GC/MS analysis of Hypericum perforatum L. (Hypericaceae) species
Автор: Saleh Basel
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 2 т.19, 2023 года.
Бесплатный доступ
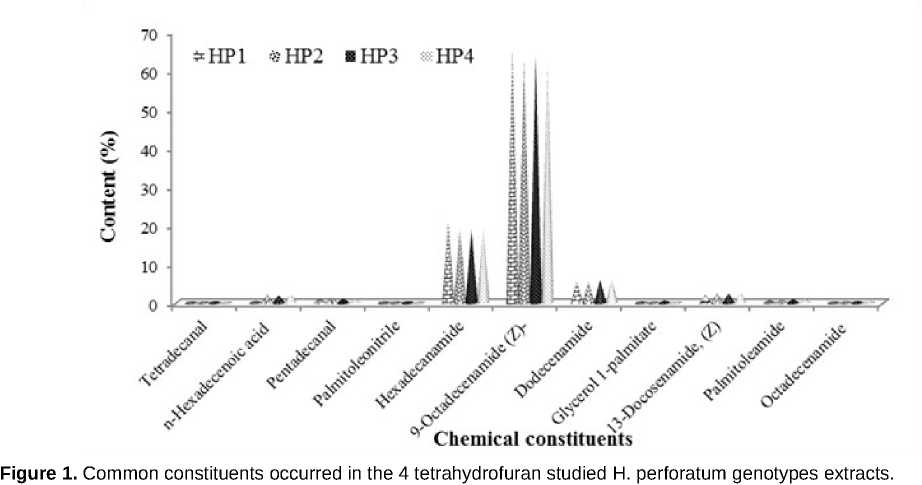
Hypericum perforatum L. aerial parts have been extracted with tetrahydrofuran solvent and their chemical composition has been investigated by Gas Chromatography-Mass Spectrometry (GC/MS) analysis. GC/MS chromatogram of the 4 tetrahydrofuran studied H. perforatum genotypes (HP) revealed that the main constituents for HP1 were: 9-Octadecenamide (Z)-(Oleic acid) (65.551%), Hexadecenamide (20.681%) and Dodecenamide (5.595%). Whereas, for HP2, they were 9-Octadecenamide (Z)- (Oleic acid) (63.117%), Hexadecenamide (19.107%) and Dodecenamide (5.585%). As for HP3, they were 9-Octadecenamide (Z)-(Oleic acid) (63.496%), Hexadecenamide (18.891%) and Dodecenamide (5.961%). Whereas, they were 9-Octadecenamide (Z)-(Oleic acid) (62.048%), Hexadecenamide (19.325%) and Dodecenamide (5.914%) for HP4. Thereby, isolation of these constituents and investigation of their biological activity is requested.
Hypericum perforatum l, phytochemical analysis, gas chromatography-mass spectrometry (gc/ms), chemical constituents
Короткий адрес: https://sciup.org/143180103
IDR: 143180103
Текст научной статьи GC/MS analysis of Hypericum perforatum L. (Hypericaceae) species
Hypericum perforatum L. species belongs to Hypericum genus and Hypericaceae family which included approximately 500 species of flowering plants (Schepetkin et al. , 2020). Wild H. perforatum L. is one of 21 Hypericum species existent in Syria (Mouterde, 1970). H. perforatum L. is commonly known as St. John’s wort and it is a perennial herb native to relatively dry temperature zones of Europe and North America (Çırak et al. , 2010) Its richness in different secondary metabolites including essential oils, amino acids, tannins, flavonoids, xanthones, naphthodianthrones, phloroglucinols, procyanidins, phenylpropanes and other water-soluble components (Çırak et al. , 2010) make them as one of the most commonly-investigated medicinal plants of the last two decades. It displayed different pharmacological properties e.g . as anticholinesterase and antioxidant properties (Božin et al. , 2013); antidepressant, antibacterial, antifungal, antiviral, relaxing smooth muscle contraction, inhibiting protein kinase C, potentiating wound healing and photodynamic effects (Ivetic et al. , 2011). It became one of the most commercially plants used due to its medicinal value beside its traditional applications in folk medicine and its importance as an ornamental plant (Saleh, 2019). Thereby, many researches have been done to investigate its extracts and essential oils chemical composition using different analytical methods; e.g . Gas chromatography-mass spectrometry (GC/MS) (Çakir et al. , 1997; Seger et al. , 2004; Chatzopoulou et al. , 2006; Çırak et al. , 2010; Helmja et al. , 2011; Chauhan et al. , 2011; Pirbalouti et al. , 2014; Đorđević, 2015; Parchin and Ebadollahi, 2016; Saleh, 2019; Schepetkin et al. , 2020); high-performance layer chromatography (HPLC) (Nuevas-Paz et al. , 2005; Božin et al. , 2013); high-performance layer chromatography (HPLC) with a diode-array detector (DAD)- mass spectrometry (MS) - (MS) coupling (HPLC–DAD–MS–MS) HPLC– (Silva et al. , 2005); liquid chromatography (LC)-with a diode-array detector (DAD)-mass spectrometry (MS) - (MS) coupling (LC-DAD-MS/MS) (Rusalepp et al. , 2017) and fourier transform raman spectroscopy ( T-Raman) (Saleh, 2020).
In the current study, tetrahydrofuran extract of wild Hypericum perforatum L. aerial parts was collected from four H. perforatum genotypes grown in Lattakia-Syria and their chemical composition has been investigated by GC/MS analysis.
MATERIALS AND METHODS
Plant Material
Wild Hypericum perforatum L. (HP) aerial parts (10 plants/sample) were collected from four genotypes in Lattakia-Syria. Sampling has been carried out during blooming stage, from four collection sites differ in their altitude (80-680 m) and annual rainfall (750-1250 mm) (Table 1). Samples were shade dried for two weeks, milled to fine powder by special electric mill and stored separately in glass bowls until extracts preparation.
Extract preparation
The fine powder for each sample was extracted with tetrahydrofuran solvent as flowing: 1 g of fine powder was extracted with 10 mL tetrahydrofuran overnight, filtrated with filter papers (Whatman no.1). Then, all extracts were kept in tightly fitting stopper bottles and stored at 4 °C. The final obtained extracts were then analyzed using GC/MS analysis.
GC/MS assay
To investigate chemical components in tetrahydrofuran H. perforatum L. aerial parts extract, GC Chromatec-Crystal 5000 system, supported with Chromatec Crystal Mass Spectrometry Detector (Chromatec, Russia) has been employed. GC/MS analysis has been carried out according to the following conditions: The range scan was 42-850 MU, the column [BP-5-MS (30 m × 0.25 mm × 0.25 μm)], carrier gas (0.695 ml/min flow of Helium gas). Oven temperature was programmed initially at 35 °C for 1 min, then an increase by 10°C /1 min till 220 °C, then increase to 230 °C by 1°C /1 min followed by 10 °C /1 min increasing till 255 °C (hold for 5 min). Injector temperature was 275 °C and detector temperature was 280 °C and ionization energy was 70 ev. Each extract component was identified by comparing retention time values of gas chromatography on polar columns and by comparing mass spectrum and NIST library databases.
RESULTS AND DISCUSSION
GC/MS analysis of the 4 tetrahydrofuran studied H. perforatum genotypes extracts has been performed. GC/MS chromatogram revealed 20, 18, 19 and 20 chemical constituents were identified in the 4 tetrahydrofuran studied H. perforatum genotypes HP1 (Table 2), HP2 (Table 3), HP3 (Table 4) and HP4 (Table 5) extracts. Of which eleven constituents commonly occurred in the 4 tetrahydrofuran studied H. perforatum genotypes extracts ( igure 1). Whereas, the remaining constituents were presented in scare amounts.
GC/MS chromatogram in the 4 tetrahydrofuran studied H. perforatum genotypes revealed that the main constituents for HP1 were: 9-Octadecenamide (Z)-(Oleic acid) (65.551%), Hexadecenamide (20.681%), Dodecenamide (5.595%), 13-Docosenamide, (Z) (2.114%) and Pentadecanal (1.25%) Whereas, for HP2, they were 9-Octadecenamide (Z)- (Oleic acid) (63.117%), Hexadecenamide (19.107%), Dodecenamide (5.585%), 13-Docosenamide, (Z) (2.514%) and Pentadecanal (1.419%). As for HP3, they were 9-Octadecenamide (Z)- (Oleic acid) (63.496%), Hexadecenamide (18.891%), Dodecenamide (5.961%), 13-Docosenamide, (Z) (2.481%) and Pentadecanal (1.041%). Whereas, they were 9-Octadecenamide (Z)-(Oleic acid) (62.048%), Hexadecenamide (19.325%), Dodecenamide (5.914%), 13-Docosenamide, (Z) (2.739%) and Pentadecanal (1.046%) for HP4.
In the current study, Glycerol 1-palmitate and 9-Octadecenamide (Z)- (Oleic acid) presented in the 4 tetrahydrofuran H. perforatum extracts and Phytol in tetrahydrofuran H. perforatum HP2 and HP4 extracts were supported by Seger et al. (2004). Moreover, n-Hexadecenoic acid as a common constituent occurred in the 4 tetrahydrofuran H. perforatum extracts in the current study, was reported in the same species and supported by Saleh (2019). Indeed, among chemical constituents, Hexadecenoic acid, Octadecanoic acid and 9-Octadecenamide (Z)- presented in the 4 tetrahydrofuran H. perforatum extracts in the current study, were reported in ethanolic Psorospermum febrifugum extracts belonging to the same family (Asogwa et al. , 2019).
Some studies deal with H. perforatum extracts phytochemical analysis. In this regards, Seger et al. (2004) reported 9 alkanes (C21–C32), four primary (C24, C26, C28 and C30) and one secondary alkanol (C28), one aldehyde (C32), alkanoic acids (C14–C32), linoleic acid, oleic acid, methyl linoleate, glyceryl-1-palmitate, as well as 42 wax esters (C29–C48). Moreover, one sesquiterpene alcohol, nerolidol, two diterpenes, neophytadiene and phytol, two pentacyclic derivatives, -amyrin and lupeol and six triterpenes, squalene, the sterols -sitosterol, -stigmasterol, and nervisterol were observed in H. perforatum supercritical fluid extracts (carbon dioxide without modifiers extract) using GC/MS. Whereas, Nuevas-Paz et al. (2005) reported protopseudohypericin, pseudohypericin, protohypericin and hypericin in methanolic H. perforatum extract using HPLC analysis. Moreover, Silva et al. (2005) reported rutinacetyl and Kaempferol 3-rutinoside were identified for the first time in total ethanolic H. perforatum extracts using HPLC–DAD–MS– MS analysis. Moreover, Božin et al. (2013) reported ethanolic H. perforatum extract using HPLC analysis. They reported that total phenolics ranged between 14.35-16.72%, whereas, total flavonoids ranged between 1.33-2.48%. Indeed, performance phenolic composition of ethanolic H. perforatum extract has been done and these phenolic constituents including phenolic acids [Chlorogenic (0.37-0.65%) and Caffeic (nd-0.07%)], lavonoids [Rutin (0.33-0.66%) and Quercitrin (0.09-0.19%)], Phloroglucinols [Hyperforin (0.76-1.71%)] and Naphtodianthrones [Hypericin (0.56-1.11%)]. Whereas, Rusalepp et al. (2017) reported phytochemical composition of methanolic H. perforatum L. aerial parts using LC-DAD-MS/MS analysis. Phytochemical analysis revealed that total flavonols ranged between 3.15 -4.72%, chlorogenic acids 0.79 - 1.27% , total phenolics ranged between 4.62 - 6.93%, total hypericins ranged between 0.26 - 0.62% and total hyperforins ranged between 2.41 - 11.91%; of which hyperoside ranged between 1.70 - 2.76% and hyperforin ranged between 2.15 - 10.60%.
Other studies however focused on H. perforatum essential oils phytochemical analysis. In this regards, Çakir et al. (1997) previously reported that α-pinene (61.7%), 3-carene (7.5%), β-caryophyllene
(5.5%), myrcene (3.6%) and cadalene (3.2%) were mainly identified in aerial parts H. perforatum L. essential oils using GC/MS analysis. Whereas, Chatzopoulou et al. (2006) reported wild and cultivated H. perforatum essential oils composition using GC/MS analysis. They reported 69 identified compounds of which Germacrene D was the main compound presented in its oils from wild (22.8%) and cultivated (16.9%) types, followed by 2-methyloctane (10.8– 17.8%), β-caryophyllene (6.6–10.3%), α-pinene (5.2– 10.1%) and bicyclogermacrene (4.1–4.8%). Indeed, 14 compounds were presented in wild type and not in cultivated one. Whereas, Çırak et al. (2010) reported H. perforatum essential oils using GC/ ID and GC/MS analyses. They reported that hydrocarbon and oxygenated sesquiterpenes such as caryophyllene oxide (6.01–12.18%), β-selinene (5.08–19.63%), α-selinene (4.12–10.42%), γ-muurolene (5.00–9.56%), β-caryophyllene (4.08–5.93%), spathulenol (2.34–5.14%) and d-cadinene (3.02–4.94%) were the main compounds occurred in H. perforatum essential oils. Whereas, monoterpenes, both hydrocarbon and oxygenated, were occurred in scarce amounts of α- and β-pinene, myrcene, linalool, cis- and trans-linalool oxide, and α-terpineol. Moreover, Helmja et al. (2011) reported 34 compounds of which Germacrene D (13.7%), Spathulenol (2.9%) and Caryophyllene oxide (2.5%) were mainly presented in H. perforatum essential oils using GC/MS. Indeed, Chauhan et al. (2011) reported 40 constituents in cultivated aerial parts H. perforatum essential oils using GC/MS analysis, of which germacrene D (22.1%), b-caryophyllene (11.3%), a-pinene (8.6%), a-cadinol (4.4%), b-pinene (3.8%), 2-methyl-octane (3.7%), terpinen-4-ol (3.3%), caryophyllene oxide (3.3%), a-muurolol (2.9%) and spathulenol (2.8%) were mainly presented. Whereas, Pirbalouti et al. (2014) reported flowers H. perforatum essential oils composition using GC/MS analysis. They reported that α-pinene (12.52-49.96%), β-pinene (6.349.70%), (E)-β-ocimene (4.44-12.54%), β-caryophyllene (1.19-5.67%), and germacrene-D (2.34-6.92%) were presented as major constituents in its essential oils. Whereas, Đorđević (2015) reported 134 compounds in H. perforatum L. essential oils using GC/MS analysis, of which germacrene D (18.6%), (E)-caryophyllene
(11.2%), 2-methyloctane (9.5%), α-pinene (6.5%), bicyclogermacrene (5.0%) and (E)-β-ocimene (4.6%) were mainly presented in its essential oils. Indeed, Parchin and Ebadollahi (2016) reported 14 chemical constituents in aerial parts H. perforatum essential oils using GC/MS analysis. They reported that Decane (59.58%), Dodecene (12.93%), ethylcyclohexane (6.84%), 5-methylnonane (4.71%), 3-methylnonane (4.32%) and tetradecane (3.82%) were mainly presented. urthermore, Saleh (2019) reported 52 chemical constituents in aerial parts H. perforatum essential oils using GC/MS. Of which, ß -Selinenol (18.13%), Elemol (12.77%), ß-Elemene (10.73%), γ-Eudesmol (6.62%), n-Hexadecenoic acid (6.46%), ß-Selinene (5.98%), Valencene (4.59%), 1S,Cis-Calamenene (3.82%), Aromadendren epoxide-(I) (3.16%) and Germacrene D (2.88%) were mainly identified.
Recently, Schepetkin et al. (2020) reported 30 compounds were detected in flowers H. perforatum essential oils using GC/MS analysis, of which 3-methoxy-2,3-dimethylcyclobutene (9.8%), cis-p-menth-3-en-1,2-diol (9.1%), terpinen-4-ol (7.4%), α-terpineol (6.1%), trans-ascaridol glycol (4.6%), 4-hydroxy-4-methyl-cyclohex-2-enone (3.4%), limonen-4-ol (3.2%), p-cymen-8-ol (2.9%), myrtenol (2.7%), and α-pinene (2.2%) were mainly presented; whereas the sesquiterpenes were found in trace amounts. While, leaves H. perforatum essential oils inversely comprised sesquiterpenes (63.2%) of which germacrene D (25.7%) and β-caryophyllene (9.5%) were mainly presented. Indeed, they also contained oxygenated monoterpenes like terpinen-4-ol (2.6%). Whereas, Saleh (2020) reported 7, 5, 6 and 6 peaks for the same HP1, HP2, HP3 and HP4 H. perforatum genotypes, respectively using T-Raman analysis. Of which three peaks were common for the four studied H. perforatum genotypes: peak of 1250 cm-1 assigned to C–O stretch-Carboxylic acids group, peak of 1600 cm-1 assigned to C=C stretch aromatic-Aromatics group and peak of 2850 cm-1 assigned to C–H stretch-Alkanes group.
Overall, the four tetrahydrofuran H. perforatum extracts showed some differences in their chemical constituents. These observed differences could be attributed to the geographical distribution where the studied samples were collected. Where, samples were collected from four collection sites differ in their altitude (80-680 m) and annual rainfall (750-1250 mm). This observation was in coherent of Tangpao et al. (2018) and Saleh (2019, 2020) findings who reported that the geographical distribution is a main factor affect plants phytochemical composition.
Table 1: Description of the 4 studied H. perforatum genotypes in the current study
|
Genotype |
Code |
Altitude (m) |
Annual rainfall (mm) |
|
H. perforatum1 |
HP1 |
80 |
750 |
|
H. perforatum2 |
HP2 |
420 |
850 |
|
H. perforatum3 |
HP3 |
546 |
1200 |
|
H. perforatum4 |
HP4 |
680 |
1250 |
|
Table 2: GC/MS analysis of the tetrahydrofuran studied H. perforatum genotype1 (HP1) extract. |
|||
|
Peak No |
RT (min) |
Name of Compound |
Peak area (%) |
|
1 |
17.7 |
Hexadecenoic acid |
0.042 |
|
2 |
19.7 |
Tetradecanal |
0.446 |
|
3 |
19.9 |
Neophytadiene |
0.128 |
|
4 |
21.4 |
n-Hexadecenoic acid |
0.598 |
|
5 |
22.3 |
Pentadecanal |
1.25 |
|
6 |
23.2 |
Palmitoleonitrile |
0.268 |
|
7 |
23.7 |
Hexadecenenitrile |
0.22 |
|
8 |
23.8 |
uranone |
0.147 |
|
9 |
24.7 |
Octadecanoic acid |
0.39 |
|
10 |
25.1 |
Hexadecenamide |
20.681 |
|
11 |
25.6 |
1-Hexacosanol |
0.008 |
|
12 |
27.3 |
Clorophene -α-Amyrin |
0.182 |
|
13 |
29.5 |
9-Octadecenamide (Z)- |
65.551 |
|
14 |
30.1 |
Dodecenamide |
5.595 |
|
15 |
32.3 |
Erucic acid |
0.658 |
|
16 |
32.6 |
Glycerol 1-palmitate |
0.477 |
|
17 |
33.6 |
Deoxyspergualin |
0.079 |
|
18 |
33.8 |
13-Docosenamide, (Z) |
2.114 |
|
19 |
33.9 |
Palmitoleamide |
0.808 |
|
20 |
34.3 |
Octadecenamide |
0.357 |
|
Table 3: GC/MS analysis of the tetrahydrofuran studied H. perforatum genotype2 (HP2) extract. |
|||
|
Peak No |
RT (min) |
Name of Compound |
Peak area (%) |
|
1 |
17.8 |
1-Octanol,3,7-dimethyl- |
0.117 |
|
2 |
19.7 |
Tetradecanal |
0.449 |
|
3 |
19.9 |
Neophytadiene |
0.237 |
|
4 |
21.4 |
n-Hexadecenoic acid |
2.279 |
|
5 |
22.3 |
Pentadecanal |
1.419 |
|
6 |
23.2 |
Palmitoleonitrile |
0.25 |
|
7 |
23.6 |
Hexadecenenitrile |
0.228 |
|
8 |
23.8 |
Phytol |
0.219 |
|
9 |
24.7 |
Pentadecanoic acid |
0.76 |
|
10 |
25.1 |
Hexadecenamide |
19.107 |
|
11 |
29.6 |
9-Octadecenamide (Z)- |
63.117 |
|
12 |
30.2 |
Dodecenamide |
5.585 |
|
13 |
32.3 |
Erucic acid |
0.772 |
|
14 |
32.6 |
Glycerol 1-palmitate |
0.319 |
|
15 |
33.6 |
Tricosane, 2-methyl- |
1.448 |
|
16 |
33.8 |
13-Docosenamide, (Z) |
2.514 |
|
17 |
33.9 |
Palmitoleamide |
0.903 |
|
18 |
34.3 |
Octadecenamide |
0.511 |
|
Table 4: GC/MS analysis of the tetrahydrofuran studied H. perforatum genotype3 (HP3) extract. |
|||
|
Peak No |
RT (min) |
Name of Compound |
Peak area (%) |
|
1 |
17.6 |
CycloDodecene |
0.102 |
|
2 |
19.7 |
Tetradecanal |
0.405 |
|
3 |
19.9 |
Neophytadiene |
0.125 |
|
4 |
21.4 |
n-Hexadecenoic acid |
1.997 |
|
5 |
22.3 |
Pentadecanal |
1.163 |
|
6 |
23.2 |
Palmitoleonitrile |
0.144 |
|
7 |
23.6 |
Octadecanenitrile |
0.163 |
|
8 |
23.8 |
Tricosane, 2-methyl- |
0.071 |
|
9 |
24.7 |
Octadecanoic acid |
0.54 |
|
10 |
25.1 |
Hexadecenamide |
18.891 |
|
11 |
27.3 |
Oxazolidine |
0.115 |
|
12 |
29.6 |
9-Octadecenamide (Z)- |
63.496 |
|
13 |
30.1 |
Dodecenamide |
5.961 |
|
14 |
32.3 |
Butanoic acid, tridec-2-ynyl ester |
0.631 |
|
15 |
32.6 |
Glycerol 1-palmitate |
0.639 |
|
16 |
33.6 |
Hexadecenoic |
1.499 |
|
17 |
33.8 |
13-Docosenamide, (Z) |
2.481 |
|
18 |
33.9 |
Palmitoleamide |
1.041 |
|
19 |
34.3 |
Octadecenamide |
0.536 |
|
Table 5: GC/MS analysis of the tetrahydrofuran studied H. perforatum genotype4 (HP4) extract. |
|||
|
Peak No |
RT (min) |
Name of Compound |
Peak area (%) |
|
1 |
17.8 |
1-Undecanol |
0.293 |
|
2 |
19.7 |
Tetradecanal |
0.31 |
|
3 |
19.9 |
Neophytadiene |
0.208 |
|
4 |
21.4 |
n-Hexadecenoic acid |
2.418 |
|
5 |
22.3 |
Pentadecanal |
0.958 |
|
6 |
23.2 |
Palmitoleonitrile |
0.168 |
|
7 |
23.6 |
Hexadecenenitrile |
0.198 |
|
8 |
23.8 |
Phytol |
0.632 |
|
9 |
24.7 |
9-Octadecenoic acid (Z)-, oxiranymethyl ester |
0.766 |
|
10 |
25.1 |
Hexadecenamide |
19.325 |
|
11 |
25.6 |
2,6,9,12,16-Pentamethylheptadeca-2,6,11,15-tetraaene-9- carboxylic acid |
0.265 |
|
12 |
27.3 |
Oixren-5(1aH)-one,2,7,9,10-tetrakis (acetyloxy) decahydro- |
0.144 |
|
13 |
29.6 |
9-Octadecenamide (Z)- |
62.048 |
|
14 |
30.2 |
Dodecenamide |
5.914 |
|
15 |
32.3 |
Erucic acid |
0.661 |
|
16 |
32.6 |
Glycerol 1-palmitate |
0.393 |
|
17 |
33.6 |
Nonadecane |
0.878 |
|
18 |
33.8 |
13-Docosenamide, (Z) |
2.739 |
|
19 |
33.9 |
Palmitoleamide |
1.046 |
|
20 |
34.3 |
Octadecenamide |
0.635 |
CONCLUSION
Chemical composition of the 4 tetrahydrofuran studied H. perforatum HP1, HP2, HP3 and HP4 genotypes extracts has been assessed using GC/MS analysis. GC/MS chromatogram revealed eleven constituents commonly occurred in the 4 tetrahydrofuran studied H. perforatum genotypes extracts In this regards, 9-Octadecenamide (Z)- (Oleic acid),
Hexadecenamide and Dodecenamide were mainly occurred in the 4 tetrahydrofuran studied H. perforatum extracts. GC/MS chromatogram showed some differences in chemical composition of the studied H. perforatum genotypes extracts, could be attributed to the geographical distribution. This study highlighted GC/MS chemical composition of H. perforatum genotypes for the first time in Syria.
ACKNOWLEDGMENT
I thank Dr. I. Othman (Director General of AECS) and Dr. N. Mirali (Head of Molecular Biology and Biotechnology Department in AECS) for their support, and also the Plant Biotechnology group for technical assistance.
CONFLICTS OF INTEREST
The authors declare that they have no potential conflicts of interest.
Список литературы GC/MS analysis of Hypericum perforatum L. (Hypericaceae) species
- Asha, S., and Rao, K.N. (2002). Effect of simulated Asogwa FC, Ibezim A, Ntie-Kang F, Asogwa CJ, Okoye COB (2019). Anti-psoriatic and immunomodulatory evaluation of Psorospermum febrifugum Spach and its phytochemicals. Sci. Afr. e00229.
- Bozin B, Kladar N, Grujic N, Anackov G, Samojlik I, Gavaric N, Conic B (2013). Impact of origin and biological source on chemical composition, anticholinesterase and antioxidant properties of some St. John's Wort species (Hypericum spp., Hypericaceae) from the central Balkans. Molecules. 18(10): 11733-11750.
- Qakir A, Duru ME, Harmandar M, Ciriminna R, Passannanti S, Piozzi F (1997). Comparison of the volatile oils of Hypericum scabrum L. and Hypericum perforatum L. from Turkey. Flavour. Fragr. J. 12(4): 285-287.
- Chatzopoulou P.S., Koutsos T.V. and Katsiotis S.T. (2006). Chemical composition of the essential oils from cultivated and wild grown St. John's Wort (Hypericum perforatum). J. Essent. Oil Res. 18(6): Cecotti R (2011). Essential oil composition of Hypericum perforatum L. from cultivated source. J. Essent. Oil Res. 23(3): 20-25.
- Çirak C, Bertoli A, Pistelli L, Seyis F (2010). Essential oil composition and variability of Hypericum perforatum from wild populations of northern Turkey. Pharm. Biol. 48(8): 906-914.
- Dordevic AS (2015). Chemical composition of Hypericum perforatum L. essential oil. Adv. Technol. 4(1): 64-68.
- Grafakou M.-E, Diamanti A, Antaloudaki E, Kypriotakis Z, Ciric A, Sokovic M, Skaltsa H (2020). Chemical composition and antimicrobial activity of the essential oils of three closely related Hypericum species growing wild on the island of Crete, Greece. Appl. Sci. 10(8): 2823.
- Helmja K, Vaher M, Pussa T, Orav A, Viitak A, Levandi T, Kaljurand M (2011). Variation in the composition of the essential oils, phenolic compounds and mineral elements of Hypericum perforatum L. growing in Estonia. Nat. Prod. Res. 25(5): 496-510.
- Ivetic V, Trivic S, Pogancev MK, Popovic M, Zlinska J (2011). Effects of St John's Wort (Hypericum perforatum L.) extracts on epiletogenesis. Molecules. 16(9): 8062-8075.
- Mouterde P (1970). Nouvlle Flore du Liban et de la Syrie, Tome II Dar el Machreq- Bayrouth. p. 519529.
- Nuevas-Paz L, Molina-Tores J, Prieto-Gonzalez S (2005). Determination of Hypericin in Hypericum species grown in Cuba. Acta Farm. Bonaerense. 24 (1): 89-90.
- Parchin RA, Ebadollahi A (2016). Biological activities of Hypericum perforatum L. essential oil against red flour beetle, Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). J. Entomol. 13: 91-97.
- Pirbalouti AG, Fatahi-Vanani M, Craker L, Shirmardi H (2014). Chemical composition and bioactivity of essential oils of Hypericum helianthemoides, Hypericum perforatum and Hypericum scabrum. Pharm. Biol. 52(2):175-81.
- Rusalepp L, Raal A, Püssa T, Mäeorg U (2017). Comparison of chemical composition of Hypericum 643-646.
- Chauhan RS, Vashistha RK, Nautiyal MC, Tava A, differences in chemical composition of the studied H. chemical composition of H. Perforatum I thank Dr. I. Othman (Director General of AECS) and Dr. N. Mirali (Head of Molecular Biology and Biotechnology Department in AECS) for their support, and also the Plant Biotechnology group for technical perforatum and H. maculatum in Estonia. Biochem. Syst. Ecol. 73: 41-46.
- Saleh B (2019). Volatile constituents of three Hypericum (Hypericaceae) species using GC-MS analysis. Int. J. Pharm. Life. Sci. 10(11-12):6349-6354.
- Saleh B (2020). FT-Raman phytochemical analysis of Hypericum perforatum L. (Hypericaceae) species. Int. J. Pharm. Life. Sci. 11(9): 6953-6958.
- Schepetkin IA, Özek G, Özek T, Kirpotina LN, Khlebnikov AI, Quinn MT (2020). Chemical composition and immunomodulatory activity of Hypericum perforatum essential oils. Biomolecules. 10(6): 916.
- Seger, C., Römpp, H., Sturm, S., Haslinger, E., Schmidt, P. C. and Hadacek, F. (2004). Characterization of supercritical fluid extracts of St. John's Wort (Hypericum perforatum L.) by HPLC-MS and GC-MS. Eur. J. Pharm. Sci. 21(4): 453-463.
- Silva BA, Ferreres F, Malva JO, Dias ACP (2005). Phytochemical and antioxidant characterization of Hypericum perforatum alcoholic extracts. Food Chem. 90: 157-167.
- Tangpao T, Chung H-H, Sommano SR (2018). Aromatic profiles of essential oils from five commonly used Thai basils. Foods. 7(11):175-187.


