Генная инженерия в онкологии, основанная на технологии CRISPR-CAS9
Автор: Полатова Д.Ш., Мадаминов А.Ю., Савкин А.В., Ибрагимова Д.А.
Журнал: Сибирский онкологический журнал @siboncoj
Рубрика: Обзоры
Статья в выпуске: 4 т.23, 2024 года.
Бесплатный доступ
Цель исследования - анализ современных научных данных, посвященных молекулярным механизмам системы CRISPR-Сas9 в редактировании генов, преимуществам и недостаткам в исследованиях рака и разработке новых методов лечения. Материал и методы. Комплексный электронный поиск соответствующих публикаций проведен в научных базах данных PubMed/MEDLINE, ScienceDirect, Wiley и Google Scholar за период с 2014 по 2024 г. Поиск был адаптирован к конкретным требованиям каждой базы данных на основе следующих ключевых слов: CRISPR-Cas9, sgРНК, редактирование генома, иммунотерапия рака, CAR-T. В результате поиска было найдено 487 публикаций по интересующей теме, из которых 54 были использованы для написания литературного обзора. Кроме того, в статье дискретно подчеркиваются важность и проблемы CRISPR-Cas9 при производстве генно-инженерных Т-клеток для их потенциального использования при лечении определенных типов рака.
Crispr-cas9, sgрнк, днк, редактирование генома, рак, cart
Короткий адрес: https://sciup.org/140307082
IDR: 140307082 | УДК: 616-006:577.21 | DOI: 10.21294/1814-4861-2024-23-4-152-161
Текст обзорной статьи Генная инженерия в онкологии, основанная на технологии CRISPR-CAS9
По оценкам ВОЗ, в 2050 г. ожидается увеличение количества впервые выявленных злокачественных новообразований (ЗНО) на 77 % по сравнению с 2022 г. [1]. Борьба со злокачественными опухолями является глобальной проблемой, которая требует новых стратегий лечения. Э. Шарпантье и Дж. Даудна удостоены Нобелевской премии по химии 2020 г. за разработку метода редактирования генома с помощью системы CRISPR-Cas9 [2]. Перед многими учеными встали вопросы о том, как изменит эта система геном человека, который сформировался в результате эволюции. Однако авторы открытия считают целесообразным редактирование генома соматических клеток по мере необходимости в конкретных исследованиях, а не половых клеток. Хотя спорадическое распространение системы CRISPR-Cas в микробиотах предполагает, что она подвергалась горизонтальному переносу генов в процессе филогенеза [3].
Система CRISPR (кластеризованные регулярно расположенные короткие палиндромные повторы, Clustered Regularly Interspaced Short Palindromic Repeats) сформировалась в результате эволюционного развития прокариот (бактерии и археи) и представляет собой механизм защиты от чужеродной ДНК вирусов и плазмид, генерирующихся за счет активации определенных родов РНК. Функциональная ассоциация белка 9 (Cas9, CRISPR-associated protein 9), члена семейства белков с рестриктазной активностью, с CRISPR приводит к двухцепочечным разрывам ДНК на уровне специфических нуклеотидных последовательностей (т.н. протоспейсеры). Следовательно, высокопроизводительные генетические маневры CRISPR-Cas9 в редактировании ДНК сгенерировали мощный импульс исследованиям и разработкам биологических методов лечения рака [4]. Трансляция технологий генной инженерии в медицинскую онкологию и успехи в генетических манипуляциях с использо- ванием системы CRISPR-Cas9 в эукариотических клетках привели к созданию эффективных иммуно-и таргетных препаратов при раке, рефрактерном к стандартным методам лечения. В настоящее время эта система эффективно используется в иммунотерапии рака, включая модификацию адаптивных Т-клеток и естественных киллеров против опухолевых клеток, выработку антител, стимуляцию секреции цитокинов и ингибирование активности иммунных контрольных точек. Например, сегодня CAR-T (chimeric antigen receptor T cells, Т-клетки с химерным антигенным рецептором)-терапия широко используется в качестве одного из основных компонентов иммунотерапии при лечении лейкемии, лимфомы и некоторых солидных опухолей [5]. CRISPR-Cas9 превзошел другие системы редактирования генома по следующим параметрам: CRISPR-Cas9 распознает комплементарный сайт ДНК посредством взаимодействия РНК-ДНК; легко проектируется; приводит к более высокой специфичности и эффективности; обеспечивает простой способ манипулирования несколькими ДНК-мишенями одновременно (высокоэффективное мультиплексирование); является экономичной технологией [6]. Исследования, направленные на дальнейшее изучение потенциала системы CRISPR-Cas9, предоставили ученым понимание микроокружения ЗНО и возможность создания модифицированных клеток, которые считаются важными движущими силами иммунотерапии. Хотя система CRISPR-Cas9 является новой технологией, в настоящее время она становится общепринятым лабораторным методом редактирования генов. Однако трансляция технологии CRISPR-Cas9 в клиническую практику сталкивается с некоторыми серьезными проблемами, которые мешают ей быть успешным терапевтическим подходом. Эти препятствия включают, но не ограничиваются, следующие проблемы: нецелевые изменения, недостаточная специфичность, ненадежная до- ставка, неожиданные делеции и сложные геномные перестройки в отредактированных клетках, генотоксичность, иммуногенность белка Cas9 и CRISPR-Cas9 могут проявлять свою способность редактирования генома только при наличии нуклеотидной последовательности PAM (protospacer adjacent motif, смежный мотив протоспейсера) (5'-NGG-3') [7].
В данном обзоре обсуждаются применение и проблемы технологии CRISPR-Cas9 в исследованиях и лечении рака, достижения и перспективы продвижения новых инженированных клеточных и субклеточных терапевтических средств за пределами иммуноонкологии. Анализируются современные молекулярные механизмы стратегии CRISPR-Cas9 в редактировании генов, преимущества и недостатки в исследованиях рака и разработке новых методов лечения. Кроме того, мы фокусируемся на важности и проблемах CRISPR-Cas9 в производстве генетически модифицированных Т-клеток для потенциального использования при лечении определенных типов рака. Комплексный электронный поиск соответствующих публикаций был проведен в научных базах данных PubMed/MEDLINE, ScienceDirect, Wiley и Google Scholar за период с 2014 по 2024 г. Поиск адаптирован к конкретным требованиям каждой базы данных на основе следующих ключевых слов: CRISPR-Cas9, sgРНК, ДНК, редактирование генома, рак, CAR-T.
Конструирование системы CRISPR-Cas9
Способность ДНК восстанавливаться после двухцепочечных разрывов позволяет генетически манипулировать системой CRISPR-Cas9. CRISPR-Cas9 представляет собой адаптивный модуль редактирования генов, имеющий преимущества перед другими технологиями, такими как интерференция РНК (RNAi), эффекторные нуклеазы, подобные активаторам транскрипции (TALENs), и нуклеазы цинковых пальцев (ZFNs) [8]. На самом деле, CRISPR-Cas9 появляется в природе как жизненно важная иммунная система прокариот, приспособленная расщеплять чужеродную или вторгающуюся ДНК вируса [9]. Прокариоты справедливо интегрируют в свой геном определенные сегменты вирусного генома (протоспейсеры), внедрившиеся в цитозоль, в качестве молекулярной памяти, что позволяет сформировать быструю защитную реакцию при последующем заражении этим вирусом. Интегрированная вирусная последовательность (спейсер) затем транскрибируется с образованием CRISPR РНК (crРНК) и мобилизуется в качестве комплементарного зонда для обнаружения последовательности PAM во вторгающейся ДНК, которая, в свою очередь, определяет специфический сайт расщепления для эндонуклеазы Cas9 [10]. Таким образом, система CPISPR-Cas9, ориентированная на молекулярную защиту прокариот, широко используется в геномной инженерии, которая в последнее время быстро интегрируется с медицинской онкологией [11, 12].
Однако исследователи разработали направляющую РНК (guide [g] РНК) в качестве искусственной замены эндогенного комплекса crРНК для распознавания целевого нуклеотида с последующей активностью нуклеазы Cas9. Кроме того, у CRISPR преобладает потенциал для мультиплексирования нескольких генов-мишеней, программирования gРНК и простоты доставки in vivo с низкой цитотоксичностью [13]. CRISPR-Cas9 можно рассматривать как РНК-управляемую эндонуклеазу (RGEN, RNA-guided endonuclease), которая включает в себя распознавание специфических коротких последовательностей-мишеней (~20 п.н.). PAM, нуклеотидная последовательность из 2–6 пар оснований, очень важно, чтобы gРНК узнала свои целевые нуклеотиды, за которыми следует рекрутирование белка Cas9. При этом gРНК направляет завербованный Cas9 через его специфические последовательности, связанные с трансактивирующей crRNA (tracrРНК). Путем формирования гибрида crРНК и tracrРНК (также называемой последовательностью одиночной направляющей РНК, sgРНК [single guide]) система CRISPR-Cas может направлять фермент Cas9 на гибридизацию с определенными последовательностями ДНК и вводить двухцепочечные разрывы с помощью спейсера (рис. 1). Связывание эндогенных crРНК и tracРНК посредством линкерных последовательностей (Linkerloop) обеспечивает образование единой искусственной олигонуклеотидной структуры, выполняющей роль направляющей РНК [14].
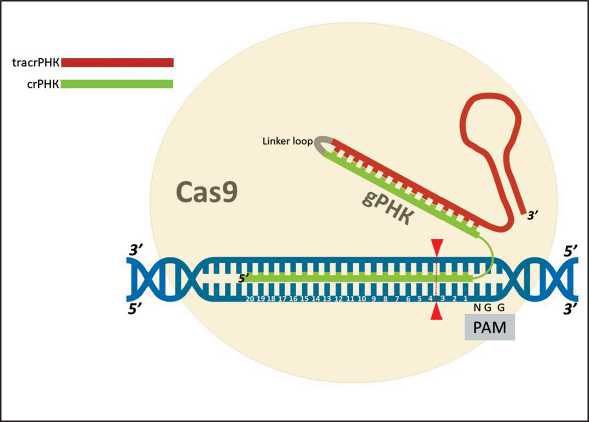
Рис. 1. Управляемый рибонуклеопротеиновый комплекс для распознавания и разрыва целевой последовательности ДНК (gРНК, однокомпонентная запрограммированная направляющая РНК; crRNA, CRISPR РНК; tracrRNA, трансактивирующая crРНК; PAM, смежный мотив протоспейсера; Linker loop, линкерная петля). Примечание: рисунок выполнен авторами
Fig. 1. A regulated ribonucleoprotein complex for recognizing and breaking the target DNA sequence (gRNA, single-component programmed guide RNA; crRNA, CRISPR RNA; tracrRNA, transactivating crRNA; PAM, protospacer adjacent motif).
Note: created by the authors
При взаимодействии sgРНК с ДНК-мишенью белок Cas9 (эндонуклеаза рестрикции) генерирует двухцепочечный разрыв из трех нуклеотидов выше последовательности PAM (рис. 1). PAM представляет собой последовательность 5'-NGG-3', где «N» – это любое азотистое основание, за которым следуют два азотистых основания гуанина («G»). Направляющие РНК могут транспортировать Cas9 на многие комплементарные сайты в геноме, но никакое редактирование не сможет произойти, если Cas9 не распознает сайт PAM. В идеале репарация ДНК в клетке может происходить посредством негомологичного соединения концов (NHEJ)-опосредованного пути ДНК или посредством репарации ДНК, направленной по гомологии (HDR) [15]. Прямое соединение двух одноцепочечных концов в механизме репарации NHEJ приводит к небольшим случайным мутациям вставки или делеции (indels), которые в конечном итоге способствуют молчанию соответствующего гена (knock-out). HDR использует комплементарные последовательности ДНК в гомологичной матрице для генерации репарации ДНК, с помощью которой конкретный ген может быть создан (knock-in) путем транспозиции конструктуриро-ванных одноцепочечных фрагментов ДНК [16]. Поскольку нацеливание системы CRISPR основано на формировании комплекса белок/рибонуклеотид, ее можно использовать для любых геномных целей путем создания различных структурных вариаций gРНК [11]. Прямая доставка РНК, кодирующей белок Cas, и специфической gРНК в клетку еще больше повышает эффективность системы CRISPR при редактировании конкретных генов, тем самым преодолевая препятствия и трудности, связанные с априорными методами, основанными на системе репарации гомологичной рекомбинации [17].
CRISPR-Cas9 в исследованиии лечении рака
Раковые клетки в основном характеризуются различными мутациями ДНК, которые провоцируют нестабильность генома и нарушение регуляции механизмов клеточного деления. В настоящее время наиболее известными мутационными изменениями являются инактивация гена TP53 и активация гена RAS вследствие миссенс-мутаций. Более 50 % всех первичных ЗНО человека связаны с мутациями гена TP53 [18] и примерно до 30 % – с мутациями гена RAS (особенно KRAS ) [19]. За исключением недавно одобренного таргетного препарата Соторасиба, нацеленного на KRAS (G12C) [20], продукты этих мутантных генов остаются наиболее трудными и безуспешными мишенями в точечной терапии рака. В этом контексте технология CRISPR-Cas9 использовалась для изучения известных генов, вызывающих рак, таких как TP53 и KRAS , и для выявления новых ответственных генов. Использование CRISPR для инактивации онкогена
KRAS на мышиной модели рака легких оказалось весьма эффективным [21]. Обладая этими знаниями, исследователи могут разрабатывать таргетные методы лечения мутантных генов и предотвращать прогрессирование рака. CRISPR-Cas9 предоставляет беспрецедентные возможности для имитации структурно аберрантных хромосом, наблюдаемых при раке у генетически модифицированных мышей, которые ранее было трудно моделировать. Этот подход, основанный на CRISPR-индуцированных двухцепочечных разрывах ДНК и последующей репарации эндогенными системами NHEJ/HDR, позволяет относительно просто вставлять или удалять фрагменты ДНК разных размеров не только в геноме мыши, но и в геноме человека [15, 22]. Кроме того, система CRISPR-Cas9 также используется для индукции и изучения мутаций белков, которые вызывают устойчивость к лекарствам и снижают их фармакологическую активность. Стратегия Drug Target SeqR, разработанная для поиска физиологических мишеней для лекарств, включает в себя сочетание высокопроизводительного секвенирования и редактирования генома с помощью CRISPR-Cas9 [23, 24].
В настоящее время для редактирования соответствующих генов-мишеней используется единственная направляющая РНК (sgRNA), состоящая из 17–20 нуклеотидов, которая образуется путем лигирования crРНК (нацеливающая последовательность) и tracrРНК (рекрутирующая нуклеаза Cas9). Нуклеаза Cas9 расщепляет примерно на уровне третьего азотистого основания выше PAM, что требует комплементарного нековалентного связывания sgРНК с 20 нуклеотидами, расположенными выше последовательности PAM (5'-NGG-3') на 3'-конце целевой ДНК [12] (рис. 1). Таким образом, последовательность PAM абсолютно необходима для расщепления фосфодиэфирной связи ферментом Cas9, но не может быть включена в последовательность sgРНК.
Скрининг объединенной библиотеки с использованием CRISPR – мощный инструмент для обнаружения новых зависимостей рака на основе модели потери функции (loss-of-functionmodel). Плазмиды в этих библиотеках CRISPR нацелены на тысячи конкретных генов, и их влияние на определенный фенотип можно впоследствии изучить в различных условиях. Первым шагом является лен-ти- или ретровирусная доставка sgRNA в клетки с последующим отбором к определенным условиям роста для отбора интересующего фенотипа. После этого этапа ДНК выделяют из клеток и подвергают секвенированию. Затем эти данные сопоставляются с библиотекой gRNA, специфичной для гена, и далее анализируются in silico с помощью вычислительных подходов. Затем фенотипы клеток и соответствующие гены-мишени сравнивают с фенотипом известных контролей в библиотеке, чтобы идентифицировать изменения, вызванные нацеливанием на представляющий интерес ген. Эти объединенные подходы к скринингу CRISPR способны выявлять новые зависимости в онкогенезе путем проверки большого количества потенциальных генов и путей, ведущих к разработке новых методов лечения [25].
В полном смысле основными компонентами стратегий лечения рака на основе СRISPR являются следующие: инактивация онкогенов (например, генов EGFR и KRAS при раке легких) [26], перепрограммирование иммунных клеток против антигенов раковых клеток (Т-клетки при гематологических и солидных опухолях) [27], коррекция генов-супрессоров ( BRCA1 при раке яичников) [28] и доставка терапевтических агентов к опухолевым клеткам (бактерии с отредактированным геномом против клеток рака поджелудочной железы, стимуляция экспрессии микроРНК в клетках рака печени) [28, 29]. Из этих стратегий наиболее распространенным и эффективно используемым в клинической практике является редактирование геномов иммунных клеток, в частности Т-лимфоцитов, с целью модулирования цитотоксического ответа на опухолеспецифические антигены [30].
CRISPR-Cas9 часто используется для модификации аутологичных Т- и NK-клеток с помощью антигенных конструкций и химерных антигенных рецепторов, а также для улучшения их сенсорных цепей с помощью сложной функциональности, способных распознавать и уничтожать опухолевые клетки [5]. При этом Т-лимфоциты периферической крови используются для создания генетически модифицированных CAR-T клеток, экспрессирующих трансгенные Т-клеточные рецепторы (TCR) и химерные антигенные рецепторы (CAR) [31]. CAR-T-клетка как «живое лекарство» исследуется уже более двух десятилетий. Хотя результаты исследований подтверждают терапевтическую эффективность CAR-T против некоторых солидных опухолей, на данный момент ни один CAR-T не был одобрен FDA для лечения солидных опухолей. Одним из основных препятствий на пути успешного использования стратегии CAR-T в лечении солидных опухолей являются сложный клеточный состав опухолевого микроокружения и диверсификация репертуара опухолеспецифических антигенов [32].
В настоящее время проводится несколько клинических испытаний с использованием технологии CRISPR-Cas9 на CAR-T-клетках. Например, NCT04037566 является первым исследованием на людях, оценивающим CD19 CAR-T-клетки с отредактированным эндогенным HPK1 у пациентов с рецидивирующей и рефрактерной (Р/Р) лейкемией или лимфомой [33]. NCT04637763 – это клиническое исследование I фазы, посвященное изучению влияния и безопасности аллогенных CD19 CAR-T-клеток, отредактированных с помощью CRISPR [33], у пациентов с Р/Р B-клеточной не- ходжкинской лимфомой. Наконец, NCT03545815 – клиническое исследование I фазы с использованием CRISPR-Cas9 для нокаута PD-1 и TCR в CART-клетках и направления их на лучение пациентов с мезотелин-позитивными множественными солидными опухолями [34].
Доставка CRISPR-Cas9 в клетку
Доставка модели CRISPR-Cas9 in vivo сталкивается с различными проблемами, включая низкую эффективность разрушения, инсерционный мутагенез, нецелевые эффекты, токсичность и иммуногенность. Максимизация специфичности и эффективности CRISPR-Cas9 можно будет обеспечить за счет разработки более надежных средств доставки, улучшения конструкции sgРНК и создания новой нуклеазы Cas9 [35]. Редактирование CAR-T-клеток с CRISPR-Cas9 может преодолеть многие проблемы, такие как использование только аутологичных Т-клеток, аллогенные реакции, тоническая сигнализация (конститутивная передача сигналов) и истощение Т-клеток, низкая производительность в микроокружении солидных опухолей и токсичность. Трансфекция CRISPR-Cas9 для редактирования интересующего генома определяется тремя отдельными стратегиями. Первый подход заключается в использовании плазмидной ДНК, кодирующей белок Cas9 и sgРНК из одного и того же вектора. Второй подход характеризуется доставкой в клетку смеси мРНК Cas9 и sgРНК. Последняя, третья, стратегия – трансфекция готового к использованию рибонуклеопротеинового комплекса, состоящего из молекул белка Cas9 и sgРНК, которая отличается высокой селективностью по сравнению с двумя вышеназванными системами [36]. Хотя первая стратегия доставки, система CRISPR-Cas9 на основе плазмид, является простым и прямым подходом, она имеет тенденцию вызывать нецелевые мутации [37]. При попадании плазмиды в нужное ядро она подвергается процессам транскрипции и трансляции для экспрессии кодируемых белков. Эти процессы требуют больше времени для эффективного нацеливания на интересующий ген. Что еще более важно, было обнаружено, что этот формат доставки приводит к необратимому нецелевому сайту расщепления [38]. Еще одним недостатком векторов на основе плазмид является их ограниченный размер, что создает препятствия для переноса крупных генов. Кроме того, распознавание трансфицированной плазмидной ДНК как чужеродного генетического материала циклической GMP-AMP-синтазой, являющейся цитозольным сенсором, приводит к развитию иммунного ответа хозяина [39]. Во второй стратегии прямая доставка комплекса мРНК Cas9 и sgРНК в клетки-мишени обеспечивает образование комплекса Cas9/sgРНК за сравнительно короткое время, минуя ядро, за счет трансляции мРНК в цитоплазме. Хотя доставка на основе мРНК характеризуется низкой частотой нецелевых эффектов по сравнению со стратегиями доставки плазмидной ДНК, мРНК может легко разрушаться во время приготовления или доставки [40]. Введение в клетку готового рибонуклеопротеинового комплекса исключает необходимость транскрипции и трансляции, обеспечивая относительно быстрое редактирование генов по сравнению с двумя предыдущими подходами [41]. Следовательно, этот тип редактирования происходит очень быстро, и вставка может произойти в течение 3–24 ч, а деградация белка Cas9 в клетках – примерно в течение 24 ч [42]. Существует несколько нановекторов для трансфекции in vitro молекулярных модулей CRISPR-Cas в клетки, которые представляют собой нитевидные наночастицы ДНК, реплицированные по принципу «катящегося кольца», катионные липидные наночастицы, наночастицы на основе золота и каркасы цеолитовых имидазолов [43] Однако лентивирусные и аденовирусные векторы часто используются для доставки компонентов CRISPR-Cas для генетической модификации Т-клеток (таблица). Эти доставки, по-видимому, неэффективны из-за низкой эффективности разрушения генов, слабой сайт-специфичной вставки и случайного нарушения нежелательных генов [44].
CRISPR-Cas-9 при редактировании генома CAR-T
В настоящее время универсальные, аллогенные или готовые Т-клетки, полученные от доноров, подвергаются генетическим манипуляциям и могут использоваться для разных пациентов. Аллогенные Т-клетки, экспрессирующие αβ TCR, могут распознавать и разрушать клетки ткани реципиента, содержащие репертуар генетически несходных антигенов человеческого лейкоцитарного антигена класса I (HLA-I), что приводит к событию «трансплантат против хозяина» [45]. Бета-2-микроглобулин (β2М) является основной субъединицей HLA-I и играет ключевую роль в распознавании чужеродного HLA-I на мембране аллогенных Т-клеток TCR-рецепторами иммунными клетками реципиента [46]. Следовательно, выключение (knock-out) генов TCR и HLA (или β2M) как двух важнейших рецепторов Т-клеток с помощью CRISPR-Cas9 может привести к развитию готовых CAR-T-клеток, свободных от реакции «трансплантат против хозяина». Однако существуют разногласия по поводу преимуществ удаления TCR. В недавнем исследовании сравнивались терапевтические результаты применения CD19 CAR-T-клеток с удаленным рецептором
Òàблицà/Table
Îñîбåннîñти ñтðàтåгии äîñтàвêи ñиñтåмы CRISPR-Cas9
Features of the CRISPR-Cas9 system delivery strategy
|
Способ/Method |
Доставка/Delivery |
Преимущества/Advantages |
Недостатки/Disadvantages |
|
ДНК-Cas9 и sgRNA/ DNA-Cas9 and sgRNA |
Электропорация/ Electroporation Микроинъекция/ Microinjection Наночастицы ААВ/ AAV nanoparticles Лентивирус/Lentivirus |
Простой/Simple Удобный/Comfortable Стабильный/Stable Избегание мультитрансфекции/ Avoidance of multitransfection |
Сложность с переносом плазмидами в ядро/ Difficulty with plasmid transfer into the nucleus Эффект замедления при редактировании генов/ Slowdown effect of gene editing Нежелательные случайные интеграция в геном хозяина/ Undesirable random integration into the host genome |
|
мРНК-Cas9 и sgRNA/ mRNA-Cas9 and sgRNA |
Электропорация/ Electroporation Наночастицы/ Nanoparticles |
Низкая цитотоксичность/ Low cytotoxicity Переходное выражение/ Transitional expression Быстрый эффект/ Quick effect |
Мультитрансфекция/ Multitransfection Высокая разлагаемость мРНК/ High mRNA degradability |
|
Cas9 и sgRNA/ Cas9 and sgRNA |
Электропорация/ Electroporation Наночастицы/ Nanoparticles |
Быстрый эффект/ Quick effect Высокая эффективность/ High efficiency |
Менее стабильный/ Less stable |
Избегание нежелательных интеграции/
Avoiding unwanted integrations
Низкая антигенность/
Low antigenicity
Избегание мультитрансфекции/
Avoiding multitransfection
Примечание: таблица составлена авторами.
Note: created by the authors
TCRβ и CD19 CAR-T-клеток с интактным рецептором TCRβ [47]. В то время как выключение гена TCR в CAR-T-клетках сильно подавляет аллореактивность, TCR-экспрессирующие CART-клетки демонстрируют высокую персистенцию и значительно усиливают контроль над лейкемией in vivo . Эти данные позволяют предположить, что, несмотря на преимущества выключения TCR в предотвращении нежелательных иммунологических реакций и в развитии готовых Т-клеток, высокоселективных к опухолевому антигену СD19, присутствие эндогенных TCR может быть лучше для длительного выживания Т-клеток.
Экспрессия PD-1 (Programmed cell death 1) клетками CAR-T имеет подавляющий эффект, так как нарушение функции PD-1 может усилить противоопухолевый ответ Т-клеток [48]. Электропорация Cas9 и sgRNA, направленная на экзон 1 гена белка PD-1 (PDCD1, Programmed cell death protein 1) в Т-клетках человека с последующей доставкой лентивирусного вектора, содержащего трансген CD19 CAR, обеспечивает высокую эффективность и минимальную токсичность СAR-T-терапии. Это открытие подчеркивает негативную роль оси PD-1/PD-L1 в формировании цитотоксического эффекта CAR-T-клеток против раковых клеток, экспрессирующих опухолеассоциированный антиген [49]. Модифицированные Т-клетки c нокаутом гена PD-1 продемонстрировали высокую персистенцию, минимизацию аллогенной токсичности и признаков активации HLA-1, кроме того, выживаемость трансгенных мышей увеличилась [50]. Результаты исследования показали, что использование плазмидной ДНК является более удобным подходом по сравнению с методами доставки РНК, белков и вирусов из-за ее низкой стоимости и простоты приготовления [51]. В недавнем исследовании система CRISPR-Cas9 использовалась для редактирования локусов PD-1, β2M и TRAC (альфа-константа Т-клеточного рецептора) в геноме CAR-T-клеток, нацеленных на EGFRvIII (III вариант рецептора эпидермального фактора роста). В результате создана новая модификация CAR-T EGFRvIII, устойчивая к супрессии PD-1, что привело к значительному увеличению выживаемости на мышиных моделях глиобластомы человека [52]. Однако критические биологические процессы, происходящие в микроокружении солидных опухолей (гипоксия, кислая среда, дефицит питательных веществ, некоординированное содержание медиаторов воспаления, иммунотолерантность), резко снижают эффективность CAR-T-терапии в реальных условиях [53]. Группа исследователей отредактировала (knock-out) ген эндогенного рецептора II трансформирующего фактора роста-бета (TGFBR2) в Т-клетках с помощью технологии CRISPR-Cas9, чтобы преодолеть негативное влияние TGF-β (основной регулятор микроокружении опухоли) на CAR-T-терапию. Также было показано, что выключение гена TGFBR2 позволит модифицированным Т-клеткам выживать, эффективно размножаться и проявлять более высокую противоопухолевую ак-
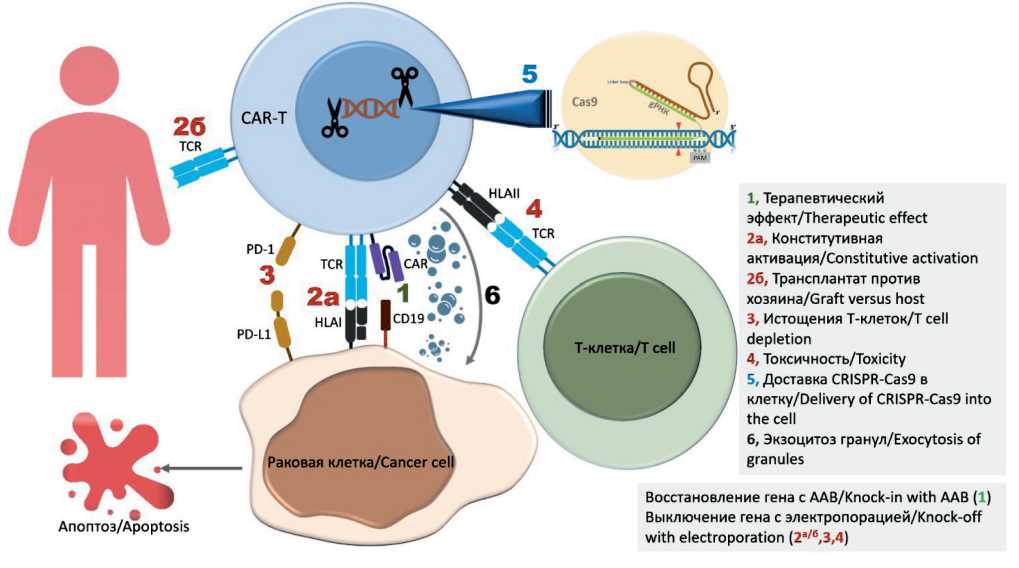
Рис. 2. Пути доставки и механизмы действия CRISPR-Cas9 для редактирования генома CAR-T-клеток: СAR – химерный антигенный рецептор; CAR-T, Т-клетка с CAR; TCR, Т-клеточный рецептор; HLA, человеческий лейкоцитарный антиген; CD19 (кластер дифференцировки 19), антиген В-клетки; PD-1, запрограммированная гибель клетки 1; PD-L1, 1 лиганд PD;
AAВ – аденоассоциированный вирус. Примечание: рисунок выполнен авторами
Fig. 2. Delivery routes and mechanisms of action of CRISPR-Cas9 for genome editing of CAR T cells. CAR, chimeric antigen receptor; CAR-T, CAR T cell; TCR, T cell receptor; HLA, human leukocyte antigen; CD19 (cluster of differentiation 19), B cell antigen; PD-1, programmed cell death 1; PD-L1, PD ligand 1; AAV, adeno-associated virus. Note: created by the authors
тивность [54]. Обнадеживает, что подавление других иммунных контрольных точек, таких как PD-1, одновременно с TGF-ß1 может привести к лучшему результату при лечении CAR-T-клетками.
Таким образом, использование технологии CRISPR-Cas9 для выявления и устранения негативно регулируемых генов в CAR-T-клетках, безусловно, улучшит лечение за счет повышения их специфической активности в отношении раковых клеток (рис. 2). Доклинические исследования CAR-T-клеток, отредактированных с помощью CRISPR, продемонстрировали многообещающие результаты, которые скоро откроют новые горизонты в иммунотерапии рака. Тем не менее еще есть возможности для дальнейшей работы над новыми соответствующими генами, которые оказывают негативное влияние на терапию CAR-T-клетками.
Заключение
Прокариотический адаптивный иммунный механизм используется для введения двухцепочечных разрывов в интересующем генетическом месте с использованием sgRNA. Редактирование генома с этической точки зрения является сложным вопро- сом и вызывает множество дискуссий. Но система CRISPR-Cas9 может открыть новые возможности в исследованиях и эффективном лечении рака. При создании новых молекулярных инструментов системы CRISPR-Cas необходимо сохранить такие качества, как высокая специфичность и направленность, отсутствие генотоксичности и иммуногенности, легкость трансфекции и высокая эффективность. Иммунный ответ против Cas9 снижает эффективность процесса редактирования и может привести к побочным эффектам. Однако обычный белок Cas9 распознает только несколько последовательностей PAM, что ограничивает широкое применение системы CRISPR. Секвенирование генома человека привело к созданию интеллекутальной версии белка хCas9, которая может распознавать больше последовательностей PAM, что позволяет системе CRISPR-Cas9 работать в более широком диапазоне. В обзоре мы рассмотрели многообещающие исследования новых технологий в лечении рака. Ожидается, что использование CRISPR-Cas9 для редактирования генома и эпигенома приведет к разработке новых, более эффективных методов лечения злокачетсвенных новообразований.
Список литературы Генная инженерия в онкологии, основанная на технологии CRISPR-CAS9
- Global cancer burden growing, amidst mounting need for services. Saudi Med J. 2024; 45(3): 326-7.
- Westermann L., Neubauer B., Köttgen M. Nobel Prize 2020 in Chemistry honors CRISPR: a tool for rewriting the code of life. Pflugers Arch. 2021; 473(1): 1-2. https://doi.org/10.1007/s00424-020-02497-9.
- Alseth E.O., Pursey E., Luján A.M., McLeod I., Rollie C., Westra E.R. Bacterial biodiversity drives the evolution of CRISPR-based phage resistance. Nature. 2019; 574(7779): 549-52. https://doi.org/10.1038/s41586-019-1662-9.
- Afolabi L.O., Afolabi M.O., Sani M.M., Okunowo W.O., Yan D., Chen L., Zhang Y., Wan X. Exploiting the CRISPR-Cas9 gene-editing system for human cancers and immunotherapy. Clin Transl Immunology. 2021; 10(6). https://doi.org/10.1002/cti2.1286.
- Sadeqi Nezhad M., Yazdanifar M., Abdollahpour-Alitappeh M., Sattari A., Seifalian A., Bagheri N. Strengthening the CAR-T cell therapeutic application using CRISPR/Cas9 technology. Biotechnol Bioeng. 2021; 118(10): 3691-705. https://doi.org/10.1002/bit.27882.
- Xu Y., Li Z. CRISPR-Cas systems: Overview, innovations and applications in human disease research and gene therapy. Comput Struct Biotechnol J. 2020; 18: 2401-15. https://doi.org/10.1016/j.csbj.2020.08.031.
- Zhang D., Hussain A., Manghwar H., Xie K., Xie S., Zhao S., Larkin R.M., Qing P., Jin S., Ding F. Genome editing with the CRISPR-Cas system: an art, ethics and global regulatory perspective. Plant Biotechnol J. 2020; 18(8): 1651-69. https://doi.org/10.1111/pbi.13383.
- Naeem M., Majeed S., Hoque M.Z., Ahmad I. Latest Developed Strategies to Minimize the Off-Target Effects in CRISPR-Cas-Mediated Genome Editing. Cells. 2020; 9(7): 1608. https://doi.org/10.3390/cells9071608.
- Manghwar H., Li B., Ding X., Hussain A., Lindsey K., Zhang X., Jin S. CRISPR/Cas Systems in Genome Editing: Methodologies and Tools for sgRNA Design, Off-Target Evaluation, and Strategies to Mitigate Off-Target Effects. Adv Sci (Weinh). 2020; 7(6). https://doi.org/10.1002/advs.201902312.
- Javed M.R., Sadaf M., Ahmed T., Jamil A., Nawaz M., Abbas H., Ijaz A. CRISPR-Cas System: History and Prospects as a Genome Editing Tool in Microorganisms. Curr Microbiol. 2018; 75(12): 1675-83. https://doi.org/10.1007/s00284-018-1547-4.
- Batool A., Malik F., Andrabi K.I. Expansion of the CRISPR/Cas Genome-Sculpting Toolbox: Innovations, Applications and Challenges. Mol Diagn Ther. 2021; 25(1): 41-57. https://doi.org/10.1007/s40291-020-00500-8.
- Singh V., Gohil N., Ramírez García R., Braddick D., Fofié C.K. Recent Advances in CRISPR-Cas9 Genome Editing Technology for Biological and Biomedical Investigations. J Cell Biochem. 2018; 119(1): 81-94. https://doi.org/10.1002/jcb.26165.
- Cao J., Wu L., Zhang S.M., Lu M., Cheung W.K., Cai W., Gale M., Xu Q., Yan Q. An easy and efficient inducible CRISPR/Cas9 platform with improved specificity for multiple gene targeting. Nucleic Acids Res. 2016; 44(19). https://doi.org/10.1093/nar/gkw660.
- Morshedzadeh F., Ghanei M., Lotfi M., Ghasemi M., Ahmadi M., Najari-Hanjani P., Sharif S., Mozaffari-Jovin S., Peymani M., Abbaszadegan M.R. An Update on the Application of CRISPR Technology in Clinical Practice. Mol Biotechnol. 2024; 66(2): 179-97. https://doi.org/10.1007/s12033-023-00724-z.
- Ray U., Raghavan S.C. Modulation of DNA double-strand break repair as a strategy to improve precise genome editing. Oncogene. 2020; 39(41): 6393-405. https://doi.org/10.1038/s41388-020-01445-2.
- Miyaoka Y., Berman J.R., Cooper S.B., Mayerl S.J., Chan A.H., Zhang B., Karlin-Neumann G.A., Conklin B.R. Systematic quantification of HDR and NHEJ reveals effects of locus, nuclease, and cell type on genome-editing. Sci Rep. 2016; 6. https://doi.org/10.1038/srep23549.
- Gruzdev A., Scott G.J., Hagler T.B., Ray M.K. CRISPR/Cas9- Assisted Genome Editing in Murine Embryonic Stem Cells. Methods Mol Biol. 2019; 1960: 1-21. https://doi.org/10.1007/978-1-4939-9167-9_1.
- Chen X., Zhang T., Su W., Dou Z., Zhao D., Jin X., Lei H., Wang J., Xie X., Cheng B., Li Q., Zhang H., Di C. Mutant p53 in cancer: from molecular mechanism to therapeutic modulation. Cell Death Dis. 2022; 13(11): 974. https://doi.org/10.1038/s41419-022-05408-1.
- Prior I.A., Hood F.E., Hartley J.L. The Frequency of Ras Mutations in Cancer. Cancer Res. 2020; 80(14): 2969-74. https://doi.org/10.1158/0008-5472.CAN-19-3682.
- Nakajima E.C., Drezner N., Li X., Mishra-Kalyani P.S., Liu Y., Zhao H., Bi Y., Liu J., Rahman A., Wearne E., Ojofeitimi I., Hotaki L.T., Spillman D., Pazdur R., Beaver J.A., Singh H. FDA Approval Summary: Sotorasib for KRAS G12C-Mutated Metastatic NSCLC. Clin Cancer Res. 2022; 28(8): 1482-6. https://doi.org/10.1158/1078-0432.CCR-21-3074.
- Lakshmanan V.K., Jindal S., Packirisamy G., Ojha S., Lian S., Kaushik A., Alzarooni A.I.M.A., Metwally Y.A.F., Thyagarajan S.P., Do Jung Y., Chouaib S. Nanomedicine-based cancer immunotherapy: recent trends and future perspectives. Cancer Gene Ther. 2021; 28(9): 911-23. https://doi.org/10.1038/s41417-021-00299-4.
- Behan F.M., Iorio F., Picco G., Gonçalves E., Beaver C.M., Migliardi G., Santos R., Rao Y., Sassi F., Pinnelli M., Ansari R., Harper S., Jackson D.A., McRae R., Pooley R., Wilkinson P., van der Meer D., Dow D., Buser-Doepner C., Bertotti A., Trusolino L., Stronach E.A., Saez-Rodriguez J., Yusa K., Garnett M.J. Prioritization of cancer therapeutic targets using CRISPR-Cas9 screens. Nature. 2019; 568(7753): 511-6. https://doi.org/10.1038/s41586-019-1103-9.
- Kasap C., Elemento O., Kapoor T.M. DrugTargetSeqR: a genomics- and CRISPR-Cas9-based method to analyze drug targets. Nat Chem Biol. 2014; 10(8): 626-8. https://doi.org/10.1038/nchembio.1551.
- Neggers J.E., Vercruysse T., Jacquemyn M., Vanstreels E., Baloglu E., Shacham S., Crochiere M., Landesman Y., Daelemans D. Identifying drugtarget selectivity of small-molecule CRM1/XPO1 inhibitors by CRISPR/Cas9 genome editing. Chem Biol. 2015; 22(1): 107-16. https://doi.org/10.1016/j.chembiol.2014.11.015.
- Yang X., Zhang B. A review on CRISPR/Cas: a versatile tool for cancer screening, diagnosis, and clinic treatment. Funct Integr Genomics. 2023; 23(2): 182. https://doi.org/10.1007/s10142-023-01117-w.
- Gong X., Du J., Peng R.W., Chen C., Yang Z. CRISPRing KRAS: A Winding Road with a Bright Future in Basic and Translational Cancer Research. Cancers (Basel). 2024; 16(2): 460. https://doi.org/10.3390/cancers16020460.
- Huang D., Miller M., Ashok B., Jain S., Peppas N.A. CRISPR/ Cas systems to overcome challenges in developing the next generation of T cells for cancer therapy. Adv Drug Deliv Rev. 2020; 158: 17-35. https://doi.org/10.1016/j.addr.2020.07.015.
- Stefanoudakis D., Kathuria-Prakash N., Sun A.W., Abel M., Drolen C.E., Ashbaugh C., Zhang S., Hui G., Tabatabaei Y.A., Zektser Y., Lopez L.P., Pantuck A., Drakaki A. The Potential Revolution of Cancer Treatment with CRISPR Technology. Cancers (Basel). 2023; 15(6): 1813. https://doi.org/10.3390/cancers15061813.
- Yang H., Bailey P., Pilarsky C. CRISPR Cas9 in Pancreatic Cancer Research. Front Cell Dev Biol. 2019; 7: 239. https://doi.org/10.3389/ fcell.2019.00239.
- Atsavapranee E.S., Billingsley M.M., Mitchell M.J. Delivery technologies for T cell gene editing: Applications in cancer immunotherapy. EBioMedicine. 2021; 67. https://doi.org/10.1016/j.ebiom.2021.103354.
- Met Ö., Jensen K.M., Chamberlain C.A., Donia M., Svane I.M. Principles of adoptive T cell therapy in cancer. Semin Immunopathol. 2019; 41(1): 49-58. https://doi.org/10.1007/s00281-018-0703-z.
- Long K.B., Young R.M., Boesteanu A.C., Davis M.M., Melenhorst J.J., Lacey S.F., DeGaramo D.A., Levine B.L., Fraietta J.A. CAR T Cell Therapy of Non-hematopoietic Malignancies: Detours on the Road to Clinical Success. Front Immunol. 2018; 9. https://doi.org/10.3389/fimmu.2018.02740.
- Ottaviano G., Georgiadis C., Gkazi S.A., Syed F., Zhan H., Etuk A., Preece R., Chu J., Kubat A., Adams S., Veys P., Vora A., Rao K., Qasim W.; TT52 CRISPR-CAR group. Phase 1 clinical trial of CRISPR-engineered CAR19 universal T cells for treatment of children with refractory B cell leukemia. Sci Transl Med. 2022; 14(668). https://doi.org/10.1126/scitranslmed.abq3010.
- Wang Z., Li N., Feng K., Chen M., Zhang Y., Liu Y., Yang Q., Nie J., Tang N., Zhang X., Cheng C., Shen L., He J., Ye X., Cao W., Wang H., Han W. Phase I study of CAR-T cells with PD-1 and TCR disruption in mesothelin-positive solid tumors. Cell Mol Immunol. 2021; 18(9): 2188-98. https://doi.org/10.1038/s41423-021-00749-x.
- Hu J.H., Miller S.M., Geurts M.H., Tang W., Chen L., Sun N., Zeina C.M., Gao X., Rees H.A., Lin Z., Liu D.R. Evolved Cas9 variants with broad PAM compatibility and high DNA specificity. Nature. 2018; 556: 57-63. https://doi.org/10.1038/nature26155.
- Luther D.C., Lee Y.W., Nagaraj H., Scaletti F., Rotello V.M. Delivery approaches for CRISPR/Cas9 therapeutics in vivo: advances and challenges. Expert Opin Drug Deliv. 2018; 15(9): 905-13. https://doi.org/10.1080/17425247.2018.1517746.
- Kornete M., Marone R., Jeker L.T. Highly Efficient and Versatile Plasmid-Based Gene Editing in Primary T Cells. J Immunol. 2018; 200(7): 2489-2501. https://doi.org/10.4049/jimmunol.1701121.
- Fujihara Y., Ikawa M. CRISPR/Cas9-based genome editing in mice by single plasmid injection. Methods Enzymol. 2014; 546: 319-36. https://doi.org/10.1016/B978-0-12-801185-0.00015-5.
- Xu X., Wan T., Xin H., Li D., Pan H., Wu J., Ping Y. Delivery of CRISPR/Cas9 for therapeutic genome editing. J Gene Med. 2019; 21(7). https://doi.org/10.1002/jgm.3107.
- Givens B.E., Naguib Y.W., Geary S.M., Devor E.J., Salem A.K. Nanoparticle-Based Delivery of CRISPR/Cas9 Genome-Editing Therapeutics. AAPS J. 2018; 20(6): 108. https://doi.org/10.1208/s12248-018-0267-9.
- Seki A., Rutz S. Optimized RNP transfection for highly efficient CRISPR/Cas9-mediated gene knockout in primary T cells. J Exp Med. 2018; 215(3): 985-97. https://doi.org/10.1084/jem.20171626.
- Kim S., Koo T., Jee H.G., Cho H.Y., Lee G., Lim D.G., Shin H.S., Kim J.S. CRISPR RNAs trigger innate immune responses in human cells. Genome Res. 2018; 28(3): 367-73. https://doi.org/10.1101/gr.231936.117.
- Wei T., Cheng Q., Min Y.L., Olson E.N., Siegwart D.J. Systemic nanoparticle delivery of CRISPR-Cas9 ribonucleoproteins for effective tissue specific genome editing. Nat Commun. 2020; 11(1): 3232. https://doi.org/10.1038/s41467-020-17029-3.
- Lino C.A., Harper J.C., Carney J.P., Timlin J.A. Delivering CRISPR: a review of the challenges and approaches. Drug Deliv. 2018; 25(1): 1234-57. https://doi.org/10.1080/10717544.2018.1474964.
- Townsend M.H., Bennion K., Robison R.A., O'Neill K.L. Paving the way towards universal treatment with allogenic T cells. Immunol Res. 2020; 68(1): 63-70. https://doi.org/10.1007/s12026-020-09119-7.
- Salas-Mckee J., Kong W., Gladney W.L., Jadlowsky J.K., Plesa G., Davis M.M., Fraietta J.A. CRISPR/Cas9-based genome editing in the era of CAR T cell immunotherapy. Hum Vaccin Immunother. 2019; 15(5): 1126-32. https://doi.org/10.1080/21645515.2019.1571893.
- Stenger D., Stief T.A., Kaeuferle T., Willier S., Rataj F., Schober K., Vick B., Lotfi R., Wagner B., Grünewald T.G.P., Kobold S., Busch D.H., Jeremias I., Blaeschke F., Feuchtinger T. Endogenous TCR promotes in vivo persistence of CD19-CAR-T cells compared to a CRISPR/Cas9-mediated TCR knockout CAR. Blood. 2020; 136(12): 1407-18. https://doi.org/10.1182/blood.2020005185.
- Seliger B. Basis of PD1/PD-L1 Therapies. J Clin Med. 2019; 8(12): 2168. https://doi.org/10.3390/jcm8122168.
- Rupp L.J., Schumann K., Roybal K.T., Gate R.E., Ye C.J., Lim W.A., Marson A. CRISPR/Cas9-mediated PD-1 disruption enhances anti-tumor efficacy of human chimeric antigen receptor T cells. Sci Rep. 2017; 7(1): 737. https://doi.org/10.1038/s41598-017-00462-8.
- Nakazawa T., Natsume A., Nishimura F., Morimoto T., Matsuda R., Nakamura M., Yamada S., Nakagawa I., Motoyama Y., Park Y.S., Tsujimura T., Wakabayashi T., Nakase H. Effect of CRISPR/Cas9-Mediated PD-1-Disrupted Primary Human Third-Generation CAR-T Cells Targeting EGFRvIII on In Vitro Human Glioblastoma Cell Growth. Cells. 2020; 9(4): 998. https://doi.org/10.3390/cells9040998.
- Hu W., Zi Z., Jin Y., Li G., Shao K., Cai Q., Ma X., Wei F. CRISPR/ Cas9-mediated PD-1 disruption enhances human mesothelin-targeted CAR T cell effector functions. Cancer Immunol Immunother. 2019; 68(3): 365-77. https://doi.org/10.1007/s00262-018-2281-2.
- Choi B.D., Yu X., Castano A.P., Darr H., Henderson D.B., Bouffard A.A., Larson R.C., Scarfò I., Bailey S.R., Gerhard G.M., Frigault M.J., Leick M.B., Schmidts A., Sagert J.G., Curry W.T., Carter B.S., Maus M.V. CRISPR-Cas9 disruption of PD-1 enhances activity of universal EGFRvIII CAR T cells in a preclinical model of human glioblastoma. J Immunother Cancer. 2019; 7(1): 304. https://doi.org/10.1186/s40425-019-0806-7.
- Yazdanifar M., Zhou R., Mukherjee P. Emerging immunotherapeutics in adenocarcinomas: A focus on CAR-T cells. Curr Trends Immunol. 2016; 17: 95-115.
- Tang N., Cheng C., Zhang X., Qiao M., Li N., Mu W., Wei X.F., Han W., Wang H. TGF-β inhibition via CRISPR promotes the long-term efficacy of CAR T cells against solid tumors. JCI Insight. 2020; 5(4). https://doi.org/10.1172/jci.insight.133977.


