Giant foam-like macrophages in advanced ovarian cancer
Автор: Rakina M.A., Kazakova E.O., Sudarskikh T.S., Bezgodova N.V., Villert A.B., Kolomiets L.A., Larionova I.V.
Журнал: Сибирский онкологический журнал @siboncoj
Рубрика: Лабораторные и экспериментальные исследования
Статья в выпуске: 2 т.21, 2022 года.
Бесплатный доступ
Introduction. Ovarian cancer (OC) is the third most common gynecological cancer with the worst prognosis and highest mortality rate. The progression of OC can be accompanied by the detrimental functions of the components of the tumor microenvironment, including tumor-associated macrophages (TAMs). The purpose of the study to analyze distribution and morphological phenotype of TAMs in tumor tissue of patients with high-grade serous ovarian cancer (HGSOC). Material and Methods. Formalin fixed paraffin embedded tissue sections were obtained from ovarian cancer patients after tumor resection. The protein expression of general macrophage marker CD68 and M2-like markers CD206, CD163 and stabilin-1, belonging to scavenger receptors, was analysed by immunohistochemical staining in tumor tissue. Histological assessment of TAM distribution was performed by pathologist. Immunofluorescent analysis/confocal microscopy was applied to establish the co-expression of CD68 with the main macrophage scavenger receptors. Results. We were able to find giant CD68-positive macrophages with foamy cytoplasm in ovarian tumor tissue. The accumulation of these TAMs was specific only for patients with advanced stage (IIIC and IV stages). The presence of foam-like TAMs had a statistical tendency to be associated with ovarian cancer progression, including metastasis and recurrence. The distribution of stabilin-1-positive macrophages was matched to CD68 expression in almost all cases, as was shown by IHC. Confocal microscopy confirmed that stabilin-1 was expressed in at least 50 % of giant TAMs. IF analysis of tumor samples also demonstrated co-expression of other scavenger receptors, CD163 and CD36, in foam-like cells. Similar to IHC, in most samples the expression of CD206 in TAMs of foam-like morphology was limited. Conclusion. For the first time we demonstrated the accumulation of giant macrophages with fluffy foam cytoplasm in the tumor tissue of treated patients with advanced ovarian cancer. Such macrophages express diverse scavenger receptors (stabilin-1, CD163, CD36), thus indicating a high clearance activity of giant TAMs.
Ovarian neoplasms, tumor-associated macrophages, foam-like cells, receptors, scavenger
Короткий адрес: https://sciup.org/140293898
IDR: 140293898 | УДК: 618.11-006.6:
Текст научной статьи Giant foam-like macrophages in advanced ovarian cancer
Ovarian cancer (OC) is the third among most common gynecological cancer after cervical and endometrial cancers and has the worst prognosis with the highest mortality rate [1, 2]. In 2020, 313,959 cases of ovarian cancer were detected, representing 3.4 % of all cancers in women and 207,252 deaths occurred [3]. The urgency of the OC research derives from the large number of issues associated with late-stage diagnosis, difficulties in choosing treatment tactics, low treatment efficacy and a high proportion of relapses [1, 4, 5]. There are no effective criteria for diagnosing OC in the early stages, and screening tests have limited sensitivity. Therefore, up to 70 % of OC cases are detected at late stages [6]. More than 80 % of pa- tients with advanced OC recur and die within 5 years [7]. However, the life expectancy of patients with the early stages of OC (stages I–II) is very favorable, and the five-year survival rate of such women is 92 % [8, 9]. Tumors usually respond to the first-line standard platinum/taxane-based chemotherapy. Despite this, the development of relapses associated with multidrug resistance is detected within a short period of time in 70 % of patients. It can be accompanied by the detrimental effect of therapeutic agents on the components of the tumor microenvironment (TME), including tumor-associated macrophages (TAM) [10].
TAMs are key cells of the innate immunity in tumors. In most cancers, including breast cancer, ovarian cancer, prostate cancer, lung cancer, gastric cancer, glioblastoma, and melanoma, TAM infiltration positively correlates with metastasis and short-term survival [11]. In ovarian cancer, the ratio of anti-tumor (M1)/pro-tumor (M2) TAMs has a prognostic value for predicting metastasis and recurrence, as shown in several patient cohorts [11–13]. However, plastic adaptation of macrophages to structural tumor development leads to TAM heterogeneity, which can significantly depend on tumor type, tumor microenvironment, and the location of TAMs in particular intratumoral compartments [11, 14, 15]. The major markers of TAMs belong to the scavenger receptors and include common marker of macrophages CD68, and M2 markers with pro-tumor polarization CD206, CD163, CD204, MARCO, stabilin-1, and others [16, 17].
In some cancers macrophages obtain the phenotype of foam cells, where the intracellular lipid content exceeds macrophage`s capacity to maintain lipid homeostasis, triggering lipid droplet formation and the foamy appearance [18–21]. Studies showed that foam cells tend to lose immune functions and induce tissue damage [18, 22, 23]. Foamy cells have been extensively studied in atherosclerosis and tuberculosis, however the role of these cells in cancer has only begun to be explored recently [19].
In the present study we analyzed M2-like TAM distribution and morphological phenotype in tumor tissue of patients with high-grade serous ovarian cancer (HGSOC). We were able to find foam-like CD68-positive macrophages with characteristics of incomplete endocytosis in advanced stage patients. We described the phenotypic profile of these cells.
The purpose of the study to analyze distribution and morphological phenotype of TAMs in tumor tissue of patients with high-grade serous ovarian cancer (HGSOC).
Material and Methods
Clinical samples
The study included 42 patients with histologically-verified high-grade serous ovarian carcinoma (HG-SOC), treated at the Department of Gynecological Oncology, Cancer Research Institute of Tomsk National Research Medical Center (Tomsk, Russia) from 2015 to 2021. The study was carried out according to Declaration of Helsinki (from 1964, revised in 1975 and 1983) and was approved by the local committee of Medical Ethics of Tomsk Cancer Research Institute; all patients signed informed consent for the study. The clinical and pathological parameters of OC patients are presented in Table 1.
Neoadjuvant chemotherapy regimens included standard platinum/taxane-based chemotherapy (cis-platin/carboplatin and paclitaxel/docetaxel). The chemotherapy response score (CRS) was used for the assessment of histological effect in ovarian cancer after neoadjuvant chemotherapy (NACT). All patients underwent surgical treatment. In adjuvant regime, patients received chemotherapy by the same schemes.
Immunohistochemical analysis
Digital IHC analysis and quantification
Tumor tissue slides were scanned by using the Leica Aperio AT2 histoscanning station (Leica, Germany) and ScanScope software (Aperio ScanScope XT Leica). QuPath software (free from https://qupath. was used to analyze and quantify marker expression. Individual tumor regions were selected and analyzed using the cell detection and cell intensity classification. “Cell: DAB OD mean” was used for the analysis of both membranous and cytoplasmic staining of a selective antibody. Intensity thresholds were set to further subclassify cells as being negative, weak, moderate or strongly positive for CD68, CD163, CD206 and stabilin-1 staining based upon mean nuclear DAB optical densities. The results of analysis were presented with H-score parameter that was automatically calculated by Qupath software for each tissue section.
Immunofluorescent staining and confocal microscopy
Immunofluorescent staining was performed using monoclonal mouse anti-CD68 (1:100, clone KP1, NBP2-44539, Novus Biologicals, USA); monoclonal rabbit anti-CD163 (1:250, clone EPR19518, ab182422, Abcam, USA), monoclonal rat anti-CD68 (1:50, clone FA-11, GTX41864, GeneTex, USA), and monoclonal mouse anti-CD36 (1:50, clone 185-1G2, MA5-14112, ThermoFisher scientific, USA). The following combinations of secondary antibodies were used: Cy3-conjugated anti-rabbit, AlexaFluor488-conjugated anti-mouse, AlexaFluor647-conjugated anti-goat and AlexaFluor647-conjugated anti-rat
Table 1/Таблица 1
|
Clinical and pathological parameters/ Клинико-патологические параметры |
Number of patients/ Количество пациенток |
|
Age, years/Возраст, лет |
61.2 ± 13.2 |
|
Stage/Стадия |
|
|
I |
2 (4.8 %) |
|
II |
4 (9.5 %) |
|
III |
22 (52.4 %) |
|
IV |
4 (33.3 %) |
|
NACT/НАХТ |
|
|
Treated /Прошедших лечение |
29 (69.0 %) |
|
Untreated /Не прошедших лечение |
13 (31.0 %) |
|
Response to NACT/Эффект от НАХТ |
|
|
CRS1 |
5 (17.2 %) |
|
CRS2 |
12 (41.4 %) |
|
CRS3 |
12 (41.4 %) |
|
Ascites/Асцит |
|
|
Yes /Да |
20 (47.6 %) |
|
No /Нет |
15 (35.7 %) |
|
Unknown /Неизвестно |
7 (16.7 %) |
|
Carcinomatosis/Канцероматоз |
|
|
Yes /Да |
28 (66.7 %) |
|
No /Нет |
14 (33.3 %) |
|
Recurrence/Рецидив |
|
|
Yes /Да |
16 (38.1 %) |
|
No /Нет |
26 (61.9 %) |
|
Progression/Прогрессирование |
|
|
Yes /Да |
9 (21.4 %) |
|
No /Нет |
33 (78.6 %) |
Foam-like macrophages/Пенообразные макрофаги
|
Yes /Да |
20 (47.6 %) |
|
No /Нет |
22 (52.4 %) |
Clinical and pathological parameters of OC patients Клинико-патологические параметры пациенток с раком яичника
antibodies (all donkey, Dianova, Germany, dilution 1:400). Samples were mounted with Fluoroshield Mounting Medium with DAPI (#ab104135, Abcam, USA) and analyzed by confocal microscopy. Confocal laser scanning microscopy was performed with Carl Zeiss LSM 780 NLO laser scanning spectral confocal microscope (Carl Zeiss, Germany), equipped with 40x objective. Data were acquired and analyzed with Black Zen software. All three- and four-color images were acquired using a sequential scan mode.
Statistical analysis
Statistical analysis was performed using STATIS-TICA 8.0 for Windows. The Mann-Whitney test was implemented to compare two independent groups. Fisher Exact Probability Test was applied for the association of recurrence with the presence of foam-like cells. Data are presented as mean±standart deviation (M±SD) (Table 1). Results were considered to be significant with p<0,05. Data with marginal significance (p value <0.1) were also discussed.
Results
We characterized TAM morphology and amount in tumor tissue of HGSOC patients. Firstly, the expression of general macrophage marker CD68 and the main M2-like markers including CD163, CD206, and stabilin-1, was assessed by quantitative IHC. All analyzed markers belong to scavenger receptors, that are essential for TAM function [25]. Ligands for scavenger receptors include modified LDL, phospholipids, apoptotic cells, amyloid proteins, ferritin, hyaluronan (HA), heparin, and others [25]. TAMs actively display endocytosis altering extracellular landscape of TME and regulating tumor development [26].
Tissue slides were scanned and then the expression of TAM markers was analyzed. The level of protein expression of CD68, CD206, CD163 and stabilin-1 was quantified using the cell detection and cell intensity classification. Statistical analysis based on the comparison of two independent groups did not show significant differences in the expression of CD68, CD206, CD163 and stabilin-1 for the ovarian cancer stages, the present of recurrence, metastasis, and ascites, and for NACT response (p>0.05).
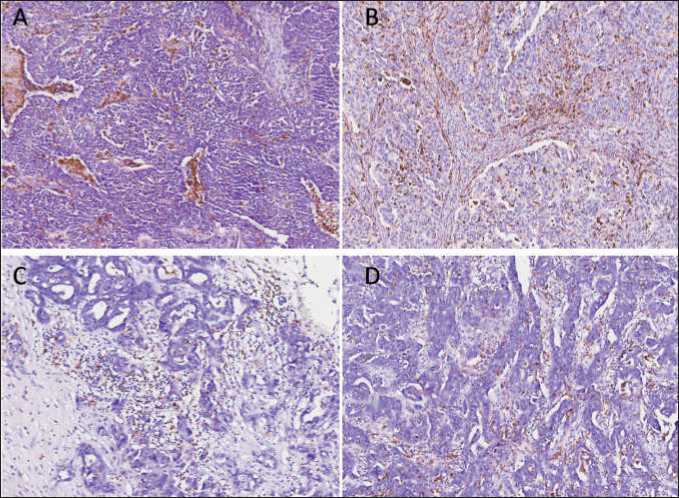
Fig. 1. Microphoto. Immunohistochemistry. The distribution of CD68-positive macrophages in tumor tissue. (A) intratumoral located macrophages with uniform and diffuse disposition, with increased amount around/inside necrosis focus and invasive front; (B) uniform disposition inside whole tumor area; (C) discrete macrophages or small groups of macrophages in stroma; (D) irregular intratumoral location of macrophages. ×40
Рис. 1. Микрофото. ИГХ-исследование. Распределение CD68-позитивных макрофагов в опухолевой ткани. (А) внутриопухо-левые макрофаги с равномерным и диффузным расположением с увеличенным накоплением вокруг/внутри очага некроза и фронта инвазии; (B) равномерное расположение в пределах всей опухоли; (C) отдельные макрофаги или небольшие группы макрофагов в строме; (D) неравномерное внутриопухолевое расположение макрофагов. ×40
Then we analyzed the distribution and morphological features of TAMs. The distribution of CD68positive TAMs varied greatly between tumors, but no correlation with clinical and pathological parameters was demonstrated. Tumors displayed diverse TAM content: a) intratumoral located macrophages with uniform and diffuse disposition, with increased amount around/inside necrosis focus and invasive front; b) irregular intratumoral location of macrophages; c) discrete macrophages or small groups of macrophages in stroma; d) uniform disposition inside whole tumor area; e) abundant accumulation of giant foam-like macrophages in stroma (Fig. 1). We were interested in the accumulation of giant CD68-positive macrophages that resemble foam-like cells. These TAMs have fluffy foamy granular cytoplasm, sometimes with inclusions. This morphology is the outcome of lipid over-intake and was found to be inherent to macrophages in atherosclerosis and tuberculosis, as well as in some cancers such as colorectal and renal cancers [21, 27, 28].
The presence of these macrophages in tumor was found in almost 50 % of patients (20/42 cases). Interestingly, all of them have advanced ovarian cancer: 9 patients with stage 3C and 11 patients with stage 4. Among 20 patients with giant fluffy macrophages, 18 patients underwent NACT, with different initial histological response (CRS1-CRS3). In most cases, foam-like macrophages form big aggregates in stroma surrounding tumor cells (Fig. 2A-C). In some patients, foam-like CD68+ cells are located in small aggregates or as discrete cells (Fig. 2F). Such cells can be merged and form 2 and 3-nuclei cells, resembling epithelioid cells (activated macrophages with pale foamy cytoplasm) [29]. The location of giant macrophages with cytoplasmic vacuolation around tumor cells after chemotherapy can indicate that they can have characteristics of incomplete endocytosis [30]. The presence of foam-like TAMs had a tendency to be associated with ovarian cancer progression, including metastasis and recurrence (Fisher’s exact test, p=0.058). Further IHC analysis allowed us to reveal that foam-like macrophages express scavenger receptor stabilin-1 in almost 100 % of cases (Fig. 3A). Stabilin-1-positive cells had the same morphology and location in tumor tissue. The expression of other M2 marker CD163 was almost the same, but CD206 expression was absent in many samples (Fig. 3B and C).
These observations prompted us to perform im-munofluorescent analysis to reveal the co-expression of CD68 and most common macrophage scavenger receptors (CD206, CD163, CD36, and stabilin-1). Using four-colour IF images we confirmed the coexpression of stabilin-1 in CD68+ macrophages (Fig. 3A). Stabilin-1 was expressed in at least 50 % of giant TAMs. IF analysis of several tumor samples also demonstrated co-expression of other SRs, CD163 and CD36, in foam-like cells (Fig. 3B). Similar to IHC, in most samples we did not find the expression of CD206 in TAMs of foam-like morphology (Fig. 3A).
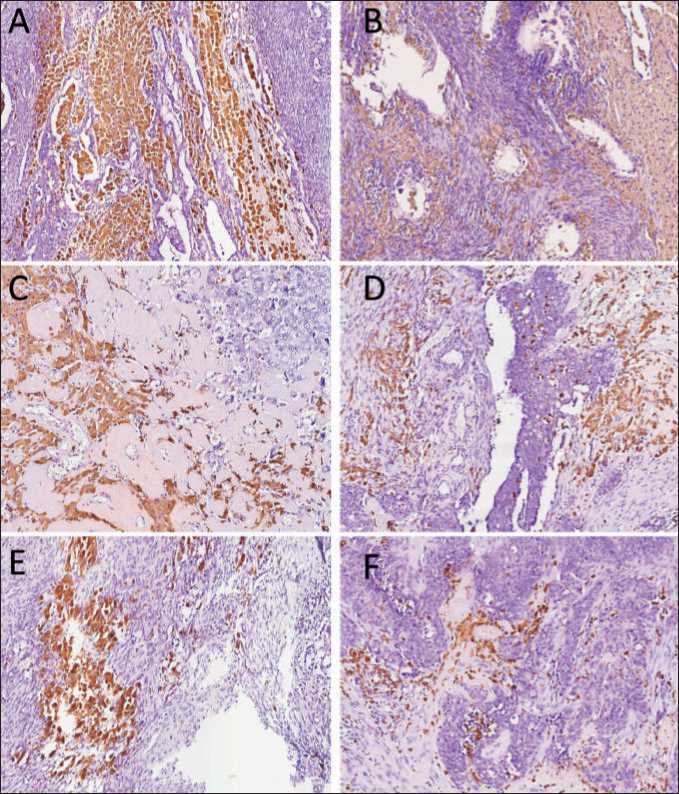
Fig. 2. Microphoto. Immunohistochemistry. The distribution of large foam-like CD68+ macrophages in tumor tissue of HGSOC. (A) and (B), big aggregates of foam-like macrophages surrounded tumor cells. (C) and (D), fluffy macrophages in fibrosis around tumor cells. (E) – medium aggregates of foam-like macrophages. (F) – small aggregates or discrete cells with foam-like morphology. ×40
Рис. 2. Микрофото. ИГХ-исследование. Распределение крупных CD68+ макрофагов с пенистой цитоплазмой в опухолевой ткани HGSOC. (А) и (B) — большие скопления макрофагов с пенистой цитоплазмой, окружающих опухолевые клетки; (C) и (D), рыхлые макрофаги в фиброзе вокруг опухолевых клеток; (E) – средние агрегаты макрофагов с пенистой цитоплазмой; (F) – мелкие агрегаты или дискретные клетки с пенообразной морфологией. ×40
Discussion
Foam macrophages are cells with extensive amount of fluffy looking cytoplasm. This morphology is due to aberrant lipoprotein metabolism and accumulation of LDL or cholesterol either via macropinocytosis or scavenger receptor-mediated pathways (mostly by SRA and CD36) [31]. In our study for the first time we found the aggregates of giant macrophages with foamy cytoplasm in patients with advanced ovarian cancer. Cancer cells were able to activate adipocytes and other stromal cells to lipolyze their triglyceride storage resulting in release of fatty acids (FA) outside of cells, that was linked to an increased risk of developing lung cancer, gastric cancer, thyroid cancer, rectal cancer, colon cancer, and ovarian cancer [27, 32]. Excessive amount of FA in intratumoral milieu prompts TAMs to uptake FA through SR-mediated phagocytosis, which in turn leads to formation of lipid-laden TAMs [28].
In colorectal cancer, the formation of lipid dropletbearing CD68+CD206+ TAMs was specifically found in tumor tissue, but not in adjacent benign tissue, indicating that specific quality of TAMs could serve as a parameter to predict the prognosis of cancer [28]. Moreover, the lipid droplet-dependent FA metabolism was shown to induce the immunosuppressive phenotype of TAMs, and targeting lipid droplets with chemical inhibitors impaired tumor growth in vivo [28]. A correlation between the presence of large tumor-associated macrophages (L-TAMs) in colorectal cancer metastasis and poor outcomes was observed. The presence of L-TAMs correlated to low 5-year disease-free survival rates, while in case of small TAMs (S-TAM) the survival rate was higher [20]. Transcrip-tomic analysis demonstrated that the gene profile of L-TAMs was associated with lipid metabolism; however, S-TAMs had a distinct profile characterized by
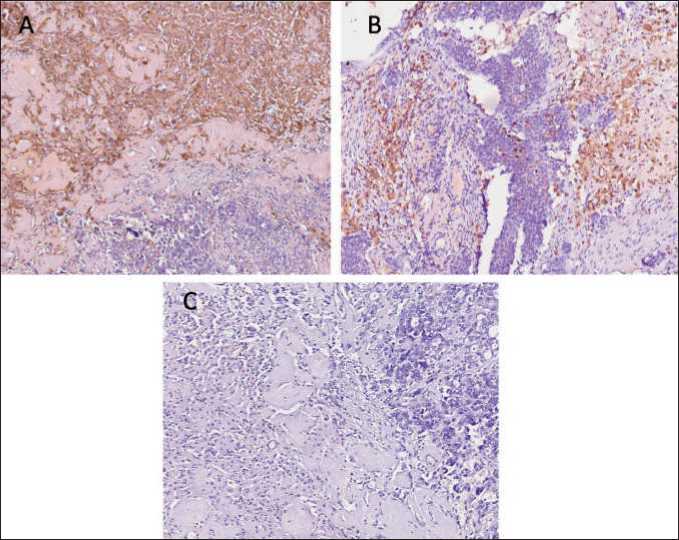
Fig. 3. Microphoto. Immunohistochemistry. Representative images of M2 foam-like macrophages in tumor tissue of HGSOC. (A) Stabilin-1-positive TAMs located in fibrosis tissue surrounding tumor cells. (B) CD163-positive TAMs located in fibrosis tissue surrounding tumor cells. (C) The absence of CD206 expression in foam-like macrophages. ×40
Рис 3. Микрофото. ИГХ-исследование. Примеры пенообразных M2 макрофагов в опухолевой ткани HGSOC. (A) Стабилин-1-положительные ОАМ, расположенные в фиброзной ткани, окружающей опухолевые клетки. (B) CD163-положительные ОАМ, расположенные в фиброзной ткани, окружающей опухолевые клетки; (C) Отсутствие экспрессии CD206 в пенообразных макрофагах. ×40
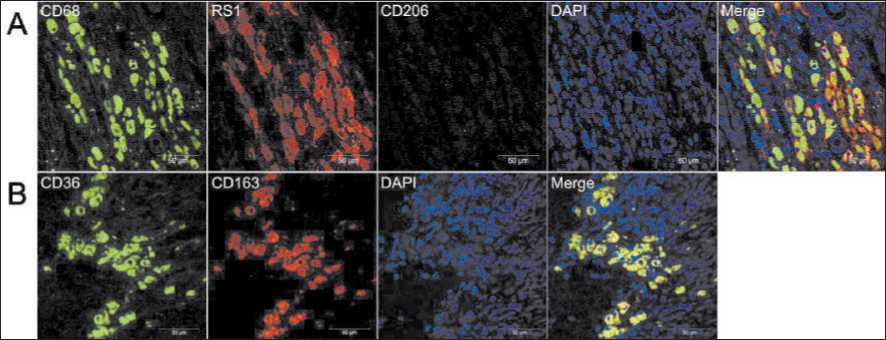
Fig. 4. Multicolor immunofluorescent analysis of scavenger receptor co-expression in CD68-positive macrophages. (A) Co-expression of CD68, CD206 and stabilin-1 in tumor samples from a patient with ovarian cancer. (B) Co-expression of scavenger receptors CD163 and CD36 in tumor samples from a patient with ovarian cancer. Scale bars correspond to 50µM
Рис. 4. Многоцветный иммунофлюоресцентный анализ ко-экспрессии скавенджер-рецепторов в CD68-позитивных макрофагах (А) Ко-экспрессия CD68, CD206 и стабилина-1 в образцах опухоли пациентки с раком яичника; (В) Ко-экспрессия скавенджер-рецепторов CD163 и CD36 в образцах опухоли пациентки с раком яичника. Шкала соответствует 50µM
inflammatory gene expression. In single-cell analysis S-TAM and L-TAM signatures were also differentially enriched in individual clusters [20].
An increase of lipids, which co-localized with CD68 staining in tissue sections of patients with breast, colon, and prostate cancers, was observed [33]. CD11b+CD68+ macrophages isolated from colon cancer tissues displayed significantly more lipid accumulation compared with macrophages from normal tissues. In addition, an increased lipid accumulation in
TAMs from multiple myeloma patients’ bone marrow was positively and significantly associated with the progression of monoclonal gammopathy of undetermined significance to multiple myeloma [33].
Conditioned medium from papillary renal cell carcinoma (pRCC) cultures skewed human monocytes toward the M2 macrophage phenotype. These macrophages became enlarged and loaded with lipids, adopting the foam cell morphology found in pRCC tissue. The formation of these cells was linked to the expression of IL-8, CXCL16, and chemerin by pRCC primary tumor cells [21].
In a study of 30 non–small cell lung carcinomas after neoadjuvant therapy the formation of foam cells/ giant cells was stated to be a histologic feature of tumor regression along with coagulative necrosis, fibrosis, and mixed inflammatory infiltrate [34]. Pretreatment non-small cell lung cancer tissue samples from all patients with hyperprogression showed tumor infiltration by M2-like CD163+CD33+PD-L1+ clustered epithelioid macrophages [35].
In the present study giant foam-like TAMs displayed high scavenging activity, that was confirmed by high expression of scavenger receptors, including stabilin-1, CD36 and CD163. The expression of CD206 was limited almost in all foam-like CD68+ macrophages. Although CD206 participates in endogenous and exogenous molecule clearance as scavenger receptor, there is no data about the involvement of CD206 in lipid metabolism, as was shown for other scavenger
Список литературы Giant foam-like macrophages in advanced ovarian cancer
- Momenimovahed Z., TiznobaikA., Taheri S., Salehiniya H. Ovarian cancer in the world: epidemiology and risk factors. Int J Womens Health. 2019; 11: 287-99. doi: 10.2147/IJWH.S197604.
- Виллерт А.Б., Коломиец Л.А., Юнусова Н.В., Иванова А.А. Асцит как предмет исследований при раке яичников. Сибирский онкологический журнал. 2019; 18(1): 116-23. [Villert A.B., KolomietsL.A., Yunusova N.V., Ivanova A.A. Ascites as a subject of studies in ovarian cancer. Siberian Journal of Oncology. 2019; 18(1): 116-23. (in Russian)]. doi: 10.21294/1814-4861-2019-18-1-116-123.
- SungH., Ferlay J., SiegelR.L., LaversanneM., Soerjomataram I., JemalA., BrayF. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021; 71(3): 209-49. doi: 10.3322/caac.21660.
- Спиридонова Н.В., Демура А.А., Щукин В.Ю. Оценка сопутствующей гинекологической патологии в группе пациенток репродуктивного возраста с опухолями и опухолевидными образованиями яичников. Медицинский алфавит. 2020; 16: 10-4. [Spiridonova N.V., Demura A.A., Schukin V.Yu. Evaluation of concomitant gynecological pathology in group of patients of reproductive age with tumors and tumor formations. Medical Alphabet. 2020; 16: 10-4. (in Russian)]. doi: 10.33667/2078-5631-2020-16-10-14.
- ВяткинаН.В., ФроловаИ.Г., Коломиец ЛА., Молчанов С.В., Виллерт А.Б. Возможности комплексного ультразвукового исследования в дооперационном стадировании диссеминированного рака яичников. Сибирский онкологический журнал. 2016; 15(4): 26-32. [Vyatkina N.V., Frolova I.G., Kolomiets L.A., Molchanov S.V., Villert A.B. Diagnostic value of ultrasound examination in preoperative staging of disseminated ovarian cancer. Siberian Journal of Oncology. 2016; 15(4): 26-32. (in Russian)]. doi: 10.21294/1814-4861-2016-15-4-26-32.
- YinM., Shen J., Yu S., Fei J., Zhu X., Zhao J., Zhai L., Sadhukhan A., Zhou J. Tumor-Associated Macrophages (TAMs): A Critical Activator In Ovarian Cancer Metastasis. Onco Targets Ther. 2019; 12: 8687-99. doi: 10.2147/OTT.S216355.
- Cortez A.J., Tudrej P., Kujawa K.A., Lisowska K.M. Advances in ovarian cancer therapy. Cancer Chemother Pharmacol. 2018; 81(1): 17-38. doi: 10.1007/s00280-017-3501-8.
- Ефимова О.А., СафоноваМ.А. Эпидемиология рака яичников на ранних стадиях. Acta Medica Eurasica. 2018; 4: 9-18. [Efimova O.A., Safonova M.A. Epidemiology of ovarian cancer in early stages. Acta Medica Eurasica. 2018; 4: 9-18. (in Russian)].
- BrandA.H., DiSilvestro P.A., Sehouli J., Berek J.S. Cytoreductive surgery for ovarian cancer: quality assessment. Ann Oncol. 2017; 28(8): 25-9. doi: 10.1093/annonc/mdx448.
- Henderson J.T., Webber E.M., Sawaya G.F. Screening for Ovarian Cancer: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2018; 319(6): 595-606. doi: 10.1001/jama.2017.21421.
- Larionova I., Tuguzbaeva G., Ponomaryova A., Stakheyeva M., CherdyntsevaN., Pavlov V., ChoinzonovE., Kzhyshkowska J. Tumor-Associated Macrophages in Human Breast, Colorectal, Lung, Ovarian and Prostate Cancers. Front Oncol. 2020; 10. doi: 10.3389/fonc.2020.566511.
- Zhang M, He Y., Sun X., Li Q, Wang W, Zhao A., Di W. A high M1/M2 ratio of tumor-associated macrophages is associated with extended survival in ovarian cancer patients. J Ovarian Res. 2014; 7: 19. doi: 10.1186/1757-2215-7-19.
- Yuan X., Zhang J., Li D, Mao Y, Mo F., Du W., Ma X. Prognostic significance of tumor-associated macrophages in ovarian cancer: A meta-analysis. Gynecol Oncol. 2017; 147(1): 181-7. doi: 10.1016/j. ygyno.2017.07.007.
- AllavenaP., Mantovani A. Immunology in the clinic review series; focus on cancer: tumour-associated macrophages: undisputed stars of the inflammatory tumour microenvironment. Clin Exp Immunol. 2012; 167(2): 195-205. doi: 10.1111/j.1365-2249.2011.04515.x.
- CassettaL., FragkogianniS., SimsA.H., SwierczakA., ForresterLM., Zhang H., Soong D.Y.H., Cotechini T., Anur P., Lin E.Y., FidanzaA., Lo-pez-YrigoyenM., MillarM.R., UrmanA., Ai Z., SpellmanP.T., HwangE.S., Dixon J.M., Wiechmann L., Coussens L.M., Smith H.O., Pollard J.W. Human Tumor-Associated Macrophage and Monocyte Transcriptional Landscapes Reveal Cancer-Specific Reprogramming, Biomarkers, and Therapeutic Targets. Cancer Cell. 2019; 35(4): 588-602. doi: 10.1016/j. ccell.2019.02.009.
- Malfitano A.M., Pisanti S., Napolitano F., Di Somma S., Marti-nelli R., Portella G. Tumor-Associated Macrophage Status in Cancer Treatment. Cancers (Basel). 2020; 12(7): 1987. doi: 10.3390/can-cers12071987.
- EisingerS., SarhanD., Boura VF., Ibarlucea-BenitezI., TyystjärviS., Oliynyk G., Arsenian-HenrikssonM., Lane D., Wikström S.L., KiesslingR., Virgilio T., Gonzalez S.F., Kaczynska D., Kanatani S., Daskalaki E., Wheelock C.E., Sedimbi S., ChambersB.J., Ravetch J.V., KarlssonM.C.I. Targeting a scavenger receptor on tumor-associated macrophages activates tumor cell killing by natural killer cells. Proc Natl Acad Sci USA. 2020; 117(50): 32005-16. doi: 10.1073/pnas.2015343117.
- Guerrini V., GennaroM.L. Foam Cells: One Size Doesn't Fit All. Trends Immunol. 2019; 40(12): 1163-79. doi: 10.1016/j.it.2019.10.002.
- Lee-RueckertM., Lappalainen J., Kovanen P.T., Escola-Gil J.C. Lipid-Laden Macrophages and Inflammation in Atherosclerosis and Cancer: An Integrative View. Front Cardiovasc Med. 2022; 9. doi: 10.3389/ fcvm.2022.777822.
- DonadonM., Torzilli G., Cortese N., Soldani C., Di Tommaso L., Franceschini B., Carriero R., Barbagallo M., Rigamonti A., Anselmo A., Colombo F.S., Maggi G., Lleo A., Cibella J., Peano C., Kunderfranco P., Roncalli M., Mantovani A., Marchesi F. Macrophage morphology correlates with single-cell diversity and prognosis in colorectal liver metastasis. J Exp Med. 2020; 217(11). doi: 10.1084/jem.20191847.
- KrawczykK.M., NilssonH., AllaouiR., LindgrenD., ArvidssonM., Leandersson K., Johansson M.E. Papillary renal cell carcinoma-derived chemerin, IL-8, and CXCL16 promote monocyte recruitment and differentiation into foam-cell macrophages. Lab Invest. 2017; 97(11): 1296-305. doi: 10.1038/labinvest.2017.78.
- OuimetM., Koster S., SakowskiE., RamkhelawonB., van Solingen C., Oldebeken S., Karunakaran D., Portal-Celhay C., Sheedy F.J., Ray T.D., Cecchini K., Zamore P.D., Rayner K.J., Marcel Y.L., Philips J.A., Moore K.J. Mycobacterium tuberculosis induces the miR-33 locus to reprogram autophagy and host lipid metabolism. Nat Immunol. 2016; 17(6): 677-86. doi: 10.1038/ni.3434.
- Nolan S.J., Romano J.D., Coppens I. Host lipid droplets: An important source of lipids salvaged by the intracellular parasite Toxoplasma gondii. PLoS Pathog. 2017; 13(6). doi: 10.1371/journal.ppat.1006362.
- Politz O, GratchevA., McCourtP.A., SchledzewskiK., GuillotP., Johansson S., Svineng G., Franke P., Kannicht C., Kzhyshkowska J., Longati P., Velten F. W., Johansson S., Goerdt S. Stabilin-1 and -2 constitute a novel family of fasciclin-like hyaluronan receptor homologues. Biochem J. 2002; 362(1): 155-64. doi: 10.1042/0264-6021:3620155.
- Taban Q., Mumtaz P.T., Masoodi K.Z., Haq E., Ahmad S.M. Scavenger receptors in host defense: from functional aspects to mode of action. Cell Commun Signal. 2022; 20(1): 2. doi: 10.1186/s12964-021-00812-0.
- Madsen D.H., Jürgensen H.J., Siersbœk M.S., Kuczek D.E., Grey CloudL., Liu S., Behrendt N., Gr0ntvedL., Weigert R., Bugge T.H. Tumor-Associated Macrophages Derived from Circulating Inflammatory Monocytes Degrade Collagen through Cellular Uptake. Cell Rep. 2017; 21(13): 3662-71. doi: 10.1016/j.celrep.2017.12.011.
- CornK.C., WindhamM.A., RafatM. Lipids in the tumor microenvironment: From cancer progression to treatment. Prog Lipid Res. 2020; 80. doi: 10.1016/j.plipres.2020.101055.
- Wu H., Han Y., Rodriguez Sillke Y., Deng H., Siddiqui S., Treese C., Schmidt F., Friedrich M., Keye J., Wan J., Qin Y., Kühl A.A., Qin Z., Siegmund B., Glauben R. Lipid droplet-dependent fatty acid metabolism controls the immune suppressive phenotype of tumor-associated macrophages. EMBO Mol Med. 2019; 11(11). doi: 10.15252/emmm.201910698.
- Shimizu R., Tanaka K., Oikawa Y., Tomioka H., Kayamori K., Ikeda T., Yoshioka T., Ebihara A., Harada H. Epithelioid cell granuloma with caseating necrosis possibly caused by periapical periodontitis: a case report. J Med Case Rep. 2018; 12(1): 365. doi: 10.1186/s13256-018-1891-9.
- Gordon S. Phagocytosis: An Immunobiologic Process. Immunity. 2016; 44(3): 463-75. doi: 10.1016/j.immuni.2016.02.026.
- Huff M.W., Daugherty A., Lu H. Chapter 18 - Atherosclerosis. Biochemistry of Lipids, Lipoproteins and Membranes (Sixth Edition). Elsevier. 2016. P. 519-48. doi: 10.1016/B978-0-444-63438-2.00018-3.
- Zhang L., Han L., He J., Lv J., Pan R., Lv T. A high serum-free fatty acid level is associated with cancer. J Cancer Res Clin Oncol. 2020; 146(3): 705-10. doi: 10.1007/s00432-019-03095-8.
- Su P., Wang Q, Bi E, Ma X., Liu L, Yang M, Qian J., Yi Q. Enhanced Lipid Accumulation and Metabolism Are Required for the Differentiation and Activation of Tumor-Associated Macrophages. Cancer Res. 2020; 80(7): 1438-50. doi: 10.1158/0008-5472.CAN-19-2994.
- Liu-Jarin X., Stoopler M.B., Raftopoulos H., Ginsburg M., Go-renstein L., Borczuk A.C. Histologic assessment of non-small cell lung carcinoma after neoadjuvant therapy. Mod Pathol. 2003; 16(11): 1102-8. doi: 10.1097/01.MP.0000096041.13859.AB.
- Lo Russo G., Moro M., Sommariva M., Cancila V., Boeri M., Centonze G., Ferro S., GanzinelliM., Gasparini P., Huber V., MilioneM., Porcu L., Proto C., Pruneri G., Signorelli D., Sangaletti S., Sfondrini L., Storti C., Tassi E., Bardelli A., Marsoni S., Torri V., Tripodo C., Colombo M.P., Anichini A., Rivoltini L., Balsari A., Sozzi G., Garassino M.C. Antibody-Fc/FcR Interaction on Macrophages as a Mechanism for Hyperprogressive Disease in Non-small Cell Lung Cancer Subsequent to PD-1/PD-L1 Blockade. Clin Cancer Res. 2019; 25(3): 989-99. doi: 10.1158/1078-0432.CCR-18-1390.
- Feinberg H., Jegouzo S.A.F., Lasanajak Y., Smith D.F., Dricka-merK., Weis W.I., TaylorM.E. Structural analysis of carbohydrate binding by the macrophage mannose receptor CD206. J Biol Chem. 2021; 296. doi: 10.1016/j.jbc.2021.100368.
- PrabhuDas M.R., Baldwin C.L., Bollyky P.L., Bowdish D.M.E., Drickamer K., Febbraio M., Herz J., Kobzik L., Krieger M., Loike J., McVicker B., Means T.K., Moestrup S.K., Post S.R., Sawamura T., Sil-verstein S., Speth R.C., Telfer J.C., Thiele G.M., Wang X.Y., Wright S.D., El Khoury J. A Consensus Definitive Classification of Scavenger Receptors and Their Roles in Health and Disease. J Immunol. 2017; 198(10): 3775-89. doi: 10.4049/jimmunol.1700373.


