Glutathione is not involved in light-, dark-, Ca- and H2O2-induced stomatal movement in Arabidopsis
Автор: Sarwar Jahan Md, Bin Che Lah Mohd Khairi, Bin Nordin Mohd Nozulaidi, Syed Kamarulzaman syed S.B.
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 3 т.8, 2012 года.
Бесплатный доступ
Glutathione (GSH), is a thiol-containing tripeptide, maintains redox homeostasis in plants under normal and stressful conditions. In this study, we investigated whether GSH involved in light-, dark-, Ca- and H2O2-induced stomatal movement in Arabidopsis. Application of GSH and a GSH decreasing chemical (CDNB; 1-chloro-2,4-dinitrobenzene) did not affect stomatal aperture in guard cells of Arabidopsis. Dark induced stomatal closure and light induced stomatal opening but pre-treatment of GSH and CDNB did not alter dark- and light-induced stomatal aperture. Treatment of guard cells with Ca and H2O2 did not affect GSH contents in guard cells but induced stomatal closure in both wild type and chorinal-1 (ch1-1) mutant plants. In addition, pre-treatment of GSH and CDNB did not affect Ca- and H2O2-induced stomatal closure in both plants. Taken together these results suggest that GSH might not directly affect light-, dark-, Ca- and H2O2-induced stomatal movement in guard cells of Arabidopsis.
Abscisic acid, monochlorobimane, stress
Короткий адрес: https://sciup.org/14323669
IDR: 14323669
Текст научной статьи Glutathione is not involved in light-, dark-, Ca- and H2O2-induced stomatal movement in Arabidopsis
Glutathione has many functions including sulfur metabolism, regulation of growth and development, cell defence, redox signalling, and regulation of gene expression (May et al., 1998; Noctor and Foyer, 1998). Different factors, atmospheric pollutants, biotic and abiotic stress, and light, affect glutathione contents in plant (Alscher, 1989; Sánchez-Fernández et al., 1997) Therefore, control of GSH content in plants can be expected to have important consequences through modification of metabolic functions in plant cells Glutathione contents in aerial parts of ch1-1 mutant plants are lower than wild type plants (Ogawa et al., 2004, Jahan et al., 2008, Jahan et al., 2011).
Increment of [Ca2+] cyt in guard cells is closely related to stomatal closure (McAinsh et al. , 1995) In previous, several authors stated that ABA and H 2 O 2 elicits I Ca currents and [Ca2+] cyt oscillation in guard cells (Allen et al. , 1999; Pei et al. , 2000;
Köhler et al. , 2003, Okuma et al., 2011). The glutathione peroxidise-3 (ATGPX3) protein involved in scavenging H 2 O 2 . Therefore, the Arabidopsis mutant atgpx3 plants are less sensitive to ABA than wild type (Miao et al. , 2006). Glutathione levels enhanced ABA sensitivity to guard cells (Jahan et al. , 2008; Okuma et al., 2011). Increment of GSH levels in guard cells decreased Allylisothiocyanate sensitivity to stomatal closure (Khokon et al., 2011) However, the effect of GSH contents on light-, dark-, Ca- and H 2 O 2 -induced stomatal movement in guard cells remained unclear.
To date, very few reports have been published on GSH function in guard cells of Arabidopsis. Recently Jahan et al. (2011) stated that GSH can be quantified in guard cells. In order to understand the function of GSH contents on light-, dark-, Ca- and H 2 O 2 -induced stomatal movement in Arabidopsis , we presented results in this report that GSH contents might not directly involve in light-, dark-, Ca- and H 2 O 2 -induced stomatal movement in Arabidopsis .
MATERIALS AND METHODS
Plant materials and growth conditions
We used Arabidopsis wild type, ecotype Columbia (Col-0), and ch1-1 mutant plants in this study. Plants were grown in a plastic pot (5.5 d × 5.5 h) according to (Jahan et al. , 2008; Jahan et al. , 2011).
Measurement of stomatal aperture
Stomatal assay was prepared and aperture was measured as described previously (Jahan et al. , 2008; Jahan et al. , 2011). Rosette leaves from 5 to 6 week-old plants were incubated on solution containing 10 mM KCl, 50 µM CaCl 2 , and 10 mM Mes-Tris (pH 6.15) under light for 2 h to facilitate stomatal opening and/or followed by 2 h light
242 incubation with GSH or CDNB or Ca or H 2 O 2 . Then stomatal aperture was measured under Leica DM5000B fluorescence microscope connected with digital imaging color camera and image analysis software. There were twenty stomatal apertures were measured for each replicate.
Measurement of GSH content in guard cells
Glutathione contents in guard cells were quantified using monochlorobimane (MCB) according to Jahan et al. (2011). Epidermal peels were incubated in a 100 μM MCB staining solution for 2 h at room temperature. Leaf was attached onto a microscope slide glass with adhesive then cuticle and upper mesophyll layer were carefully removed. Fluorescence intensity of GSB in guard cells was observed under a fluorescent microscope (Leica DM5000B fluorescence microscope , Germany). The fluorescence of guard cell image was captured and pixels/intensity of the fluorescence was measured using Adobe Photoshop CS3 software (Adobe Systems Inc. San Jose, CA).
Statistical analysis
Student’s t-test was used to assess significance of differences between mean values.
Accession numbers
Arabidopsis Genome Initiative number for the genes discussed in this article is as follows: CH1-1 , At1g44446.
RESULTS
Increment or depletion of GSH in guard cell did not affect stomatal aperture
Whether GSH content directly affect stomatal aperture, we measured stomatal aperture after guard cells were treated with GSH which increases GSH and CDNB which decreases GSH. When guard cells were treated with different concentration of
GSH, stomatal apertures were similar to GSH untreated guard cell (Fig. 1, open bars). Whether depletion of GSH affects stomatal aperture, we measured CDNB-induced stomatal aperture. Figure 1 (closed bars) showed that CDNB-induced stomatal aperture was similar to CDNB-untreated guard cells. These results suggest that increment or depletion of GSH in guard cells might not affect stomatal aperture in guard cells of Arabidopsis plants.
Effects of GSH content on dark- and light-induced stomatal aperture
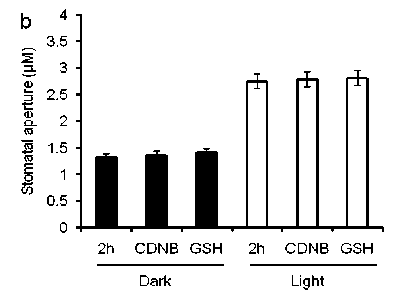
Dark induced stomatal closure and light induced stomatal opening (Shimazaki et al., 2007). Whether GSH content affects dark- and light-induced stomatal aperture in guard cells, we tested dark-and light-induced GSH content and stomatal aperture in guard cells of Arabidopsis. We found that the treatment of dark decreased but light increased GSH content in guard cells of Arabidopsis plants (Fig 2a). We measured dark- and light-induced stomatal aperture after guard cells were treated with CDNB and GSH. Figure 2b showed that dark-induced stomatal closure and light-induced stomatal opening were similar to that of guard cells were pre-treated with CDNB and GSH. These results indicate that GSH might not affect light- and dark-induced stomatal movement in guard cells of Arabidopsis.
Effects of GSH content in Ca+2- and H2O2-induced stomatal aperture
In previous, several authors stated that many signalling compounds enhanced Ca+2 concentration and H 2 O 2 production in guard cell. Whether GSH content mediates Ca+2- and H 2 O 2 -induced stomatal closure, we measure Ca and H 2 O 2 induced GSH content and stomatal closure in guard cells. The treatment of Ca and H 2 O 2 did not affect GSH content in guard cells of wild types and ch1-1 mutant plants (Fig 3a). But both Ca and H 2 O 2 treatments significantly enhanced stomatal closure in both plants (Fig. 3b) which were consistent with previous results (Munemasa et al. , 2006). In addition, pre-treatment of GSH and CDNB did not affect Ca- and H 2 O 2 -induced stomatal closure (Fig. 3c). These results indicated that GSH content in guard cells did not increase Ca+2 and H 2 O 2 sensitivity to guard cells of Arabidopsis. Taken together, our results suggest that GSH might not affect Ca- and H 2 O 2 -induced stomatal movement in guard cells of Arabidopsis.
-
□ GSH eCDNB
Illi
0 1 10 100
Concentration (pM)
Figure 1. Glutathione and CDNB induced stomatal aperture. Excised leaves of wild type plants were treated with different concentration of GSH. Averages from three independent experiments (60 total stomata per bar) are shown.
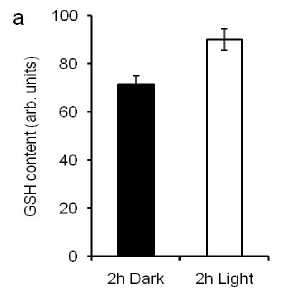
Figure 2. GSH contents in guard cells and dark- and light-induced stomatal aperture
-
a , Glutathione contents in guard cells of wild type plants incubated for 2h dark (closed bar) and light (open bar). Glutathione contents were measured using MCB fluorescence dye. Error bars represent standard error (n=5).
-
b , Glutathione and CDNB did not affect stomatal aperture in dark- (closed bars) and light- (open bars) induced stomatal aperture in guard cells of wild type plants. Averages from three independent experiments (60 total stomata per bar) are shown. Error bars represent standard error.
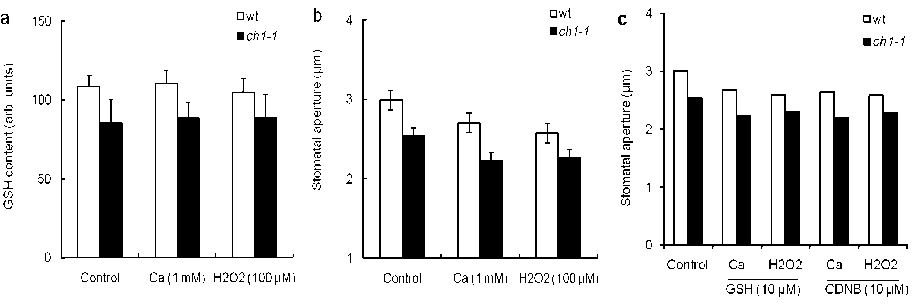
Figure 3. Effects of GSH and CDNB on Ca- and H 2 O 2 -induced stomatal closure
a , Excised leaves treated with Ca and H 2 O 2 to induce GSH contents in the wild types (open bars) and ch1-1 mutant plants (closed bars). Glutathione contents were measured using MCB dye Error bars represent standard error (n = 5). b , Excised leaves were treated with Ca and H 2 O 2 to induce stomatal closure in the wild types (open bars) and ch1-1 mutant plants (closed bars) Averages from three independent experiments (60 total stomata per bar) are shown. Error bars represent standard error. c , Leaves were treated with Ca and H 2 O 2 in GSH and CDNB pretreated guard cells of wild types (open bars) and ch1-1 mutant plants (closed bars). Averages from three independent experiments (60 total stomata per bar) are shown. Error bars represent standard error.
DISCUSSION
Glutathione has many functions in plant e.g sulfur metabolism, growth, development, cell defence, redox signalling, and regulation of gene expression (Noctor and Foyer, 1998; Jahan et al., 2008). Glutathione peroxidases (GPXs) are enzymes that involve in scavenging oxyradicals in animal cells (Arthur, 2000). In Arabidopsis, GPX3 play an important role as a H2O2 scavenger therefore atgpx3 plants is more sensitive to ABA-induced stomatal closure than wild-type plants (Miao et al., 2006). Deficient of intercellular GSH contents in guard cells increased ABA sensitivity to guard cell
(Jahan et al. , 2008). This result stated that reduced GSH level increased ABA sensitivity to guard cells Our study was conducted to find the clue whether GSH directly affect light-, dark-, Ca- and H 2 O 2 -induced stomatal movement in Arabidopsis which finally leads ABA sensitivity to guard cells. Several factors in ABA signalling are regulated by redox conditions in guard cells (Meinhard et al. , 2002). In this study, we showed that treatment of guard cells with GSH and CDNB did not affect stomatal aperture (Fig. 1). In addition, GSH and CDNB treatment did not affect dark/light-induced stomatal aperture (Fig. 2). We chemically (CDNB) and genetically ( ch1-1 mutant ) confirmed that GSH content did not increase Ca+2 and H 2 O 2 sensitivity to guard cells (Fig. 3). Our result confirms that reduction or increment of GSH in guard cells might not activate Ca+2 and H 2 O 2 sensitivity to guard cells of Arabidopsis .
In conclusion, plant responses stress condition through different physiological processes. Plant accumulates ABA in guard cells to reduce water loss through stomatal closing during drought condition It is also proved that GSH helps in many plant defence functions. We stated here that GSH might not directly affect light-, dark-, Ca- and H 2 O 2 -induced stomatal movement in Arabidopsis .
ACKNOWLEDGEMENT
This work was supported in part by SEED fund project (UniSZA/09/BR-008), Universiti Sultan Zainal Abidin, Kuala Terengganu, Malaysia.
Список литературы Glutathione is not involved in light-, dark-, Ca- and H2O2-induced stomatal movement in Arabidopsis
- Allen, G.J., Kwak, J.M., Chu, S.P., Llopis, J., Tsien, R.Y., Harper, J.F., Schroeder, J.I. (1999). Cameleon calcium indicator reports cytoplasmic calcium dynamics in Arabidopsis guard cells. Plant J., 19, 735-747.
- Alscher, R.G. (1989). Biosynthesis and antioxidant function of glutathione in plants. Physiolog. Planta., 77, 457-464.
- Arthur J.R. (2000). The glutathione peroxidases. Cell. Mol. Life Sci., 57, 1825-1835.
- Jahan M.S., Nakamura, Y., Murata, Y., (2011) Histochemical Quantification of GSH Contents in Guard Cells of Arabidopsis Thaliana. Science Asia, 37, 291-295.
- Jahan, M.S., Ogawa, K., Nakamura, Y., Shimoishi, Y., Mori, I.C., Murata, Y. (2008). The Deficient Glutathione in Guard Cells Facilitates Abscisic Acid-Induced Stomatal Closure but Does Not Affect Light-Induced Stomatal Opening. Biosci. Biotech. Biochem., 72, 2795-2798.
- Khokon, M.A.R., Jahan, M.S., Rahman, T., Hossain, M.A., Munemasa, S., Mori, I.C., Nakamura, Y., Murata, Y. (2011). Allylisothiocyanate (AITC) induces stomatal closure in Arabidopsis. Plant Cell Environ., 34, 1900-1906.
- Köhler, B., Hills, A., Blatt, M.R. (2003). Control of guard cell ion channels by hydrogen peroxide and abscisic acid indicates their action through alternate signaling pathways. Plant Physiol., 131, 385-388.
- May, M.J., Vernoux, T., Leaver, C., Van Montagu, M., Inze, D. (1998). Glutathione homeostasis in plants: implications for environmental sensing and plant development. J. Expt. Bot., 49, 649-667.
- McAinsh, M.R., Clayton, H., Mansfield, T.A., Hetherington, A.M. (1996). Changes in stomatal behavior and guard cell cytosolic free calcium in response to oxidative stress. Plant Physiol., 111, 1031-1042.
- Meinhard, M., Rodriguez, P.L., Grill, E. (2002). The sensitivity of ABI2 to hydrogen peroxide links the abscisic acid-response regulator to redox signalling. Planta, 214, 775-782.
- Miao, Y., Lv, D., Wang, P., Wang, X.C., Chen, J., Miao, C., Song, C.P. (2006). An Arabidopsis glutathione peroxidase functions as both a redox transducer and a scavenger in abscisic acid and drought stress responses. Plant Cell, 18, 2749-2766.
- Munemasa, S., Oda, K., Watanabe-Sugimoto, M., Nakamura, Y., Shimoishi, Y., Murata, Y. (2007). The coronatine-insensitive 1 mutation reveals the hormonal signaling interaction between abscisic acid and methyl jasmonate in Arabidopsis guard cells. Specific impairment of ion channel activation and second messenger production. Plant Physiol., 143, 1398-1407.
- Noctor, G., Foyer, C.H. (1998). Ascorbate and glutathione: keeping active oxygen under control. Annu. Rev. Plant Physiol. Plant Mol. Biol., 49, 249-279.
- Ogawa, K., Hatano-Iwasaki, A., Yanagida, M., Iwabuchi, M. (2004). Level of glutathione is regulated by ATP-dependent ligation of glutamate and cysteine through photosynthesis in Arabidopsis thaliana: mechanism of strong interaction of light intensity with flowering. Plant Cell Physiol., 45, 1-8.
- Okuma, E., Jahan, M.S., Munemasa, S., Ogawa, K., Watanabe-Sugimoto, M., Nakamura, Y., Shimoishi, Y., Mori, I.C., Murata, Y. (2011). Negative regulation of abscisic acid-induced stomatal closure by glutathione in Arabidopsis. J. Plant Physiol., 168, 2048-2055
- Pei, Z.M., Murata, Y., Benning, G., Thomine, S., Klüsener, B., Allen, G.J., Grill, E., Schroeder, J.I. (2000). Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature, 406, 731-734.
- Sánchez-Fernández, R., Fricker, M., Corben, L.B., White, N.S., Sheard, N., Leaver, C.J., Van Montagu, M., Inzé, D., May, M.J. (1997). Cell proliferation and hair tip growth in the Arabidopsis root are under mechanistically different forms of redox control. Proc. Natl. Acad. Sci. USA, 94, 2745-2750.
- Shimazaki, K., Doi, M., Assmann, S.M., Kinoshita, T. (2007). Light regulation of stomatal movement. Ann. Rev. Plant Bio., 58, 219-247.


