ. 2010;4 (25):4-8.
- Marshall H.G., Pomeranz Y. Buckwheat: Description, breeding, production and utilization. Pomeranz Y. (ed.) Advances in Cereal Science and Technology. St. Paul, Minnesota : American Associationof Cereal Chemists Incorporated. 1982;(5):157-210.
- Мартынов А.И. Слагаемые урожая. Пчеловодство. 1984;(6):9-10.
- Мартынов С.П. Оценка экологической пластичности сортов сельскохозяйственных культур. Сельскохозяйственная биология. 1989;3:124-128
- Чудаков Н. Гречиха: увеличение урожайности при минимуме затрат. Аграрное обозрение. 2016;3(55) 56-59.
- Афонин А.Н., Грин С.Л., Дзюбенко Н.И., Фролов А.Н. Агроэкологический атлас России и сопредельных стран: экономически значимые растения, их вредители, болезни и сорные растения. [Интернет-версия 2.0] 2008. URL: http://www.agroatlas.ru (дата обращения: 20.09.2021).
- Потемкина В.И., Кузнецова А.В., Лелей А.С., Коротяев Ю.А., Тимошинов Р.В. Гречишный долгоносик - опасный вредитель гречихи в Приморском крае. Защита и карантин растений. 2008;(6):38.
- Кузнецова А.В., Клыков А.Г. Оценка сортов гречихи на поврежденность гречишным долгоносиком в условиях Приморского края. Зерновое хозяйство России. 2015;(3):37-40.
- Кузнецова А.В., Клыков А.Г. Биология гречишного долгоносика (Rhinoncus sibiricus Faust, 1893) в Приморском крае. Научные ведомости Белгородского государственного университета. Серия Естественные науки. 2014;26(3(174):58-61.
- Хайруллин Р.М., Кутлубердина Д.Р. Распространенность грибов рода Fusarium в зерне яровой пшеницы в южной лесостепи Республики Башкортостан. Вестник Оренбургского государственного университета. 2008;(12):32-36.
- Методы экспериментальной микологии. Под ред. В.И. Билай. Киев: Наукова думка. 1982. 550 с.
- Методы почвенной микробиологии и биохимии. Под ред. Д.Г. Звягинцева. М.: Изд-во МГУ. 1991. 303 с.
- Нетрусов А.И., Егорова М.А., Захарчук Л.М. Практикум по микробиологии. М.: Академия. 2005. 608 с.
- Nelson P.E., Toussoun T.A., Marasas W.F.O. Fusarium species: an illustrated manual for identifications. Pennsylvania State University Press; 1983.
- Singleton L.L., Mihail J.D., Rush C.M. (еds). Methods for research on soil borne phytopathogenic fungi. St. Paul, Minnesota: APS Press; 1992.
- Leslie J.F., Summerell B.A. The Fusarium laboratory manual. USA: Blackwell Publishing; 2006.
- Гагкаева Т.Ю., Ганнибал Ф.Б., Гаврилова О.П. Зараженность зерна пшеницы грибами Fusarium и Alternaria на юге России в 2010 году. Защита и карантин растений. 2012;(1):37-41.
- Greenhalgh R., Levandier D. Adams W., Miller J.D., Blackwell B.A., McAlees A.J., Taylor A. Production and characterization of deoxynivalenol and other secondary metabolites of Fusarium culmorum (CMI 14764, HLX 1503). Journal agriculture food chemistry. 1986;34(1):98102.
- Билай В.И. Фузарии. Киев: Наукова думка. 1977. 443 с.
- Соколова Г.Д. Клональная изменчивость токсигенности и вегетативная совместимость Fusarium graminnearum. Микология и фитопатология. 2000;34(2):63-66.
- Гагкаева Т.Ю., Гаврилова О.П., Левитин М.М., Новожилов К.В. Фузариоз зерновых культур. Приложение к журналу «Защита и карантин растений». 2011;(5):70-112.
- Литовка Ю.А. Видовой состав и представленность грибов рода Fusarium на зерновых культурах (пшеница и ячмень), выращиваемых в условиях Средней Сибири. Вестник КрасГАУ. 2017;6(129):140-149.
- Goyal S., Ramawat K.G., Mérillon J.M. Different shades of fungal metabolites: An overview. Fungal metabolites. 2016:1-29. https://doi.org/10.1007/978-3-319-19456-1_34-1
- Иващенко В.Г., Шипилова Н.П., Нефедова Л.И. Биоэкологические и фитосанитарные аспекты исследования фузариоза колоса. Микология и фитопатология. 1997;31(2):58-63.
- Левитин М.М., Иващенко В.Г., Шипилова Н.П., Нестеров А.Н., Гагкаева Т.Ю., Поторочина И.Г., Афанасьева О.Б. Возбудители фузариоза колоса зерновых культур и форм проявления болезни на северо-западе России. Микология и фитопатология. 1994;28(3):5864.


