Growth modulation, photosynthetic attributes and proline accumulation led enhancement to Oryza sativa (L.) seedlings exposed to NaCl stress
Автор: Mir R.A., Husna K.T., Somasundaram R.
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 2 т.20, 2024 года.
Бесплатный доступ
One of the main environmental factors that limits plant performance and lowers production is soil salinity, such as NaCl stress. Therefore, to cultivate salt-tolerant crops, it is essential to understand the physiological aspects of plant to NaCl stress and use the natural genetic resource linked with salt tolerance. Rice is relatively a salt-sensitive crop that shows a significant variation in metabolic activities towards NaCl salt stress. In this view, the present investigation was carried out to find the NaCl stress tolerance of rice (ASD-16) variety. The whole experiment was accomplished in pot culture under varying NaCl concentrations (0, 25, 50, 75, and 100mM). The salt stress was imposed on the 13th days after sowing and sampling were done after five days of treatment i.e, 18th DAS. Salt stress results in a decline in growth traits, biomass production, and also reduction in carotenoids and soluble protein contents were observed with hike in NaCl treatments. However, Chlorophyll a, b, (a+b), proline contents, were found to be increased under increasing NaCl concentrations. Thus, enhancing pigment constituents and osmolytes accumulation provides salinity stress tolerance to rice seedlings.
Compatible solutes, growth, nacl stress, rice, pigment composition
Короткий адрес: https://sciup.org/143182782
IDR: 143182782
Текст научной статьи Growth modulation, photosynthetic attributes and proline accumulation led enhancement to Oryza sativa (L.) seedlings exposed to NaCl stress
The world population is expected to reach 6.0-9.3 billion by the year 2050 and during that time demand for crop productivity would also rise to meet the hunger of the expected population by 2050. The main reason is anthropogenic activities besides natural phenomenon such as abiotic stresses including drought, heat, heavy metals, salinity etc. One of these abiotic factors that has been linked to limiting plant growth performance and productivity is saline stress (Mbarki et al. , 2018). High salinity effects both cultivated agricultural lands (20 percent) and irrigated agricultural fields (33 percent) globally. Furthermore, by the year 2050, the majority of the world’s arable lands (>50%) are assumed to be salinized (Shrivastava and Kumar, 2015). In south sia an approx. 52 million hectares of land is salinized. However, in India an approx. 6.73 MHa of arable land is degraded by salinity. Therefore, creating varieties that can withstand stress is a top priority to address the looming issue of food security globally, towards expanding population and shrinkage of arable lands (Jini and Joseph, 2017).
Saline stress counteracts on plants by imposing osmotic stress, ionic toxicity, and together these lead to oxidative stress (Ceccarini et al. , 2019). However, plants defend themselves by enhancing and or activating antioxidant defense mechanisms that play a prime role in mitigating salt stress consequences. Oxidative stress is a secondary stress induced by excess Na+ and Cl-ion(s) of the cell that causes ROS production and such ROS species (H 2 O 2 ; 1O 2 , OH, O 2 .-) attack membranes, results degradation of lipids, proteins, nucleic acids and disrupts essential enzyme activities of the cell (Das et al. , 2016). ntioxidants such as enzymatic and non-enzymatic are the prime antioxidant defense systems of a cell to fight against ROS-accrued consequences in plants under abiotic stress (Mir et al. , 2019; Yang and Guo, 2018).
In reaction to saline environments, cells change their metabolism and produce suitable solutes that are dispersed throughout other organisms. Quaternary amino acid derivatives including proline (Pro), glycinebetaine, -alanine-betaine, and proline are among the substances acting as suitable solutes that are found in a variety of stressed plants (Nahar et al., 2016). Glycinebetaine and Pro are the most prevalent quaternary amino acid derivatives that plants make when they are under stress conditions (Nahar et al., 2016).
The rice plant (n=12) is a semiaquatic annual plant of the family Poaceae . It is consumed daily as a staple food by higher than half of the world’s population, while China and India account for approximately 90% of global rice production (Fahad et al. , 2019). The build-up of Na+ and Cl- has been shown to generate hyperosmotic, hyperionic, and ultimately oxidative stress in rice plants, which inhibits germination, vegetative growth, reproductive phase, and finally results in plant mortality (Yang and Guo, 2018). Moreover, the germination stage is considered crucial phases in the life cycle of a plant. Therefore, in the current study’s analysis of the rice variety ( SD-16) a salt-sensitive variety was conducted at the seedling stage at varied NaCl salt stress concentrations viz., 25, 50, 75 and 100 mM respectively.
MATERIALS AND METHODS
The rice variety namely SD-16 was purchased from the gronomy Department, griculture faculty, nnamalai University, Tamilnadu.
Experimental site
The pot culture was carried at the Botanical Garden and the experimental part was done at Stress Physiology Lab, Department of Botany, nnamalai University, Tamil Nadu, India. Healthy seeds of rice SD-16 variety were sterilized with 0.1% sodium hypo-chloride for 3 minutes, then thoroughly washing with sterile water to get rid of any chemical traces. Subsequently, the soaked seeds were sown in black plastic pots (Height = 8.5 cm and Diameter = 9.5 cm), filled with half kg of a soil mixture as red soil, sand, and farmyard manure in the ratio 1:1:1. fterwards, plants were divided into five groups with three replicates (n=3) to each treatment: (I) Control (0mM-Non-Saline) (II) NaCl (25mM) (III) NaCl (50mM) (IV) NaCl (75mM) (V) NaCl (100mM) was imposed in treatments on 13th day after germination. However, control plants were irrigated routinely with tap water. The samples were collected for observations on five days after salt imposition, i.e., 18th days after sowing (D S) (Fig.1).
Morphological AttributesTotal root length and Shoot length
Measuring root and shoot height was done manually by using a wooden scale and below the point of rootshoot transition to the fibrous root tip and similarly from base of shoot to the shoot tip of rice seedlings and values were recorded and then expressed in cm plant-1.
Fresh weight and dry weight
The fresh uprooted rice plant seedlings were immediately brought to the lab and rinsed with normal water and blotted dry with tissue paper gently. Thereafter, the whole fresh weight was noted by the help of an electronic balance and the values were recorded and expressed in g plant-1.
fter weighing fresh weight, the plant material was dried at 60ºC in a hot air oven for 72 hours. fter completion of time duration, the dry weights were measured and the material was kept in the same oven until the constant dry weight was obtained and finally the values were recorded and expressed in g plant-1.
Biochemical ConstituentsChlorophylls and Carotenoid contents
Pigment composition (Chlorophyll and carotenoid contents) were extracted from the fresh leaves and determined by following the protocol of ( rnon, 1949) For the quantification of the extract, three ml aliquots of the extracts were transferred to a cuvette and the reading was taken at different wavelengths ≅ 645, 663, and 480 nm in a Spectrophotometer (U-2001–Hitachi) using acetone as a blank.
Carotenoid contents were calculated by the formula of Kirk and llen (1965), and were expressed in mg g-1 fresh weight (FW).
Estimation of Soluble Protein Content and Proline content
The protein concentration of unknown samples was determined by the calibration curve resulting from BS sol. and expressed as mg gm-1 fresh weight (FW) by the protocol of Bradford (1976). The absorbance of the reaction mixture was read at 595 nm against a reagent blank. Proline content was quantified by following the protocol of Bates et al. (1973) at 520 nm in a UV-VIS spectrophotometer (Model-118, Systronic India Limited,
Gujarat, India) using a reagent blank. The proline content was determined from a standard curve with proline and the results were expressed in mg g-1 dry weight.
Statistical Analysis
The data collected to all the characters studied were statistically analysed using SPSS-22 Version. Statistical analysis was performed for mean of values (n=3) and (±) SD for three samples in each group.
RESULTS
Salinity impact on plants including osmotic and oxidative stress (caused by ion toxicity) leads to physiobiochemical variations and thus, significantly reduces crop productivity. To increase crops resilience to abiotic stresses, it is therefore, essential to tackle the hurdles led by salt stress at various growth stages (Mir and Somasundaram, 2023). Thus, the present study was investigated to find out the tolerance range and metabolic adaptation changes in Oryza sativa L. at varied concentrations of NaCl stress. Because germination and reproductive stages of rice plant development are thought to be the delicate phases of the life cycle (Chen et al. , 2021). Undoubtedly, rice is the most common staple food and is sensitive to abiotic factors such as NaCl stress.
Effect of NaCl Stress on morphological attributesRoot length and Shoot length
It is clear from fig. 2 which shows that root length is significantly decreased in rice plants with increasing NaCl salt treatments in comparison to non-stressed plants. The lowest root length was found in 75 and 100 mM NaCl treatments on 18th D S. When compared with control decreased root length was 7.67, and 6.5 cm on 18th D S.
Effect of NaCl Stress on biomassFresh weight and dry weight
Biomass is an indicator of growth performance in plants especially subjected to any abiotic stress such as salt stress. In our study it was found that fresh weight of rice seedlings was significantly declined with upsurging salt-stress treatments (fig. 3 ). However, a profound reduction in fresh weight was particularly noted for 75 mM and 100 mM NaCl. It was 0.19, and 0.17 g plant-1 FW respectively, relative to control which was 0.32 g plant-1 FW.
The reduction in dry biomass of Oryza sativa , was also adversely impacted by salt-stress treatments (Fig 3B). However, with raising NaCl treatment, a sharp decline was recorded in dry biomass. But higher reduction was noted for 75 mM and 100 mM NaCl treatment. It was 0.07, and 0.04 g plant-1 DW respectively, relative to control which was 0.12 g plant-1DW.
Effect of NaCl stress on pigment contentsChlorophyll contents
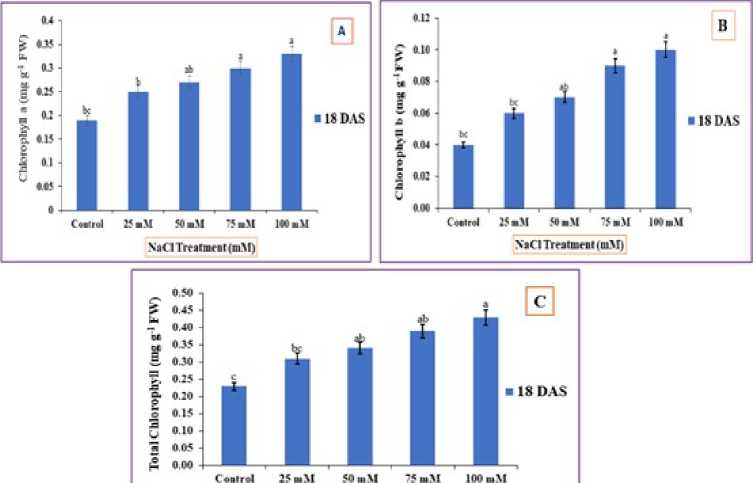
Under increased NaCl treatments a tremendous upsurge in chlorophyll contents were observed. The maximum increase in Chl. a and Chl. b were found in 75 and 100mM NaCl treatments. The recorded upsurge was 0.30 and 0.33 mg g-1 FW for Chl. a, and 0.09 and 0.10 mg g-1 FW for Chl. b, compared to control (fig. 4 -C). Besides, the levels of total chlorophyll content (a+b) also enhanced during the stress period generated by 75 and 100 mM NaCl and was noted 0.39 and 0.43 mg g-1 FW respectively, relative to control.
Carotenoid contents
While increased salt concentrations cause a tremendous decline in carotenoids for 75 and 100 mM NaCl, and were recorded 0.34 and 0.27 mg g-1 fresh weight respectively, relative to control plants in which carotenoid contents noted was 0.53 mg/g fresh weight respectively (fig. 5).

Figure 1: Oryza sativa L. Pot Culture under different NaCl concentrations
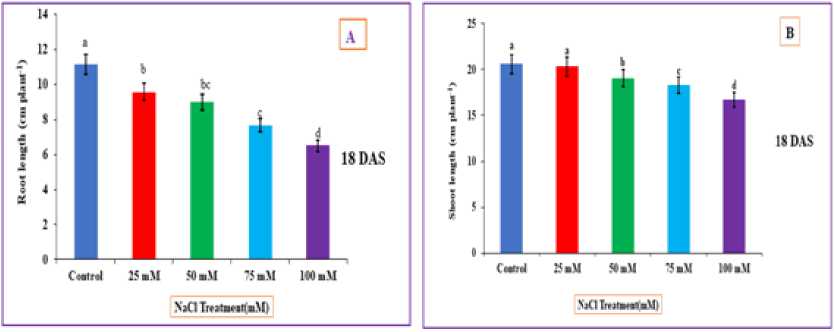
Figure 2 : Impact of NaCl stress on morphology of rice ( ) Root length and (B) Shoot length. The error bars on each column denotes the standard deviation (±) (SD) and the letters in the lower case on the bars denotes a significant difference among each treatment (p<0.05).
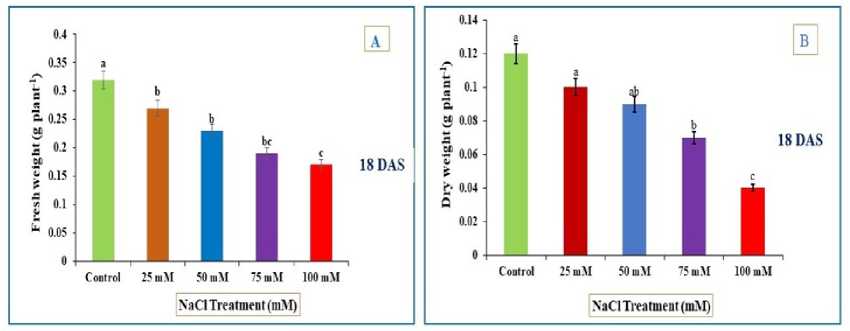
Figure 3 : Impact of NaCl stress on Biomass of rice ( ) Fresh weight and (B) Dry weight. The error bars on each column denotes the standard deviation (±) (SD) and the letters in the lower case on the bars denotes a significant difference among each treatment (p<0.05).
S«CITmtmr»l(niM|
Figure 4 : Impact of NaCl stress on ( ) chlorophyll ‘a’ (B) chlorophyll ‘b’ and (C) Total chlorophyll (a+b) contents. The error bars on each column denotes the standard deviation (±) (SD) and the letters in the lower case on the bars denotes a significant difference among each treatment (p<0.05).
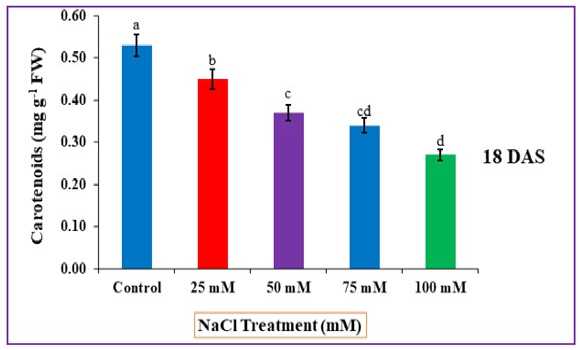
Figure 5 : Impact of NaCl stress on carotenoid contents. The error bars on each column denotes the standard deviation (±) (SD) and the letters in the lower case on the bars denotes a significant difference among each treatment (p<0.05).
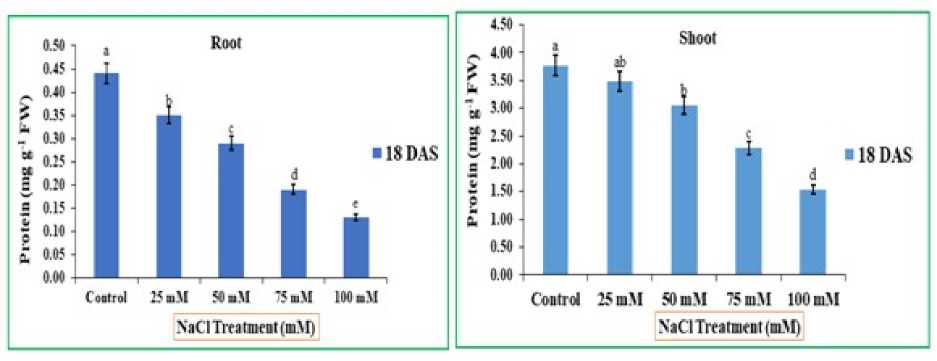
Figure 6 : Impact of NaCl stress on protein content of shoot and root of rice seedlings. The error bars on each column denotes the standard deviation (±) (SD) and the letters in the lower case on the bars denotes a significant difference among each treatment (p<0.05).
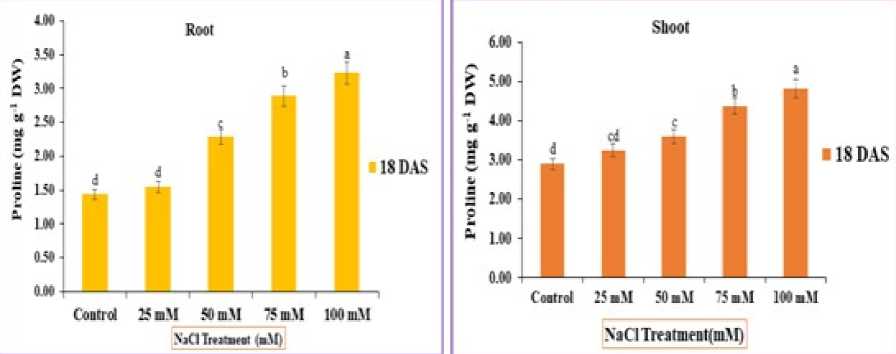
Figure 7 : Impact of NaCl stress on the proline content of the root and shoot of rice seedlings. The error bars on each column denotes the standard deviation (±) (SD) and the letters in the lower case on the bars denotes a significant difference among each treatment (p<0.05).
Effect of NaCl stress on Biochemical ConstituentsProtein Content
It is apparent from fig. 6 that the protein content decreased progressively in the both the parts of Oryza sativa L. with increased salt concentrations than those of control plants. This decrease was observed higher on 75mM and 100 mM, on 18th D S respectively, in comparison to control, it was noted 2.28 and 1.53 mg g-1 FW. Protein content was decreased very much at all growth stages in shoots under salt stress treatments than control 3.77 mg g-1 FW. similar a negative correlation was found between increased NaCl concentrations and protein content in the root. nd this decrease was noted higher on 75 and 100 mM, on 18th D S respectively. The decreased rate observed was 0.19 and 0.13 mg g-1 FW relative to control.
Proline Content (pro)
Salt stress significantly increased the proline content in all organs of rice plants with increased NaCl levels. It is evident from the fig. 7 that there was a directly proportional relationship between the proline contents and NaCl stress of Oryza sativa . However, this increased amount was found higher in 75 and 100 mM on 18th D S respectively, in comparison to control and was 4.38 and 4.83 mg g-1 DW respectively in the shoot In root proline compared with control was 2.89 and 3.32 mg g-1 DW respectively in 75 and 100mM saline solution.
DISCUSSION
In the modern world, soil salinity has emerged as a significant agricultural issue, among abiotic stresses (Noreen et al. , 2021). ccording to Liang et al. (2018), the production of significant crops may decrease by about 50% if the salinization of agricultural fields continues at the current rate.
In the current study, sodium chloride stress caused a drastic reduction of plant growth attributes like root height and shoot height. However, this reduction was seen more at under 75 mM and 100 mM NaCl exposure at 18th D S (fig. 1). Our results agreed with those of Hassan et al. (2021) for barley, Mir and Somasundaram (2021a) for cowpea, and ryendu et al. (2022a) for groundnut respectively, wherein these authors reported declined root length under varied NaCl concentrations The suppression of meristematic activity and cell expansion brought on by salt stress is the cause for the decline in root growth. s a result, the plant is unable to collect water from the soil's surface for further utilization (Kamran et al., 2020) and thereby preventing whole plant growth performance.
Plant growth reduction and less accumulation of drymatter under saline conditions have been well addressed in several grain legumes, and other crops including cabbage ( hmad et al. , 2019), tomato (Tanveer et al. , 2020) and B. napus cultivars (Naheed et al. , 2021). similar kind of determination on maize was done by bdElgawad et al. (2016), and documented that salinity led leaf biomass reduction by 20%. The built up of excess Na+ ions by salinity results decline in K+ ions that causes loss of cell turgor and thereby prevents cell expansion and disrupts other essential cellular enzymatic activities and ultimately reducing growth development and biomass of rice plant (Carvalho et al. , 2018).
Our results regarding the increment in Chlorophyll pigments (Fig. 4) with increasing NaCl doses @25mM increment are in line with results reported by (Mir and Somasundaram, 2020), wherein 18 days old little millet seedlings ( Panicum sumatrense L.) exposed at higher doses of sodium chloride resulted increased chlorophyll content. Besides, chlorophyll content (a+b) enhanced also results by enhanced osmolyte like proline that assists to stabilize the turgor pressure of cells and thereby prevents osmotic shock that in turn declines ROS level by activating and enhancing other enzymes particularly chlorophyllase activity.
Our results represented (Fig. 5) coincide with the results earlier reported in cowpea (Mir and Somasundaram, 2021a; Osman et al., 2019), and tomato ( lves et al., 2021) found decreased carotenoid contents under NaCl stress alone. Taïbi et al. (2016) also found decreased carotenoids in two Phaseolus vulgaris varieties, with increased salt treatments (50, 150, and 200 mM). Carotenoids are the antioxidants formed under stress conditions in the chloroplasts and prevents chloroplast pigments against harmful environmental factors by scavenging ROS (Ramel et al., 2012). However, a decrease in carotenoids causes carotene to break down and turn into zeaxanthins, which help to protect the chloroplast apparatus from photo-inhibition (Sharma and Hall, 1991).
The soluble protein contents were found reduced and the effect was determined higher with the rise of NaCl treatments (Fig. 6). Yao et al. (2022) in three rice plants found reduction in soluble protein content when exposed from medium to higher NaCl treatment. Similarly, Khosravinejad et al. (2009) from their study on Hordeum vulgare (L.) seedlings, observed reduced protein content exposed to NaCl stress. Frukh et al. (2020) reported soluble protein content reduced in all ten rice cultivars with increased NaCl treatments. The major reason in the drop being less availability of N-based compounds and the denaturation of enzymes required for protein synthesis that contribute for salinity led reduction of proteins (Hassanpour et al. , 2013).
Salt stress increased proline content significantly in all parts of Oryza sativa . However, it was observed that there is a direct proportionality of the proline content of roots, shoot and NaCl concentration than control one Our results are in line with earlier research done by Mir and Somasundaram (2020) in Panicum sumatrense and Sofy et al. (2020) in common bean, who reported an upsurge in proline contents under salinity. In recent research, Chiconato et al. (2019) found that sugarcane proline content increased when salt concentration doses were increased under various salinity conditions (0, 40, 80, and 160 mM). In order to sustain cellular turgor under various abiotic stresses, osmolytes accumulation, particularly proline, is crucial (Mir and Somasundaram, 2020). Since, their hydrophilic nature helps to retain water by bonding at the surface of proteins and membranes, which clearly explains their role as osmoprotectants and as chaperones. mong osmolytes, proline is known as a vital osmolyte, maintaining low osmotic potential in stressed plants (Farooq et al. , 2015).
CONCLUSION
NaCl stress caused a great reduction in growth-related characteristics including, growth traits, biomass production of rice plants cultivated under varied NaCl treatments. The increased NaCl levels caused a major reduction in those growth attributes. However, increased chlorophyll pigments, but reduction in carotenoids and protein contents were found with varying salinity levels. Osmolytes accumulation such as proline has shown a positive trend with increasing NaCl concentrations Thus, increasing proline plays a frontline role under abiotic stresses by maintaining osmotic potential of cell Therefore, it can be concluded that rice variety SD-16 tend to tolerate moderate NaCl stress and can be used for further investigations in salinity tolerance mechanism.
ACKNOWLEDGMENT
The first author is thankful and acknowledge Department of Science and Technology (DST) Delhi, India for funding, in the form of DST PURSE-II and nnamalai University authorities for their support to complete this work.
CONFLICTS OF INTEREST
The authors declare that they have no potential conflicts of interest.
REFERENCES bdElgawad, H., G. Zinta, M.M. Hegab, R. Pandey, H.
sard, and W. buelsoud. (2016). High salinity induces different oxidative stress and antioxidant responses in maize seedlings organs. Frontiers in plant science . 7:276.
hmad, W., C.M. yyub, M. . Shehzad, K. Ziaf, M. Ijaz, . Sher, T. bbas, and J. Shafi. (2019)
Supplemental potassium mediates antioxidant metabolism, physiological processes, and osmoregulation to confer salt stress tolerance in cabbage ( Brassica oleracea L.). Horticulture, Environment, and Biotechnology . 60:853-869.
lves, R.d.C., D.R. Rossatto, et. al., (2021). Seed priming with ascorbic acid enhances salt tolerance in micro-tom tomato plants by modifying the antioxidant defense system components. Biocatalysis and Agricultural Biotechnology 31:101927.
rnon, D.I. (1949). Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris Plant Physiol . 24:1.
ryendu, Mir, R , Kathiravan M, and R. Somasundaram. (2022a). lleviating NaCl Stress by Improving Growth and Yield in Arachis hypogaea L. by Exogenous pplication of Brassinolide and Paclobutrazol. Indian Journal of Natural Sciences . 13:46608-46619.
Bates, L., R.a. Waldren, and I. Teare. (1973). Rapid determination of free proline for water-stress studies. Plant Soil. 39:205-207.
Bradford, M.M. (1976). rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem . 72:248-254.
Carvalho, M.E. ., F. . Piotto, M.L. Nogueira, F.G. Gomes-Junior, H.M.C.P. Chamma, D. Pizzaia, and R. . zevedo. (2018). Cadmium exposure triggers genotype-dependent changes in seed vigor and germination of tomato offspring. Protoplasma 255:989-999.
Ceccarini, C., F. ntognoni, S. Biondi, . Fraternale, G. Verardo, . Gorassini, and V. Scoccianti. (2019) Polyphenol-enriched spelt husk extracts improve growth and stress-related biochemical parameters under moderate salt stress in maize plants. Plant Physiol. Biochem . 141:95-104.
Chen, T., S. Shabala, Y. Niu, Z.-H. Chen, L. Shabala, H. Meinke, G. Venkataraman, . Pareek, J. Xu, and M. Zhou. (2021). Molecular mechanisms of salinity tolerance in rice. The Crop Journal . 9:506-520.
Chiconato, D. ., G.d.S.S. Junior, D.M.M. dos Santos, and R. Munns. (2019). daptation of sugarcane plants to saline soil. Environ. Exp. Bot . 162:201211.
Das, S.K., J.K. Patra, and H. Thatoi. (2016) ntioxidative response to abiotic and biotic stresses in mangrove plants: review. Int. Rev. Hydrobiol. 101:3-19.
Fahad, S., M. dnan, M. Noor, M. rif, M. lam, I. . Khan, H. Ullah, F. Wahid, I. . Mian, and Y. Jamal. (2019). Major constraints for global rice production In Advances in rice research for abiotic stress tolerance . Elsevier. 1-22.
Farooq, M., M. Hussain, . Wakeel, and K.H. Siddique. (2015). Salt stress in maize: effects, resistance mechanisms, and management. review Agronomy for Sustainable Development . 35:461481.
Frukh, ., T.O. Siddiqi, M.I.R. Khan, and . hmad. (2020). Modulation in growth, biochemical attributes and proteome profile of rice cultivars under salt stress. Plant Physiol. Biochem . 146:55-70.
Hassan, ., S.F. mjad, M.H. Saleem, H. Yasmin, M Imran, M. Riaz, Q. li, F. . Joyia, S. hmed, and S.
li. (2021). Foliar application of ascorbic acid enhances salinity stress tolerance in barley ( Hordeum vulgare L.) through modulation of morpho-physio-biochemical attributes, ions uptake, osmo-protectants and stress response genes expression. Saudi J. Biol. Sci . 28:4276-4290.
Hassanpour, H., R. . Khavari-Nejad, V. Niknam, F. Najafi, and K. Razavi. (2013). Penconazole induced changes in photosynthesis, ion acquisition and protein profile of Mentha pulegium L. under drought stress. Physiol. Mol. Biol. Plants . 19:489-498.
Jini, D., and B. Joseph. (2017). Physiological mechanism of salicylic acid for alleviation of salt stress in rice. Rice Science . 24:97-108.
Kamran, M., K. Xie, J. Sun, D. Wang, C. Shi, Y. Lu, W. Gu, and P. Xu. (2020). Modulation of growth performance and coordinated induction of ascorbate-glutathione and methylglyoxal detoxification systems by salicylic acid mitigates salt toxicity in choysum ( Brassica parachinensis L.) Ecotoxicol. Environ. Saf . 188:109877.
Khosravinejad, F., R. Heydari, and T. Farboodnia. (2009). Effect of salinity on organic solutes contents in barley. Pakistan Journal of Biological Sciences: PJBS . 12:158-162.
Kirk, J.T.O., and R.L. llen. (1965). Dependence of chloroplast pigment synthesis on protein synthesis:
effect of actidione. Biochem. Biophys. Res. Commun . 21:523-530.
Liang, W., X. Ma, P. Wan, and L. Liu. (2018). Plant salttolerance mechanism: review. Biochem. Biophys. Res. Commun . 495:286-291.
Mbarki, S., O. Sytar, . Cerda, M. Zivcak, . Rastogi, X. He, . Zoghlami, C. bdelly, and M. Brestic. (2018). Strategies to mitigate the salt stress effects on photosynthetic apparatus and productivity of crop plants. Salinity Responses and Tolerance in Plants, Volume 1: Targeting Sensory, Transport and Signaling Mechanisms :85-136.
Mir, R. ., and, and R. Somasundaram. (2023). Salt Stress and tolerance mechanisms by plants: Review. Iranian Journal of Plant Physiology. 13:4569-4587.
Mir, R. ., and R. Somasundaram. (2020). Effect of NaCl stress on pigment composition, membrane integrity and proline metabolism of little millet ( Panicum sumatrense L.) CO-4 variety. International Journal of Botany Studies ,. 5:586-593.
Mir, R. ., and R. Somasundaram. (2021a). Growth improvement and pigment composition in cowpea ( Vigna unguiculata (L.) Walp.) by foliar spray of salicylic acid and ascorbic acid under NaCl stress. International Journal of Botany Studies . 6:490-496.
Mir, R. ., R. Somasundaram, and R. Panneerselvam. (2019). Changes in antioxidant enzymes activities mitigates deleterious effects of ROS in Panicum miliaceum (L.) under drought stress. Journal of Stress Physiology & Biochemistry . 15:81-91.
Nahar, K., M. Hasanuzzaman, and M. Fujita. (2016) Roles of osmolytes in plant adaptation to drought and salinity. Osmolytes and plants acclimation to changing environment: Emerging omics technologies :37-68.
Naheed, R., H. slam, H. Kanwal, F. Farhat, M.I. . Gamar, . . l-Mushhin, D. Jabborova, M.J. nsari, S. Shaheen, and M. qeel. (2021). Growth attributes, biochemical modulations, antioxidant enzymatic metabolism and yield in Brassica napus varieties for salinity tolerance. Saudi J. Biol. Sci. 28:5469-5479.
Noreen, S., M. Sultan, M.S. khter, K.H. Shah, U Ummara, H. Manzoor, M. Ulfat, M.N. lyemeni, and P. hmad. (2021). Foliar fertigation of ascorbic acid and zinc improves growth, antioxidant enzyme activity and harvest index in barley ( Hordeum vulgare L.) grown under salt stress. Plant Physiol. Biochem . 158:244-254.
Osman, M.E., . . Mohsen, . . Nessim, M.S. El-Saka, and W. Mohamed. (2019). Evaluation of biochar as a soil amendment for alleviating the harmful effect of salinity on Vigna unguiculata (L.) Walp. Egyptian Journal of Botany . 59:617-631.
Ramel, F., S. Birtic, C. Ginies, L. Soubigou-Taconnat, C. Triantaphylidès, and M. Havaux. (2012) Carotenoid oxidation products are stress signals that mediate gene responses to singlet oxygen in plants. Proceedings of the National Academy of Sciences. 109:5535-5540.
Sharma, P.K., and D.O. Hall. (1991). Interaction of salt stress and photoinhibition on photosynthesis in barley and sorghum. J. Plant Physiol. 138:614-619.
Shrivastava, P., and R. Kumar. (2015). Soil salinity: serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 22:123-131.
Sofy, M.R., N. Elhawat, and T. lshaal. (2020). Glycine betaine counters salinity stress by maintaining high K+/Na+ ratio and antioxidant defense via limiting Na+ uptake in common bean ( Phaseolus vulgaris L.). Ecotoxicol. Environ. Saf. 200:110732.
Taïbi, K., F. Taïbi, L. . bderrahim, . Ennajah, M. Belkhodja, and J.M. Mulet. (2016). Effect of salt stress on growth, chlorophyll content, lipid peroxidation and antioxidant defence systems in Phaseolus vulgaris L. S. Afr. J. Bot . 105:306-312.
Tanveer, K., S. Gilani, Z. Hussain, R. Ishaq, M. deel, and N. Ilyas. (2020). Effect of salt stress on tomato plant and the role of calcium. J. Plant Nutr. 43:2835.
Yang, Y., and Y. Guo. (2018). Unraveling salt stress signaling in plants. Journal of integrative plant biology . 60:796-804.
Yao, D., J. Wu, Q. Luo, D. Zhang, W. Zhuang, G. Xiao,
Q. Deng, and B. Bai. (2022). Effects of salinity stress at reproductive growth stage on rice (Oryza sativa L.) composition, starch structure, and physicochemical properties. Frontiers in Nutrition. 9:926217.
JOURNAL OF STRESS PHYSIOLOGY & BIOCHEMISTRY Vol. 20 No. 2 2024
Список литературы Growth modulation, photosynthetic attributes and proline accumulation led enhancement to Oryza sativa (L.) seedlings exposed to NaCl stress
- AbdElgawad, H., G. Zinta, M.M. Hegab, R. Pandey, H. Asard, and W. Abuelsoud. (2016). High salinity induces different oxidative stress and antioxidant responses in maize seedlings organs. Frontiers in plant science. 7:276.
- Ahmad, W., C.M. Ayyub, M.A. Shehzad, K. Ziaf, M. Ijaz, A. Sher, T. Abbas, and J. Shafi. (2019). Supplemental potassium mediates antioxidant metabolism, physiological processes, and osmoregulation to confer salt stress tolerance in cabbage (Brassica oleracea L.). Horticulture, Environment, and Biotechnology. 60:853-869.
- Alves, R.d.C., D.R. Rossatto, et. al., (2021). Seed priming with ascorbic acid enhances salt tolerance in micro-tom tomato plants by modifying the antioxidant defense system components. Biocatalysis and Agricultural Biotechnology. 31:101927.
- Arnon, D.I. (1949). Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 24:1.
- Aryendu, Mir, RA, Kathiravan M, and R. Somasundaram. (2022a). Alleviating NaCl Stress by Improving Growth and Yield in Arachis hypogaea L. by Exogenous Application of Brassinolide and Paclobutrazol. Indian Journal of Natural Sciences. 13:46608-46619.
- Bates, L., R.a. Waldren, and I. Teare. (1973). Rapid determination of free proline for water-stress studies. Plant Soil. 39:205-207.
- Bradford, M.M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254.
- Carvalho, M.E.A., F.A. Piotto, M.L. Nogueira, F.G. Gomes-Junior, H.M.C.P. Chamma, D. Pizzaia, and R.A. Azevedo. (2018). Cadmium exposure triggers genotype-dependent changes in seed vigor and germination of tomato offspring. Protoplasma. 255:989-999.
- Ceccarini, C., F. Antognoni, S. Biondi, A. Fraternale, G. Verardo, A. Gorassini, and V. Scoccianti. (2019). Polyphenol-enriched spelt husk extracts improve growth and stress-related biochemical parameters under moderate salt stress in maize plants. Plant Physiol. Biochem. 141:95-104.
- Chen, T., S. Shabala, Y. Niu, Z.-H. Chen, L. Shabala, H. Meinke, G. Venkataraman, A. Pareek, J. Xu, and M. Zhou. (2021). Molecular mechanisms of salinity tolerance in rice. The Crop Journal. 9:506-520.
- Chiconato, D.A., G.d.S.S. Junior, D.M.M. dos Santos, and R. Munns. (2019). Adaptation of sugarcane plants to saline soil. Environ. Exp. Bot. 162:201211.
- Das, S.K., J.K. Patra, and H. Thatoi. (2016). Antioxidative response to abiotic and biotic stresses in mangrove plants: A review. Int. Rev. Hydrobiol. 101:3-19.
- Fahad, S., M. Adnan, M. Noor, M. Arif, M. Alam, I.A. Khan, H. Ullah, F. Wahid, I.A. Mian, and Y. Jamal. (2019). Major constraints for global rice production. In Advances in rice research for abiotic stress tolerance. Elsevier. 1-22.
- Farooq, M., M. Hussain, A. Wakeel, and K.H. Siddique. (2015). Salt stress in maize: effects, resistance mechanisms, and management. A review. Agronomy for Sustainable Development. 35:461481.
- Frukh, A., T.O. Siddiqi, M.I.R. Khan, and A. Ahmad. (2020). Modulation in growth, biochemical attributes and proteome profile of rice cultivars under salt stress. Plant Physiol. Biochem. 146:55-70.
- Hassan, A., S.F. Amjad, M.H. Saleem, H. Yasmin, M. Imran, M. Riaz, Q. Ali, F.A. Joyia, S. Ahmed, and S. Ali. (2021). Foliar application of ascorbic acid enhances salinity stress tolerance in barley (Hordeum vulgare L.) through modulation of morpho-physio-biochemical attributes, ions uptake, osmo-protectants and stress response genes expression. Saudi J. Biol. Sci. 28:4276-4290.
- Hassanpour, H., R.A. Khavari-Nejad, V. Niknam, F. Najafi, and K. Razavi. (2013). Penconazole induced changes in photosynthesis, ion acquisition and protein profile of Mentha pulegium L. under drought stress. Physiol. Mol. Biol. Plants. 19:489-498.
- Jini, D., and B. Joseph. (2017). Physiological mechanism of salicylic acid for alleviation of salt stress in rice. Rice Science. 24:97-108.
- Kamran, M., K. Xie, J. Sun, D. Wang, C. Shi, Y. Lu, W. Gu, and P. Xu. (2020). Modulation of growth performance and coordinated induction of ascorbate-glutathione and methylglyoxal detoxification systems by salicylic acid mitigates salt toxicity in choysum (Brassica parachinensis L.). Ecotoxicol. Environ. Saf. 188:109877.
- Khosravinejad, F., R. Heydari, and T. Farboodnia. (2009). Effect of salinity on organic solutes contents in barley. Pakistan Journal of Biological Sciences: PJBS. 12:158-162.
- Kirk, J.T.O., and R.L. Allen. (1965). Dependence of chloroplast pigment synthesis on protein synthesis: effect of actidione. Biochem. Biophys. Res. Commun. 21:523-530.
- Liang, W., X. Ma, P. Wan, and L. Liu. (2018). Plant salt-tolerance mechanism: A review. Biochem. Biophys. Res. Commun. 495:286-291.
- Mbarki, S., O. Sytar, A. Cerda, M. Zivcak, A. Rastogi, X. He, A. Zoghlami, C. Abdelly, and M. Brestic. (2018). Strategies to mitigate the salt stress effects on photosynthetic apparatus and productivity of crop plants. Salinity Responses and Tolerance in Plants, Volume 1: Targeting Sensory, Transport and Signaling Mechanisms:85-136.
- Mir, R.A., and, and R. Somasundaram. (2023). Salt Stress and tolerance mechanisms by plants: A Review. Iranian Journal of Plant Physiology. 13:4569-4587.
- Mir, R.A., and R. Somasundaram. (2020). Effect of NaCl stress on pigment composition, membrane integrity and proline metabolism of little millet (Panicum sumatrense L.) CO-4 variety. International Journal of Botany Studies,. 5:586-593.
- Mir, R.A., and R. Somasundaram. (2021a). Growth improvement and pigment composition in cowpea (Vigna unguiculata (L.) Walp.) by foliar spray of salicylic acid and ascorbic acid under NaCl stress. International Journal of Botany Studies. 6:490-496.
- Mir, R.A., R. Somasundaram, and R. Panneerselvam. (2019). Changes in antioxidant enzymes activities mitigates deleterious effects of ROS in Panicum miliaceum (L.) under drought stress. Journal of Stress Physiology & Biochemistry. 15:81-91.
- Nahar, K., M. Hasanuzzaman, and M. Fujita. (2016). Roles of osmolytes in plant adaptation to drought and salinity. Osmolytes and plants acclimation to changing environment: Emerging omics technologies:37-68.
- Naheed, R., H. Aslam, H. Kanwal, F. Farhat, M.I.A. Gamar, A.A. Al-Mushhin, D. Jabborova, M.J. Ansari, S. Shaheen, and M. Aqeel. (2021). Growth attributes, biochemical modulations, antioxidant enzymatic metabolism and yield in Brassica napus varieties for salinity tolerance. Saudi J. Biol. Sci. 28:5469-5479.
- Noreen, S., M. Sultan, M.S. Akhter, K.H. Shah, U. Ummara, H. Manzoor, M. Ulfat, M.N. Alyemeni, and P. Ahmad. (2021). Foliar fertigation of ascorbic acid and zinc improves growth, antioxidant enzyme activity and harvest index in barley (Hordeum vulgare L.) grown under salt stress. Plant Physiol. Biochem. 158:244-254.
- Osman, M.E., A.A. Mohsen, A.A. Nessim, M.S. El-Saka, and W. Mohamed. (2019). Evaluation of biochar as a soil amendment for alleviating the harmful effect of salinity on Vigna unguiculata (L.) Walp. Egyptian Journal of Botany. 59:617-631.
- Ramel, F., S. Birtic, C. Ginies, L. Soubigou-Taconnat, C. Triantaphylides, and M. Havaux. (2012). Carotenoid oxidation products are stress signals that mediate gene responses to singlet oxygen in plants. Proceedings of the National Academy of Sciences. 109:5535-5540.
- Sharma, P.K., and D.O. Hall. (1991). Interaction of salt stress and photoinhibition on photosynthesis in barley and sorghum. J. Plant Physiol. 138:614-619.
- Shrivastava, P., and R. Kumar. (2015). Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 22:123-131.
- Sofy, M.R., N. Elhawat, and T. Alshaal. (2020). Glycine betaine counters salinity stress by maintaining high K+/Na+ ratio and antioxidant defense via limiting Na+ uptake in common bean (Phaseolus vulgaris L.). Ecotoxicol. Environ. Saf. 200:110732.
- Taibi, K., F. Taibi, L.A. Abderrahim, A. Ennajah, M. Belkhodja, and J.M. Mulet. (2016). Effect of salt stress on growth, chlorophyll content, lipid peroxidation and antioxidant defence systems in Phaseolus vulgaris L. S. Afr. J. Bot. 105:306-312.
- Tanveer, K., S. Gilani, Z. Hussain, R. Ishaq, M. Adeel, and N. Ilyas. (2020). Effect of salt stress on tomato plant and the role of calcium. J. Plant Nutr. 43:2835.
- Yang, Y., and Y. Guo. (2018). Unraveling salt stress signaling in plants. Journal of integrative plant biology. 60:796-804.


