Гидротермический синтез нанотрубок диоксида титана из концентрата титановой руды Пижемского месторождения (Россия)
Автор: Котова О.Б., Понарядов А.В., Гмзе Л.А.
Журнал: Вестник геонаук @vestnik-geo
Рубрика: Научные статьи
Статья в выпуске: 1 (253), 2016 года.
Бесплатный доступ
Высокая стоимость наночастиц TiO2 и их востребованность в промышленности определяют актуальность синтеза нанотрубок диоксида титана гидротермическим методом из недорогого минерального сырья. Исходный материал (немагнитная фракция гравитационного концентрата титановой руды Пижемского месторождения) и синтезированные образцы были исследованы с помощью сканирующей электронной микроскопии, рентгенодифракции, состав определен рентгенофлуоресцентным методом.
Нанотрубки tio2, гидротермический синтез, титановая руда, пижемское месторождение
Короткий адрес: https://sciup.org/149129181
IDR: 149129181 | УДК: 547.992.3 | DOI: 10.19110/2221-1381-2016-1-34-36
Текст научной статьи Гидротермический синтез нанотрубок диоксида титана из концентрата титановой руды Пижемского месторождения (Россия)
Titanium is one of the strategic metals of modern industry. The demand for titanium has been steadily increasing, and this trend will obviously continue. In Russia there are mainly undeveloped deposits, titanium concentrate is not produced. On the territory of Komi Republic the prospects of mining industry are associated with the largest deposits of titanium (Yaregckoe and Pizhemskoe). New applications of titanium ore based on its mineralogical and processing characteristics have a real commercial interest: the list of commodity products is expanding (and consequently the prospects for production), energy costs are reducing, environmental risks are decreasing, efficiency of sustainable development of region is increasing. One of the promising directions of solution of this problem can be the synthesis of titanium dioxide nanotubes from mineral raw materials (for example from titanium ore of Pizhemskoe deposit).
In recent decades one-dimensional nanostructure materials, derived from titanium dioxide, have been widely used in photocatalysis, as a base for catalysts, implants, reagents enhancing blood coagulability, batteries etc. [1–5].
Due to a high cost of commercially available TiO2 nanoparticles their synthesis from cheap natural raw materials by hydrothermal method becomes very important.
The aim of this article: the development of scientific basics of synthesis of titanium dioxide nanotubes based on the mineral raw materials using a simple hydrothermal method.
Experimental Procedure
Titanium nanotubes can be obtained by several methods, such as sol-gel, electrospinning, template synthesis, chemical vapor deposition (CVD), etc. Each method has its advantages and disadvantages depending on the implementation. After the pioneer works by T. Kasuga et al. [6] the hydrothermal method became one of the most applied methods for the production of one-dimensional (1D) nanostructured TiO2 products. The authors previously published a number of works on the synthesis of TiO2 nanotubes from synthetic analogs of titanium minerals and their application [7–9].
TiO2 nanotubes were produced by a simple hydrothermal method. The procedure for the preparation of nanostructures from synthetic TiO2 is described elsewhere [7, 9]. In this work this procedure is adapted for the nonmagnetic fraction of gravity concentrate of titanium ore of Pizhemskoe deposit.
In a typical preparation 0.8 g of starting ore (reduced size) was placed in 100 ml autoclave, where 80 ml of 10 M NaOH solution were added. The autoclave was kept at 110 °C
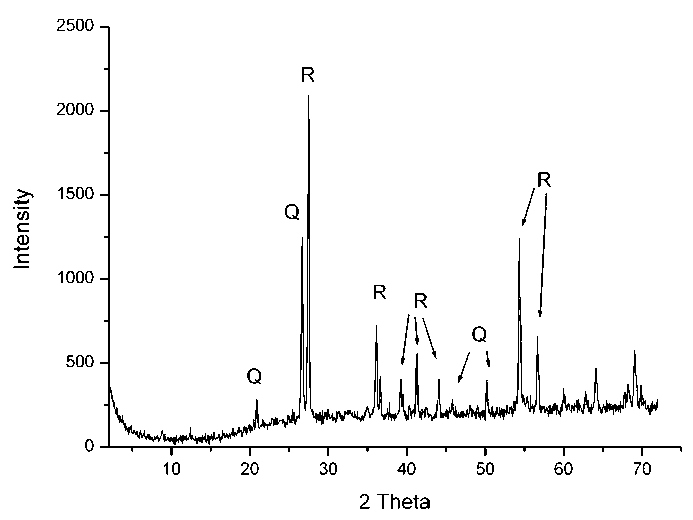
Fig. 1. Diffraction pattern of the starting material (Q – quartz, R – rutile)
Ðèñ. 1. Äèôðàêòîãðàììà èñõîäíîãî ñûðüÿ (Q – êâàðö, R – ðóòèë)
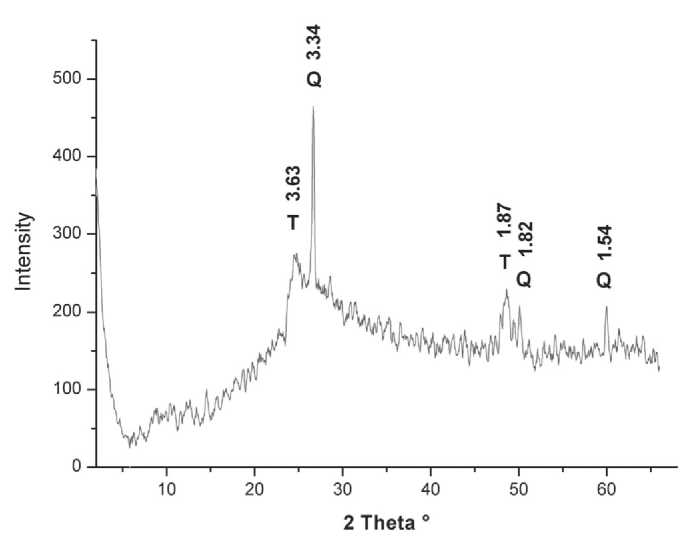
Fig. 2. Diffraction pattern of synthesized sample (Q – quartz, T – sodium titanate)
Ðèñ. 2. Äèôðàêòîãðàììà ñèíòåçèðîâàííîãî îáðàçöà
(Q – êâàðö, T – òèòàíàò íàòðèÿ)
during 24 h (temperature sensor was mounted on the stove, not inside the autoclave). After the hydrothermal reaction theautoclavewascooledtoroomtemperature,andtheresulting flaky precipitate was washed successively by distilled water and solution of hydrochloric acid (0.1 M) until neutral pH (6.5-7). The washed samples were dried in the oven at 90 °C for 12 hours.
The shape and size of the starting ore and synthesized samples were obtained by scanning electron microscopy (TESCAN Vega 3). The crystalline structures of the initial ore and synthesized samples were analyzed by diffractometer (Shimadzu XRD-6000), the material composition was studied by X-ray fluorescence (XRF Shimadzu-1800).
Results and Discussion
Until recently the starting material for TiO2 nanotubes synthesis was only high-purity synthetic analogs [1–5, 7]. In 2011 a group of scientists published a paper [10], which provided data on the production of titanium dioxide nanotubes by the hydrothermal method from Thai leucoxene. According to those data, Thai leucoxene was enriched. The starting material for nanotubes was a rutile concentrate with a TiO2 content more than 93 %.
Mineral and chemical composition of bulk concentrates of titanium ore of Pizhemskoe deposit is given in [11]. Beside leucoxene pseudorutile, Fe-ilmenite, ilmenite and siderite were found. Chemical composition (wt. %): TiO2 – 50.07, SiO2 – 30.52, Fe2O3 – 13.37, MnO – 0.26, CaO – 0.04, MgO – 0.17, Al2O3 – 2.16, K2O – 0.77, Na2O – 0.02, P2O5 – 0.16, ZrO2 – 0.10, S – 0.09, CO2 – 1.90, H2O+ – 0.39.
In comparison with bulk concentrates of titanium ore of Pizhemskoe deposit characterized in [11] the used starting material – non-magnetic fraction of gravity concentrate of titanium ore of Pizhemskoe deposit – the content of Fe2O3 and TiO2 is lower, while content of SiO2 and Al2O3 – higher. Chemical composition (wt. %): TiO2 – 42.12, SiO2 – 46.57, Fe2O3 – 1.04, Al2O3 – 7.57, K2O – 1.61, MnO – 0.06, CaO – 0.13, MgO – 0.37, SO3 – 0.06, P2O5 – 0.17, ZrO2 – 0.05, NbO – 0.11. The average particle size after grinding — 20—40 ц т.
The X-ray diffraction pattern (Fig. 1) indicates that the starting ore is mainly a mixture of two phases: rutile and quartz. The peaks are clear, that testifies to a high crystallinity of these phases. The weak reflections of clay minerals, ilmenite and anatase are present.
The synthesized sample (Fig. 2) is a mixture of two phases:quartzandhydrogentitanate(NaxH2–xTi3O7),which is consistent with the results of [1–3]. Chemical composition (wt. %): TiO2 – 74.68, SiO2 – 12.64, Fe2O3 – 5.44, Al2O3 – 4.71, Na2O – 0.14, K2O – 0.93, MnO – 0.64, CaO – 0.12, MgO – 0.25, P2O5 – 0.09, ZrO2 – 0.08, NbO – 0.14.
The formation of titanium dioxide nanotubes takes place in several stages: the slow dissolution of raw is accompanied by the epitaxial growth of layered sodium titanate nanosheets ^ exfoliation of nanosheets ^ folding of nanosheets into tubes ^ growth of nanotubes along X axis ^ exchange of sodium ions by protons during washing and separating of nanotubes from each other. The crystalline lattice of initial rutile is converted into amorphous product
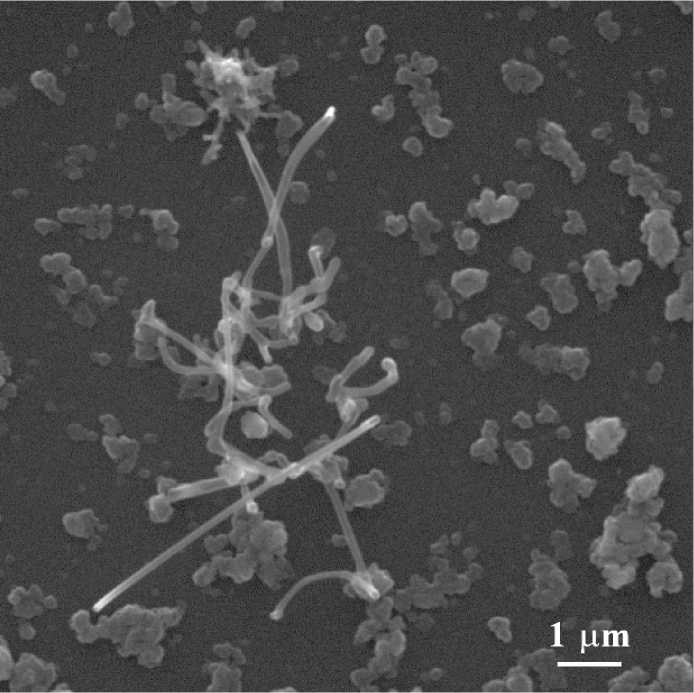
Fig. 3. Titanium dioxide nanotubes Ðèñ. 3. Íàíîòðóáêè äèîêñèäà òèòàíà at alkali processing, after treatment with distilled water and solution of hydrochloric acid the titanium dioxide nanotubes are formed. According to [7], the nanotubes consist of layers of titanate, which composition depends on such synthesis conditions as temperature and duration of treatment, ratio of solid and liquid phases.
Fig. 3 shows SEM image of synthesized TiO2 nanotubes. The channels inside the produced nanotubes are clearly visible. The resolution of scanning electron microscope allows evaluating their outside diameter (70– 100 nm) and length (up to 4500 nm). The synthesized TiO2 nanotubes have a large surface area, which is, according to [7], by orders higher than the specific surface of the starting raw, which provides high sorption properties.
Conclusion
The TiO2 nanotubes were obtained using simple hydrothermal method in Laboratory of mineral raw technology in Institute of Geology Komi SC UB RAS. An inexpensive natural raw material – non-magnetic fraction of gravity concentrate of titanium ore of Pizhemskoe deposit – was used as a starting material. The synthesized titanium dioxide nanotubes have outer diameter 70–100 nm and length up to 4500 nm. The synthesized TiO2 nanotubes have a large surface area that results in a good sorbent material.
Acknowledgements
The authors express gratitude to the common use center Geonauka for their help in analytical work. This work was supported by the Program of UB RAS (project 15-18-5-33).
-
8. Kotova O. B., Ponaryadov A. V., Vayon J. Nanostructured mineral surface: sorption properties // Vestnik of Institute of Geology of Komi SC UB RAS, 2007. No. 10. P. 8–10. (in Russian)
-
9. Ponaryadov A., Kotova O. Leucoxene and TiO2 photocatalysts for water purification // Materials Science and Engineering, 2013. Vol. 47. P. 198–202.
-
10. Aphairaja D., Wirunmongkol T., Pavasupree S., Limsuwan P. Synthesis of Titanate Nanotubes from Thai Leucoxene Mineral // Procedia Engineering, 2012. Vol. 32. P. 1068–1072.
-
11. Makeev A. B., Lyutoev V. P. Spectroscopy in technological mineralogy, mineral composition of concentrates of titanium ore from Pizhemskoó deposit (Middle Timan) // Obogashchenie rud, 2015. No. 5. P. 33–41. (in Russian)
Список литературы Гидротермический синтез нанотрубок диоксида титана из концентрата титановой руды Пижемского месторождения (Россия)
- Kuo H.-L., Kuo C.-Y., Liu C.-H., Chao J.-H., Lin C.-H. A highly active bi-crystalline photocatalyst consisting of TiO2 (B) nanotube and anatase particle for producing H2 gas from neat ethanol//Catalysis Letters, 2007. Vol. 113. P. 7-12.
- Chang L.-H., Sasirekha N., Chen Y.-W. Au/MnO2-TiO2 catalyst for preferential oxidation of carbon monoxide in hydrogen stream//Catalysis Communications, 2007. Vol. 8. P. 1702-1710.
- Kubota S., Johkura K., Asanuma K., Okouchi Y., Ogiwara N., Sasaku K. Titanium dioxide nanotubes for bone regeneration//Journal of Material Science: Materials in Medicine, 2004. Vol. 15. P. 1031-1035.
- Roy SC, Paulose M., Grimes CA The effect of TiO2 nanotubes in the enhancement of blood clotting for the control of hemorrhage//Biomaterials, 2007. Vol. 28. P. 4667-4672.
- Xu J., Jia C., Cao B., Zhang WF Electrochemical properties of anatase TiO2 nanotubes as an anode material for lithium-ion batteries//Electrochimica Acta, 2007. Vol. 52. P. 8044-8047.


