Hepatoprotective role of sodium selenite against oxidative damage induced by mercuric chloride in rat albinos Wistar
Автор: Necib Youcef, Bahi Ahlem, Zerizer Sakina, Abdennour Cherif, Boulakoud Mohamed Salah
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 4 т.9, 2013 года.
Бесплатный доступ
Background: the present study was undertaken, to evaluate the protective effect of sodium selenite against mercuric chloride induced oxidative stress in experimental rats. Female Albinos Wistar rats randomly divided into four groups, were the first was served as a control, whereas the remaining groups respectively treated with: sodium selenite (1mg/ kg b.w; ip), mercuric chloride (1 mg/kg body weight i.p) and combination of sodium selenite and HgCl2. Change in liver enzyme activities, thiobarbituric acid reactive substances (TBARS) level, antioxidants and reduced glutathione (GSH) contents were determined after 10 days experimental period. Results: Exposure of rats to mercuric chloride caused a significant increase the lipid peroxidation level along with corresponding decrease in the reduced glutathione and various antioxidant enzymes in liver. And increase in serum: glucose level, APL and transaminases activities and decreased in total protein and albumin levels. Furthermore, treatment with mercuric chloride caused a marked elevation of liver weight and decreased body weight. Supplementation of sodium selenite resulted in decreased of lipid peroxidation level and in the serum: AST, ALT and APL activities were decreased along with increase in total protein, albumin and liver GSH levels. The activities of antioxidants enzymes: glutathione peroxidase (GSH -Px) and glutathione –S-transferase (GST) were also concomitantly restored to near normal level by sodium selenite supplementation to mercuric chloride intoxicated rats. Liver histological studies have confirmed the changes observed in biochemical parameters and proved the beneficial role of sodium selenite. Conclusion: The results clearly demonstrate that sodium selenite treatment augments the antioxidants defense mechanism in mercuric chloride induced toxicity and provides evidence that it may have a therapeutic role in free radical mediated diseases.
Antioxidant enzymes, sodium selenite, mercury, oxidative stress, female rat
Короткий адрес: https://sciup.org/14323804
IDR: 14323804
Текст научной статьи Hepatoprotective role of sodium selenite against oxidative damage induced by mercuric chloride in rat albinos Wistar
Mercury a highly toxic metal, results in avariety of adverse health effects including neurological, renal, respiratory, immune, dermatologic, reproductive and developmental sequelle (Risher-John et al ., 2005). Due to wide use of mercury in agriculture, industrial, medical and other fields, its exposure is cannot be avoided. The toxicity the mercury compounds (elemental, inorganic and organic). Inorganic mercury present in the environment is a well- established toxicant to human health (WHO, 1991).
Exposure to mercury promotes the reactive oxygen species (ROS) formation such as hydrogen peroxides, these ROS enhances the subsequent iron and copper induced production of lipid peroxides and highly reactive hydroxyl radicals (Miller et al ., 1991; Hussain et al ., 1999). Detrimental effects caused by free radicals occur when there is an imbalance between free radical production and radical scavenging capacity of antioxidant system in favour of former (Garg et al ., 2005). Mercury induced oxidative stress; make an important contribution to molecular mechanism for liver injury (Farima et al ., 2004). Recent evidences also show that mercury causes severe oxidative damage ( im et al ., 2005), thus mercury is proved to be a potential oxidant in the category of environmental factors. Therefore, there is a need to provide protection against mercury induced toxicity. It is important to develop an effective drug for mercury to prevent the mercury induced cellular damages. Historically, plants have been used as folk medicine against various type of disease.
The biological important of selenium is at least 3-fold. First, it forms the prosthetic group of some critical selenocysteine containing enzymes, such as glutathione peroxidase, iodothyronine 5’-deiodinase, and thioredoxinreductase (Stadtman,
-
1996 ). Second, sodium selenite is protective against a number of toxicants. Third, selenium excessive intake cause toxic potential (Combs and Gray, 1998). The purpose of this study was to evaluate the protective role of selenium on mercury chloride induced oxidative stress in rats.
MATERIALS AND METHODS
All chemicals used in this work were purchased from sigma chemical company. Laboratory animals, Albinos Wistar female rats, were brought from the Algiers Pasteur institute at the age of 8 weeks, with an average live weight of 200g. They were located in a room with an ambient temperature of 21±1°C and up to 12h of light daily. The rats were divided into four experimental groups; each consists of six rattes. The first group was served as the control. The second group was given sodium selenite at a dose of 1mg/kg body weight, while the third group (HgCl 2 ) was intraperitoneally given mercuric chloride at a dose of 1 mg/kg body weight. Finally, the fourth group: sodium selenite was given (1mg/kg body weight) 10min before HgCl 2 (1 mg/kg body weight) and continued up to 10 days after mercuric chloride treatment. The treatment of all groups was lasted for 10 consecutive days.
Twenty four hour after the last administration the blood was collected by retro- orbital sinus punction from each anesthetized rats. After centrifugation at 3000 rpm for 10min, the serum was separated immediately and stored at ̶ 20°c until determination of: glucose, albumin and total protein levels and enzymes (AST, ALT and ALP) activities. Subsequently, rats were decapitated and liver were removed.
Tissue preparation
About 500mg of liver was homogenized in 4ml of buffer solution of phosphate buffered saline
(w/v: 500mg tissue with 4ml PBS, PH 7.4) homogenates were centrifuged at 10.000xg for 15min at 4°c. The resultant supernatant was used for determination of: reduced GSH, Thiobarbituric acid- reactive substance (TBARS) levels, and the activities of: GSH-PX and GST.
Determination of glucose, total protein and albumin levels and enzymes
Serum glucose, total protein and albumin levels and AST, ALT and ALP activities were determined using commercial kits (Spinreact).
Determination of lipid peroxidation (LPO)
Lipid peroxidation level in the liver was measured by the method of Buege and Aust (1978). 125µl of supernatant were homogenized by sonication with 50 µl of PBS, 125 µl of 20% TCA + BHT 1% (TCA-BHT) in order to precipitate proteins, and centrifuged (1000xg, 10min, 4°c), afterwards, 200µl of supernatant were mixed with 40µl of HCl (0,6M), and 160µl of TBA dissolved in tris (120 mM). And the mixture was heated at 80°c for 10min; the absorbance was measured at 530nm. The amount of TBARS was calculated by using a molar extinction coeffient of 1.56x105M/Cm.
Determination of reduced glutathione (GSH)
GSH content in liver was measured spectrophotometrically by using Ellman’s reagent (DTNB) as a colouring reagent, following the method described by Weeckbekeretcory (1988).
Determination of glutathione-S-transferase (GST) (EC2.5.1.18)
The cytosolic glutathione-S-transferase activity was determined spectrophotometrically at 37°c by method of Habig et al (1974). The reaction mixture (1ml) contained 0.334ml of 100mM phosphate buffer (PH 6.5), 0.033ml of 30mM CDNB and 0.33ml of reduced Glutathione. After pre-incubating for
2min, the reaction was started by adding 0.01ml of diluted cytosol and the absorbance was followed for 3min at 340 nm. The specific activity of GST is expressed as µmole of GSH-CDNB conjugate formed/ min /mg protein using extinction coefficient of 9.6 Mm-1 cm-1
Determination of GSH-Px (E.C.1.11.1.9)
Glutathione peroxidase (EC 1.11.1.9) activity was modified from the method of Flohe and Gunzler (1984). for the enzyme reaction, 0.2ml of the supernatant was placed into a tube and mixed with 0.4ml GSH (reduced glutathione, sigma product, analytical grade), and the mixture was put into an ice bath for 30min. Then the mixture was centrifuged for 10min at 3000rpm, 0.48ml of the supernatant was placed into a cuvette, and 2.2ml of 0.32M Na 2 HPO 4 and 0.32ml of 1m mol/l 5,5’-dithio-bis(2-nitrobenzoic acid) (DTNB, sigma) were added for color development. The absorbance at wavelength 412nm was measured with a UV spectrophotometer after 5min. The enzyme activity was calculated as a decrease in GSH within the reaction time as compared with the non-enzyme reaction.
Protein quantification
Protein was measured by the method of Bradford (1976), using bovine serum albumin as the standard.
Histopathological examination
Liver from autopsied animals were excised out and fixed in formalin (10%). five micron think section were prepared by using microtome and these section were stained with hematoxyline and eosin. For histological alterations these slides were observed under light microscope.
Statistical analysis
The data were subjected to student t test for comparison between groups. The values are expressed as mean ± SEM. Significance level was set at P<0.05, P<0.01, P<0.001.
RESULTS
Effects of treatments on body, absolute and relative liver weights
The variations in body and relative liver weights of animals subjected to different treatments were shows in Table 1. During the course of present investigations, it was observed that the control body weight and sodium selenite treated group have increased progressively, contrary in HgCl 2 treated rats, results revealed significant decrease in body weight gain as compared to the control. Besides, a significant increase of HgCl 2 treated group in absolute and relative liver weights and in sodium selenite treated group as compared to the control.
Effects of treatments on serum biochemical Parameters
Treatment with HgCl2 caused a significant (P≤ 0.01) increase in the activities of AST, ALT and ALP as compared to the control. Only sodium selenite treatment did not show any significant alteration. However, the combined treatment of sodium selenite with mercuric chloride results in gradual recovery in AST, ALT and ALP activities as compared to the control (table 2). The content of serum glucose of the HgCl2 treated group tented to be higher compared to the control. Albumin and protein levels in HgCl2 treated animals were decreased, but the co-administration of sodium selenite with HgCl2 has produced a recovery in the above mentioned biochemical variables.
Effects of treatments on hepatic oxidative stress parameters
Mercuric chloride exposure a highly significant depleted in reduced glutathione (GSH) level, GPx and GST activities. And a significant increase in liver lipid peroxidation level in mercury intoxicated rats was noticed. Sodium selenite alone treatment did not show any significant decline. In combined treatment of sodium selenite with mercuric chloride a highly significant increase in reduced glutathione (GSH) level, GPx and GST activities. And a significant depletion in lipid peroxidation level was recorded with respect to mercury intoxicated rats (Figs. 1 and 2).
Histological studies
Mercuric chloride induces various pathological alterations in liver of rats. These alterations were characterized by centrilobular necrosis, degranulation, destruction of membrane cells, cytoplasmic vacuolization (Fig. 3C). In combination group were sodium selenite was administered with mercuric chloride showed reparative changes. Liver showed prominent recovery in the form of normal hepatocytes and very less centrilobular necrosis. Pronounced sinusoid. With granular hepatocytoplasm were also evident (Fig.3D). Liver of the control group had a regular histological structure with a characteristic pattern of hexagonal lobules (Fig. 3A).Furthermore, no histological alterations were observed in the liver of sodium selenite treated group (Fig. 3B).
Table 1. Changes in body and absolute and relative liver weights of control and rats treated with selenium (Se), mercuric chloride, and combined treatment of mercuric chloride with selenium after 10 days of treatment.
|
Parameters |
Treatment groups |
|||
|
Control |
Se |
HgCl 2 |
Se + HgCl 2 |
|
|
Initial body weight (g) |
226.6±26.5 |
222.5±12.5 |
221±35.6 |
224.6±25.5 |
|
Final body weight (g) |
227±18.4 |
225.8±18.9 |
193.1±31.6 |
218.83±15.0 |
|
Absolute Liver weight (g) |
4.96±1.25 |
5.87±1.36 |
9.63±0.19*** |
9.21±1.73*** |
|
Relative Liver weight (g/100g.b.w) |
2.18±0.06 |
2.59±0.07 |
4.98±0.04*** |
4.2±0.11*** |
Values are given as mean ± SEM for group of 6 animals each. *P≤0.05, compared to controls. **P≤0.01, compared to controls. ***P≤0.001, compared to controls.
Table 2. Changes in biochemical parameters of control and rats treated with selenium (Se), mercuric chloride, and combined treatment of mercuric chloride withselenium after 10 days of treatment.
|
Parameters |
Treatment groups |
|||
|
Control |
Se |
HgCl 2 |
Se + HgCl 2 |
|
|
Glucose (mg/dl) |
131.8±23.4 |
129.1±37.16 |
145.69±39.66 |
136.35±34.81 |
|
Total protein (g/dl) |
10.91±0.31 |
10.76±0.4 |
9.21±1.05 ** |
9.93±0.45*** |
|
Albumin (g/dl) |
3.86±0.71 |
3.61±0.39 |
2.73±0.41* |
3.42±0.36 |
|
AST (UI/l) |
290.7±208 |
258.59±51.45 |
323.8±8.7** |
295.3±34.14 |
|
ALT (UI/l) |
262.1±42.2 |
270.8±15.8 |
330.32±13.7 ** |
287.21±66.5 |
|
ALP (UI/l) |
230.48±84.6 |
313.4±93.5 |
329.5±72.3* |
420.3±84.6 |
Values are given as mean ± SEM for group of 6 animals each. *P≤0.05, compared to controls. **P≤0.01, compared to controls. ***P≤0.001, compared to controls.
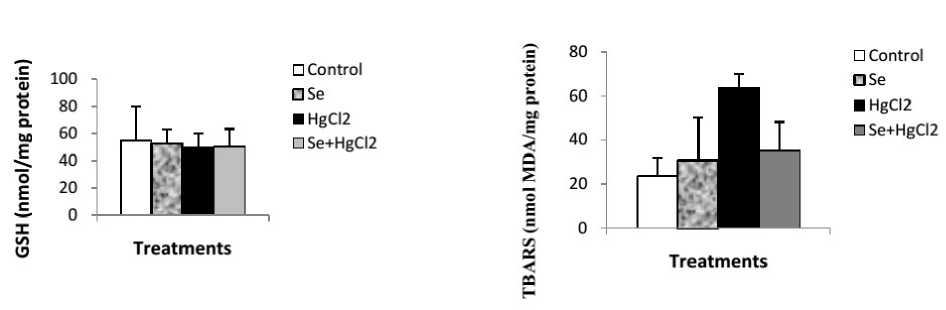
Figure 1. Reduced glutathione (nmol/ mg protein) and TBARS (nmol MDA /mg protein) levels in Liver of control and rats treated with selenium, mercuric chloride, and combined treatment of mercuric chloride with selenium after 10days of treatment. Values are given as mean ± SEM for group of 6 animals each significant difference: * compared to controls (*P≤0.05; **P≤0.01; ***P≤0.001).
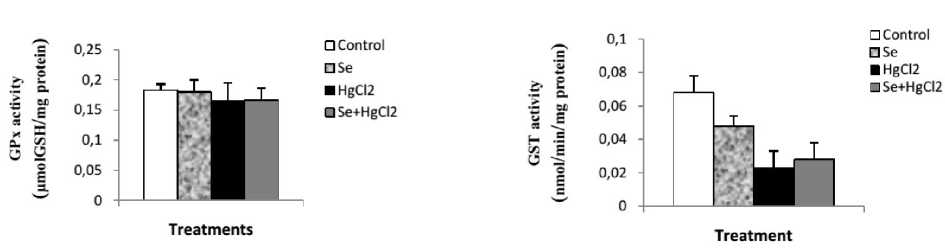
Figure 2. Enzyme activities of GPx (µmol GSH/ mg protein) and GST (nmol /min/mg protein) in Liver of control and rats treated with selenium, mercuric chloride, and combined treatment of mercuric chloride with selenium after 10 days of treatment. Values are given as mean ± SEM for group of 6 animals each significant difference: * compared to controls (*P≤0.05; **P≤0.01; ***P≤0.001).
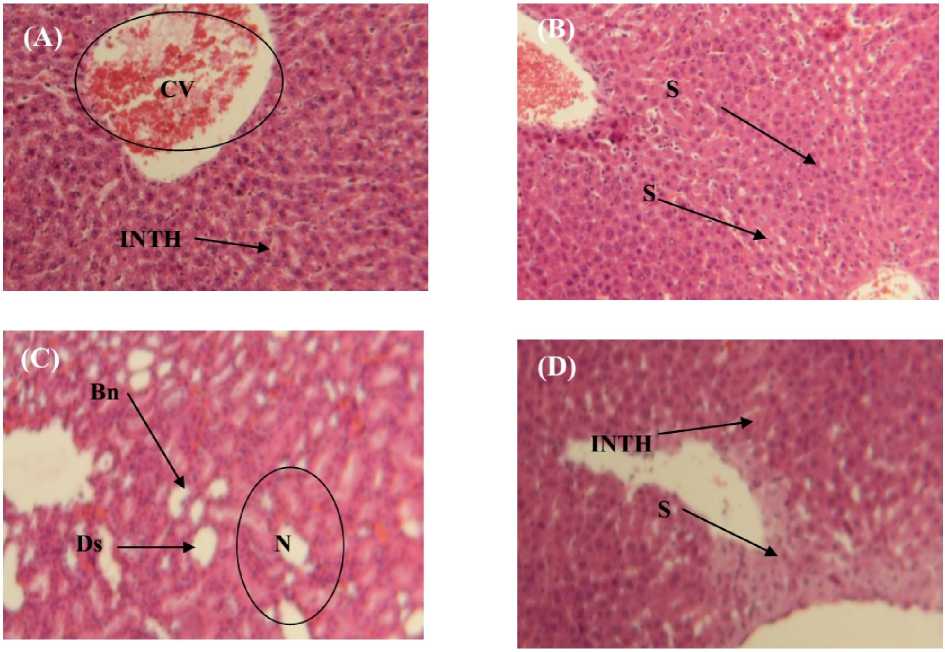
Figure 3. Microscop evaluation of hepatic tissue from (A) control, (B) treated with sodium selenite, (C) mercuric chloride and (D) sodium selenite pre-and post-treated with mercuric chloride after 10 days of treatment, section were stained using the hematoxylin-eosin method (100X). Bn: Bright nuclei, Ds: destruction of membrane cells, N: Necrosis, INTH: Intact hypatocyte cells, CV: central vein, S: Sinusoid. Sodium selenite coadministrated with mercuric chloride shows granular cytoplasm and normal hepatocytes.
DISCUSSION
The present investigation revealed that mercuric chloride intoxication causes significant increase in lipid peroxidation and glucose levels, AST, ALT and
ALP activities, and significant decrease in the serum albumin and total protein also in reduced glutathione, glutathione peroxidase and glutathione-s-transferase in liver.
The principal toxic effects of mercury involve interaction with a large number of cellular processes, including the formation of complexes with free thiols and protein thiol groups, which may lead to oxidative stress (Stacey et al., 1982). Due to its sulfhydryl group binding capability, HgCl2 can also inhibit the activities of many enzymes, especially those involved in the cellular glucose uptake, gluconeogenesis, fatty acid. Oxidation and production of glutathione. In the present study, a significant decrease in serum protein and albumin levels was recorded. The decreased in the protein concentration of Hg treated rats might be due to changes in protein synthesis and/or metabolism. Once absorbed in the cell, both Hg+2 and MeHg from covalent bonds with GSH and the cysteine residue of proteins. GSH, the primary intracellular antioxidant and the conjugating agent, was shown to be depleted and to have impaired function in Hg toxicity. A single Hg ion can bind to and cause irreversible excretion of up to two GSH molecules (QUIG, 1998). In fact, GSH serves as a primary line of cellular defense against Hg compounds. Released Hg ions form complexes with GSH and cysteine results in greater activity of the free Hg ions, disturbing GSH metabolism and damaging cells (Hultberg et al., 2001). As a result of binding of mercury to glutathione, levels of GSH are lowered in the cell and decrease the anti-oxidant potential of the cell. Antioxidant enzymes such as glutathione peroxidase and glutathione –S-transferase play a major role in the intracellular defense against oxygen radical damage to aerobic cells. Chung et al (1982) demonstrated that 10mg/kg of mercury caused time dependent decreases in the activities of the enzyme of the glutathione metabolism pathway in the rat kidney. Girardi and Elias (1995) reported that mercury inhibits the activities of redox cycles enzymes. Recent finding of Bando et al and Jadhav et al (2006) proved our point that this antioxidant enzymes show decreased level following mercury intoxication. Because of the low activity of antioxidant enzymes in the liver and decreased content of GSH, the liver is hypothesized to be highly susceptible to oxidative stress. Mercury induced oxidative stress turn to severe; the inbuilt mechanism of body fails to alleviate the damage. It has been demonstrated that mercury decreases the anti-oxidative systems and produces oxidative damages via H2O2 generation therby leading to lipid peroxidation (Cheng et al., 2006; Jadhav et al., 2007). All these possible mechanisms of mercuric chloride toxicity may lead to the formation of reactive oxygen species (ROS), as found in the present investigation. Therefore, an increase in the formation of ROS by mercuric chloride may induce membrane biochemical and functional alterations and thus induced liver cell damage.
Further, mercury intoxication also induces a significant elevation in serum and: AST, ALT and ALP activities. This increase may be due to cellular necrosis of hepatocytes, which causes increase in the permeability of cell resulting release of transaminases and ALP in blood stream (Vandenberghe, 1995; Rana et al ., 1996; umar et al ., 2005). This confirms our earlier reports on histopathological alterations in liver induced by mercury intoxication ( umar et al ., 2005; Sharma et al ., 2000).
It was observed that sodium selenite when given in combination with mercuric chloride significantly increases liver GSH level, GSH-Px and GST activities as antioxidant potential and thereby declines the level of lipid peroxidation, which in turn reduces the transaminases and ALP activities and glucose, total protein and albumin levels in serum. In present investigation, the elevated level of GSH protects cellular proteins against oxidation through glutathione redox cycle and directly detoxifies reactive species ( etterer, 1998). Glutathione, as both a carrier of mercury and an antioxidant, has specific roles in protecting the body from mercury toxicity. Glutathione, specifically bind with methylmercury, forms a complex that prevents mercury from binding to cellular proteins and causing damage to both enzymes and tissue ( romidas et al., 1990). Glutathione-mercury complexes also reduce intracellular damage by preventing mercury from entering tissue and cells, and becoming an intracellular toxin. The elevated level of GSH-Px and GST by sodium selenite as compared to the HgCl2 may have facilited the conjugation reaction of xenobiotics metabolism and may have increased the availability of non-critical nucleophile for inactivation of electrophiles and therefore might be playing a major role in metalloprotection. The protective effect of Se against Hg induced Hepatotoxicity my be related to the formation of a Se-Hg complex. This conclusion is based on previuos studies demonstrating that pretreatment with sodium selenite increased whole retention of Hg, conceivably due to the formation of inert Se-Hg complexes (Perottoni et al., 2004b). and the complexes reduced the availability of Hg (Sasakura and Suzuki, 1998). Yoneda and Suzuki (1997) also demonstrated that Se forms an equimolar complex with Hg in the plasma which subsequently binds to selenoprotein P. simultaneous administration equimolar doses of sodium selenite prevented not only methyl mercury induced increased of oxidized glutathione, inhibition of GSH-Px in liver (Hoffman and Heinz, 1998), but also histological and functional damage in liver as well (Magos et al., 1987; Perottoni et al.,
2004a,b) Se can enhance antioxidant ability by enhancing activities of antioxidant enzymes and by increasing contents of the antioxidants. Rotruck et al . (1973) and Xia et al . (2003) reported that Se is crucial in several enzymes with physiological antioxidant properties, including GSH-Px and thioredoxin. Besides, the ability of Se to reduce Hg toxicity has been extensively investigated (Sasakura and Suzuki, 1998; Perottoni et al ., 2004 a).
It may be concluded that combined treatment of sodium selenite has a preventive and protective effect on mercuric chloride induced oxidative stress. More-over, it protects from HgCl 2 induced hepatic dysfunction and executes its modulatory role in mercury induced free radical production.
Список литературы Hepatoprotective role of sodium selenite against oxidative damage induced by mercuric chloride in rat albinos Wistar
- Bando, I., Rens, M. I., Andres, D., Cascales, M. (2005) Endogenous antioxidant defence system in rat liver following mercuric chloride oral intoxication. J of Biochem Mol and Toxicol. 19(3), 154-161
- Bradford, M.A. (1976) Rapid and sensitive method for the quantities of microgram quantities of protein utilizing the principale of protein-dye binding. Anal Biochem. 72, 248-54
- Buege, J.A., Aust, S.D. (1984)Microsomal lipid peroxidation, Methods enzymol. 105, 302-10
- Cheng, J.P., Hu, W.X., Lin, X.J.M., Shi, w., Wang, W.H. (2006) Expression of C-fos and oxidative stress on brain of rats reared on food from mercury-selenium coexisting mining area. J of Environ Scie (china). 18(4), 788-792
- Chung, A.S., Maines, M.D., Reynolds, W.A. (1982) Inhibition of the enzymes of glutathione metabolism by mercuric chloride in the rat kidney: reversal by selenium. Biochem and pharmacol. 31, 3093-3100
- Combs, G.F. and Gray, W.P. (1998) Chemopreventive agents: selenium. Pharmacol Ther. 79, 179-192
- Farima, M. F., Soares, A., Zeni, G., Souza, D.O., Rocha, J.B. (2004) Additive prooxidative effect of methylmercury and ebselen in liver from suckling rat pups. Toxicol let. 146(3), 227-235
- Flohe, L., Gunzler, W.A. (1984)analysis of glutathione peroxidase. Methods enzymol. 105, 114-21
- Garg, M.C., Chaudhary, D.P., Bansal, D.D. (2005) Effect of vitamin E supplementation on diabetes induced oxidative stress in experimental diabet in rats. Ind J Exp Biol. 43, 177-180
- Girardi, G., Elias, M.M. (1995) Mercury chloride effects on rat renal redox enzymes activities: SOD protection. Free rad and Biol Med. 18(1), 61-66
- Habig, W.H., Pabst Jakoby, W.B. (1974) Glutathione-S-transferase the first step in mercapturic acid formation. J of Biol and Chemical. 249, 7130-9
- Hoffman, D.J., Heinz, G.H. (1998). Effects of mercury and selenium on glutathione metabolism and oxidative stress in mallard ducks. Environ Toxicol Chem. 17, 161-166
- Hultberg, B., Andersson, A., Isaksson, A. (2001) Interaction of metals and thiols in cell damage and glutathione distribution: potentiation of mercury toxicity by dithiothreitol. Toxicol. 156, 93-100
- Hussain, S., Atkinson, A., Thompson, S.J., Khan, A.T. (1999) Accumulation of mercury and its effect on antioxidant enzymes in brain, liver and kidneys of mice, J Environ Scie and Health, Part-B. 34(4), 645-660
- Jadhav, S.H., Sarkar, S.N., Aggarwal, M., Tripathi, H.C. (2007) Induction of oxidative stress in erythrocytes of male rats subchronically exposed to a mixture of eight metals found as ground water contaminants in different parts of india. Arc Environ Contam and Toxicol. 52(1), 145-151
- Ketterer, B. (1998) Glutathione-stransferase and prevention of cellular free radical damage. Free rad Rese. 28, 647-658
- Kim, S.H., Sharma, R.P. (2005) Mercury alters endotoxin induced inflammatory cytokine expression in liver: differential role of P38 and extra cellular signal-regulated mitogen activated protein kinases. Immunopharmacol and Immunotoxicol. 27(1), 123-135
- Kromidas, L., Trombetta, L.D., Jamall, I.S. (1990) The protective effects of glutathione against methylmercury cytotoxicity. Toxicol let. 51, 67-80
- Magos, L., Clarkson, T.W., Spanow, S., Hudson, A.R. (1987). Comparison of the protection given by selenite, selenomethionine and biological selenium against the renotoxicity of mercury. Arch Toxicol. 60, 422-426
- Miller, O.M., Lund, B.O., Woods, J.S. (1991) Reactivity of Hg (II) with superoxide: evidence for catalytic dismutation of superoxide by Hg (II), J Biochem Toxicol. 6, 293
- Perottoni, J., Lobato, L.P., Silveira, A., Rocha, J.B.T., Emanuelli, T. (2004a) effects of mercury and selenite on δ-aminolevulinatedehydratase activity and on selected oxidative stress parameters in rats. Environ Res. 95, 166-173
- Perottoni, J., Rodrigues, O.E.D., Paixao, M.W., Zeni, G., Labato, L.P., Braga, A.L., Rocha, J.B.T., Emanuelli, T. (2004b). Renal and hepatic ALA-D activity and selected oxidative stress parameters of rats exposed to inorganic mercury and organoselenium compounds. Food Chem Toxicol. 42, 17-28
- Quig, D. (1998) Cysteine metabolism and metal toxicity. Alt Medical Rev. 3, 262-270
- Risher-John, F., Amler-Sherlita, N. (2005) Mercury exposure: evaluation and intervention, the inappropriate use of chelating agents in diagnosis and treatment of putative mercury poisoning, Neurotoxicol. 26(4), 691-699
- Rotruck, J.T., Pope, A.L., Ganther, H.E., Swanson, A.B., Hafeman, D.G., Hoekstra, W.G. (1973). Selenium: biochemical role as a component of glutathione peroxidase. Sci. 179, 558-560
- Sasakura, C., Suzuki, K.T. (1998) Biological interaction between transition metals (Ag, Cd and Hg), selenide/sulfide and selenoprotein P. J Inorg Biochem. 71, 159-162
- Sharma, M. K., Kumar, M., Kumar, A. (2000) (study of mercury induced toxicity in liver and its modulation by spirulinafusiformis and ocimum sanctum). In: proceeding of third word congress on cellular and molecular biology held at jena, Germany. Cell and Mol Biol. 46, 227
- Stacey, N.H., Kappus, H. (1982) Cellular toxicity and lipid peroxidation in response to mercury. Toxicol and App Pharmacol. 63(1), 29-35
- Stadtman, T.C. (1996) Selenocysteine. Annu Rev Biochem. 65, 83-100
- Vandenberghe, J. (1995) Hepatotoxicology: mechanisms of liver toxicity and methodological aspects. In: niesink JM, vries JD, CRC press, bocaroton. P.718
- Weckbercker, G., Cory, J.G. (1988) Ribonucleotide reductase activity and growth of glutathione-depended mouse leukaemia L 1210 cells in vitro. Cancer let. 40, 257-264
- WHO, (1991) Environmental health criteria 118: inorganic mercury-environmental aspects. World healthorganization, Geneva, Switzerland. P. 115-119
- Yoneda, S., Suzuki, K.T. (1997). Detoxification of mercury by selenium by binding of equimolar Hg-Se complex to a specific plasma protein. Toxicol Appl Pharmacol. 143, 274-280


