Hereditary diffuse gastric cancer: genetic aspects and prophylactic total gastrectomy
Автор: Lyubchenko Liudmila N., Filippova Margarita G., Anurova Olga A., Nazliev Pavel B., Stilidi Ivan S.
Журнал: Сибирский онкологический журнал @siboncoj
Рубрика: Клинические исследования
Статья в выпуске: 4 т.17, 2018 года.
Бесплатный доступ
For patients with an identified germline E-cadherin-1 ( CDH1 ) mutation, prophylactic gastrectomy is the treatment of choice to eliminate the high risk of developing diffuse gastric cancer. The case report describes a rare case of hereditary diffuse gastric cancer (HDgC) associated with CDH1 gene mutation, which is reported in the Russian population for the first time. In 2013, a 28-year-old woman was referred to Clinical Oncogenetics Laboratory with a family history of gastric cancer. Molecular genetic analysis revealed CDH1 gene mutation. The lifetime risk of cancer in mutation positive members is more than 80. Histological examination of gastric biopsy specimens obtained during endoscopy revealed isolated signet ring cells in the lamina propria. Spleen-preserving D2-lymphodissection and total gastrectomy with Roux-en-Y reconstruction with a jejunal reservoir formation were performed at the Abdominal Oncology Surgery Department.
Hereditary diffuse gastric cancer (hdgc), cdh1 gene, gastrectomy, molecular genetic diagnostics
Короткий адрес: https://sciup.org/140254198
IDR: 140254198 | УДК: 616.33-006.6-056.7:575.113:577.21 | DOI: 10.21294/1814-4861-2018-17-4-48-52
Текст научной статьи Hereditary diffuse gastric cancer: genetic aspects and prophylactic total gastrectomy
Most cases of gastric cancer are sporadic, but 1–3 % cases occur in syndromes with a high hereditary predisposition to gastric cancer [1]. The most frequent hereditary gastric cancer is a diffuse type or litinitis plastica, it is commonly referred to hereditary diffuse gastric cancer (HDGC). To classify a familial case as HDGC, the international gastric cancer consortium (IGCLC) formulates the following criteria: (1) two or more documented cases of diffuse gastric cancer diagnosed in first- or second-degree relatives of age under 50 years old, in one case, at least (2) three or more documented cases of diffuse gastric cancer in relatives of the first - or second – degree relatives, regardless of the age of the disease oneset [2, 3].
HDGC is associated with germinal mutations in E-cadherin 1 ( CDH1 gene), that encodes the cell adhesion protein E-cadherin [4]. About 25–30 % of the families meeting the HDGC criteria according to the criteria of international consortium (Internatinal Gastric Canser Linkage Consortium – IGCLC) have constitutional aberrations in CDH1 gene [4]. More than 100 mutations in CDH1 gene have been described now in the families originating from different ethnic groups [5].
Although there are no major mutational hotspots, some mutations, including 1003C>T [6–9], 1901C>T [7, 10, 11], and 1137G>A [7, 11, 12], (2398delC) [7] have been observed in several unrelated families. The most common types of mutation are small insertions or deletions (35 %). The other mutations are missense (28 %), nonsense (16 %), splice site (16 %), and large exonic deletions (5 %) [13]. In addition to these major mutations, two regulatory sequence variants,-160C->A [14] and the intron 2 variant 163+37235G>A [15] have been associated with an elevated risk of DGC, although these polymorphisms are rarely associated with a strong familial clustering.
No correlations between the location or type of germline CDH1 mutation and phenotype have been ascertained. Particularly, there is no obvious correlation between genotype and the presence of lobular breast cancer in HDGC families [16]. However, somatic CDH1 mutations in sporadic DGC are predominantly splice site mutations resulting in exon skipping – particularly of exons 8–9, whereas most CDH1 mutations identified in sporadic lobular breast cancer result in premature stop codons [17, 18].
The CDH1 gene mutations originated de novo, are also identified in sporadic cases of early gastric cancer, at least, in 4 % of the patients with manifestation age under 35 years. Germinal mutations in the CDH1 gene have a high penetrance: cancer risk throughout the life is 67 % in men and 83 % - in women. The average age at diagnosis is 38-40 years old, it varies from 14 to 85 years old [19].
The female patient of 28 years old was consulted on familial history of gastric cancer cases among her young relatives (Figure 1). The study of familial anamnesis followed by molecular genetic analysis - determination of CDH1 gene sequence encoded suppressor E-cadherin, involved in
HDGC carcinogenesis was performed at Clinical Oncogenetics Laboratory.
PCR analysis of DNA isolated from peripheral blood lymphocytes followed by conformationsensitive gel electrophoresis and sequencing has revealed a germinal mutation c.1005delA - deletion in exon 7 of CDH1 gene, resulting in open reading frame shift.
Conclusion: given the high penetrance of CHD1 gene, lifetime gastric cancer risk is 80 %. Risk of infiltrative lobular breast cancer risk is much higher (60%) than the common population risk. The risk of colon cancer is also significantly higher than common population risk. This points to the need for dynamic follow up in N.N. Blokhin Medical Research Center of Oncology.
The risk of the germinal mutation inheritance by first-degree relatives is 50 %. According to the recommendations on gastric cancer of the IGCLC, a total gastrectomy is justified as a preventive measure. Ultrasound, CT, mammography and/or MRI of the breast; consultations of geneticist, gastroenterologist, mammologist, proctologist, nutritionist; DNA - diagnostics in first-degree relatives are recommended for follow-up of patients.
A planned EGDS, which was performed simultaneously with the molecular genetic study detected a gastric tumor in its antral part. Biopsy also revealed gastric tumor – isolated signet ring cells in mucosa of lamina propria. For further examination and treatment, the patient was hospitalized at the Abdominal Oncology Surgery Department. Total gastrectomy with Roux-en-Y reconstruction with formation of jejunal reservoir and D2 limphodis-section were performed on 14.03.2013. Histological verification and immunohistochemical examination of the surgical material obtained on 05.04.2013 confirmed the invasive ring-cell gastric cancer of the pyloric part within mucous membrane own plate - early gastric cancer of 0.9×0.2×0.1 cm, without vascular invasion and without metastases (Figure 2, 3). The Her2-neu overexpression was not detected («0»), the proliferation index Ki 67 was about 22 %.
This patient was monitored for 60 months. In the first 6 months there was a moderate weight loss and dumping syndrome of moderate severity, which were then compensated. According to clinical examination from 20.03.2018, no progression was observed.
Discussion
The patient pedigree represents a rare case of families that fully meets the HDGC criteria (Figure 1). Germinal mutation 1005delA in 7 exon of CDH1 gene, which the patient most likely inherited from her mother, was described in Russia for the first time [20]. Molecular genetics testing of available relatives for CDH1 1005delA mutation was performed for the first- and second-degree siblings (IV-2, IV-3); the mutation was not detected.
Thirty-eight publications on gastrectomy in carriers of CDH1 gene mutations were summarized. From
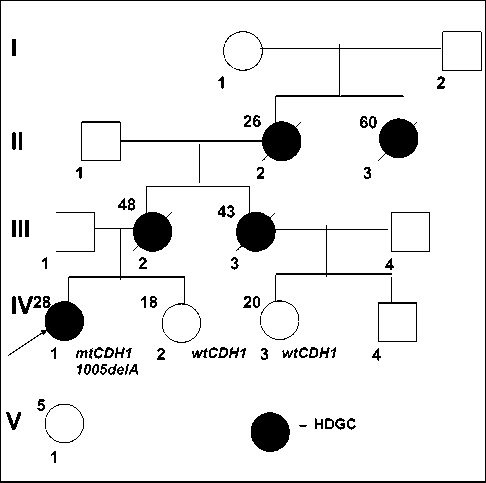
Figure 1. HDGC pedigree of proband. mtCDH1 – CDH1 1005delA mutation wtCDH1 – wild type СDH
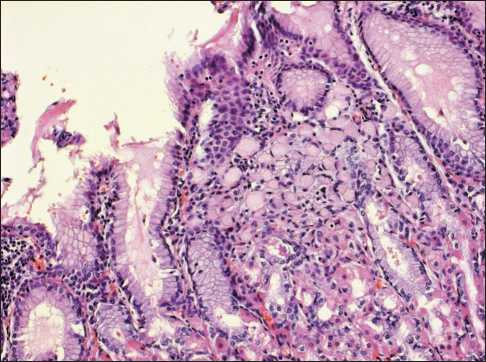
Figure 2. Signet ring cells carcinoma
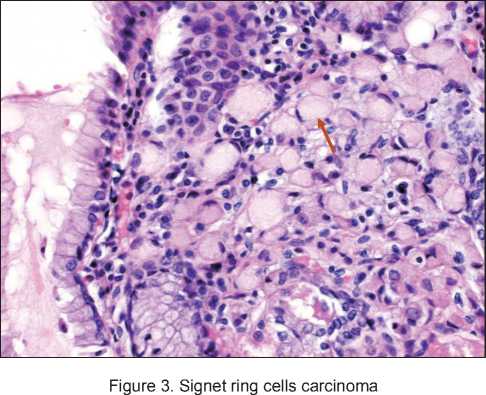
the 169 patients, the symptoms at the preoperative stage were absent in 106 (62.7 %) cases. Diagnosis of HDGC was confirmed in 21 (12.4 %) patients before operation, there were no data for 42 patients. According to postoperative histological examination, ring-cell cancer was detected in 147 (87 %) patients and it was not revealed in 17 patients only [21]. Similar data were obtained in a recent study that described postoperative outcomes of total gastrectomy in 41 patients. Thirty-five patients (85 %) demonstrated 1 or more foci of intramucosal signet ring cell gastric cancer in the examined specimen [22].
Laparoscopic total gastrectomy with jejunal pouch reconstruction as a novel approach that may be especially suitable in these patients have been performed by the surgeons from the Netherlands. A total of 11 patients with a median age of 40 (22–61) years were included. The 60-day mortality rate was 0 %. Multiple foci of intramucosal diffuse gastric signet ring cell carcinoma were found in the resection specimen of 9/11 (82 %) patients. All 11/11 (100 %) resections were microscopically radical. Thus, they proved that prophylactic laparoscopic total gastrectomy with jejunal pouch reconstruction in patients with a CDH1 germline mutation is feasible and safe [23]. In gastric cancer, laparoscopic total gastrectomy showed diminished blood loss, fewer postoperative complications, and shorter postoperative hospital stay [13]. This technique may therefore be especially suitable for prophylactic surgery [24].
This is a rare clinical case, when the diagnosis of HDGC was confirmed during routine EGDS with dynamic follow-up based on a family history and young age of disease onset in affected patient relatives.
However, the published data show that in most cases, ring-cell cancer remains undiagnosed even by repeated EGDS and biopsies. Two-year survival of the patients without any symptoms before gastrectomy was 100 %, it was 40 % among the patients with HDGC revealed before surgery [25].
High risk (>80 %) of HDGC occurrence, insufficient effectiveness of the regular EGDS and the proof of the microscopic foci with ring-shaped cells in all the CDH1 gene mutation carriers with family histories fully justifies preventive gastrectomy in this group [21, 25].
The optimal age of preventive gastrectomy in such patients is under discussion. The following aspects should be taken into account. Prophylactic gastrectomy is not warranted before the final body formation, at least, up to 20 years old. However, the patients with detected signet ring cell carcinoma at the age of about 40 years old, have only 10 % chance of the favorable disease course [26].
Mortality associated with preventive gastrectomy varies from 0 to 6 %. Mortality rate among young patients without symptoms before surgery is less than 1 %. Thus, prophylactic gastrectomy is recommended for carriers of CDH1 gene mutations with family accumulation of HDGC, taking into account the high disease risk during their life, late clinical detection and unfavorable prognosis.
Список литературы Hereditary diffuse gastric cancer: genetic aspects and prophylactic total gastrectomy
- Le Vecchia C., Negri E., Franceeschi S., Gentile A. Family history and risk of stomach and colorectal canser. Canser. 1992; 70: 50-58.
- Caldas C., Carneiro F., Lynch H.T., Yokota J., Wiesner G.L., Powell S.M., Lewis F.R., Huntsman D.G., Pharoah P.D., Jankowski J.A., MacLeod P., Vogelsang H., Keller G., Park K.G., Richards F.M., Maher E.R., Gayther S.A., Oliveira C., Grehan N., Wight D., Seruca R., Roviello F., Ponder B.A., Jackson C.E. Family gastric canser: overview and guidelines for management. J Med Genet. 1999; 36: 873-80.
- Fitzgerald R.C., Hardwick R., Huntsman D., Carneiro F., Guilford P., Blair V., Chung D.C., Norton J., Ragunath K., Van Krieken J.H., Dwerryhouse S., Caldas C.; International Gastric Cancer Linkage Consortium. Hereditary diffuse gastric cancer: updated consensus guidelines for clinical management and directions for future research. J Med Genet. 2010 Jul; 47(7): 436-44. DOI: 10.1136/jmg.2009.074237
- Guilford P., Hopkins J., Harraway J., McLeod M., McLeod N., Harawira P., Taite H., Scoular R., Miller A., Reeve A.E. E-cadgerin germline mutations in familiar gastric cancer. Nature. 1998; 392: 402-405.
- Corso G., Marrelli D., Pascale V., Vindigni C., Roviello F. Frequency of CDH1 germline mutations in gastric carcinoma coming from high- and lo-risk areas: metanalysis and systematic rewiew of the literature. BMC Canser. 2012; 2: 8.


