Histological and hormonal study of the protective effect of the Calotropis procera against the toxicity of mercury chloride
Автор: Belfarhi Leila, Chouba Ibtissem, Amri Naziha, Boukris Nadia, Tahraoui Abdelkrim
Журнал: Природные системы и ресурсы @ns-jvolsu
Рубрика: Экология и биология
Статья в выпуске: 2 т.10, 2020 года.
Бесплатный доступ
We undertook this study with the aim of investigating the detoxification of an extreme toxic metalmercury chloride by the Calotropis procera plant taken from the Algerian Sahara. We studied the protective effectsof the plant Calotropis procera against renal toxicity and Mercury chloride-induced hepatic. Ten male and femalealbino rats Wistar were divided into four equal groups. Group (I) served as a healthy control group, group (II) wereintra-peritoneal administered with 10 ml of Calotropis procera, group (III) were intra-peritoneal administrated withboth 10 ml of the plant Calotropis procera and 0.2 mg of mercuric chloride (HgCl2) and group (IV) were intra-peritoneal administrated with both 0.2 mg of mercuric chlorid (HgCl2) and 10 ml of the plant Calotropis. All groupswere treated for 20 days. Mercury chloride causes a slight increase in glomerular cellularitis in the kidneys of maleand female rats. Treatment with Calotropis procera had significantly improving protective effects of kidney offemale rats from toxicity of mercuric chloride. Calotropis procera causes a thyroid-like appearance in the glomeruliof the male kidneys to hide the lesions of mercury chloride. Our results have shown that the plant Calotropisprocera completely protects the liver of female rats against the toxicity of mercury chloride. In the liver of male rats,mercury chloride causes macro-vacuolar steatosis. Treatment with Calotrpois procera hid the hepatic steatosis ofmale rats and centralized them in the center under the aspect of peri-centro-lobular medio-vacuolar steatosis.Mercuric chloride caused a decrease in the secretion of the hormone ACTH in the group of male and female rats.Treatment with Calotropis procera caused increased ACTH levels in female rats and did not cause ACTH changesin male rats. Our results demonstrate from hormone analyzes of the hormone ACTH that female rats are resistantmore than male rats via the toxicity of mercury chloride.
Mercury, acth, histology, kidney, liver, glomerular cellularity, discrete lesion, thyroid-like condition
Короткий адрес: https://sciup.org/149131478
IDR: 149131478 | УДК: 582.923.6:581.6 | DOI: 10.15688/nsr.jvolsu.2020.2.1
Текст научной статьи Histological and hormonal study of the protective effect of the Calotropis procera against the toxicity of mercury chloride
DOI:
Heavy metals are toxic elements found in the environment, food, vaccines and in the area we breathe. The main sources of heavy metals are food and vaccines. The long-term risks of heavy metals are enormous. Studies have shown that aluminum-based vaccines are responsible for neurological deficits such as Alzheimer’s disease, the Guamanian variant, ALS – PDC and Autism Spectrum disorders in children [20]. Heavy metals are accumulated in the intestinal cells, perforating the mucous membranes. This causes the passage of all toxic elements, viruses, and food debris. Among the most toxic metals is mercury chloride. Its toxicity depends on several factors: its volatility, its viscosity and its speed. It is a metal that can cause poisoning even at low doses. It acts in a discreet and slow manner. Mercury chloride targets specific sites in the body. Studies on the toxicology of mercury chloride have shown that it accumulates in the vocal cords [16], the thyroid gland [25]. It acts on lipids and transforms them into glucose and energy. They activate the metabolism and increase the energy of the cells to weaken them afterwards. Studies have shown that it is able to alter the function of mitochondria in the liver of poisons [14].
The toxicity of mercury chloride also affects the liver and can lead to the fall of liver enzymes such as alanine aminotransferase, aspartate aminotransferase, γ -glutamyl-transferase, and lactate dehydrogenase [23]. Mercury chloride can also increase lipid peroxidation in the liver and kidney of female rats and decreases the antioxidant reserves such as glutathione [5]. It can also cause hepatic necrosis even at low doses [5]. Mercury chloride also accumulates in the kidneys and causes alterations which can lead to kidney failure.
Mercury sufferers are generally subjected to a drug treatment which is supposed to relieve the anomalies on the damaged organs and tissues but do not act directly on the disappearance of the toxicity and its effects. In other words, drugs cure illnesses that produce mercury but do not eliminate it. It is for this reason that new voices call to consider the potential effect of medicinal plants which act on mercury according to a rather reconstructive mechanism, in particular on tissue recovery and regeneration.
This article suggests testing the efficacy of the medicinal plant Calotropis procera on the toxicity of mercury from a histological study of the tissues of the kidneys of female rats suffering from the toxicity caused by mercury. The sample consists of sections obtained by a histological study on ten rats. The objective is to test the effectiveness of the plant in eliminating the toxicity of mercury on Wistar rats. This research aims to assess the protective effect of the plant Calotropis procera against the toxicity of mercury chloride.
Background. Detoxification of mercury chloride is accomplished by chelators like demercaptan; however, they have become ineffective due to the toxicity of mercury chloride. As a result, resorting to natural methods has become the solution to deal with mercury chloride poisoning. To detoxify the mercury chloride, it is necessary to use a more toxic plant similar to it. To do this, we have chosen the Calotropis procera plant. It is a plant which has adapted to the heat period. In Algeria, it grows in hot regions of the Sahara like Illizi, Adrare, Bechar and Tendouf. It is a plant which is not edible by animals. Camels detect its toxicity and flee from this plant. It is a plant characterized by the presence of a white liquid which circulates in all parts of the plant. This liquid is the latex of Calotropis procera. It contains cysteine proteins, rich in thiol groups, which have been widely implicated in the detoxification of heavy metals. It is a plant characterized by strong odor, giant green leaves and filled inside with white milk. This plant is characterized by the presence of several chemical components. It contains sweet components like cardinolides glycosides. It is even made up of energetic molecules like caffeine. The objective of this study is to seek new natural ways to decrease the toxicity of mercury chloride. This research also aims to decrease the toxicity of mercury chloride using this plant.
Methods and Material
Experimental animal design. Ten male and female albino rats Wistar weighting 250g were kept in the laboratory under constant conditions of temperature (24 ± 2°C) at one month before and through the experimental work, being maintained on a standard diet and water were ready ad-libitum. The experimental rats were being divided into four groups (see Table).
Sample collection. At the end of the experimental interval, the animals were kept fasting for 12 hours and the blood samples were collected of the retro-orbital venous plexus under diethyl ether anesthesia. The blood samples from each animal were added in tubes containing EDTA and plasma was separated after centrifugation at 4000 rpm for ten minutes at 5°C. The clear plasma samples were stored at -20°C until analysis. After blood collection, all animals were sacrificed by cervical dislocation and liver and kidney of each rat was rapidly excised, washed in isotonic saline, then cut into small pieces (0.5 x 0.5 cm) and fixed in 10 % formalin saline solution for histological examination.
Hormonal analysis . Basal ACTH plasma concentrations were determined by the “IMMULATE / ACTH” kit using the minarine method.
Histopathological examination. After fixation of kidneys and liver tissues in 10% saline buffered formalin, the kidneys and liver tissues were dried in ascending grades of ethanol, cleared in xylol, and then immersed in paraffin. Impregnated kidneys and liver tissues were treated three times in pure paraffin to be established in blocks. Sections (5 µm thick) were preparatory using Leica microtome and stained by hematoxylin and eosin (H&E) for histopathological investigation [4].
Statistical analysis. In the present study, the results were analyzed using the ANOVA statistics to compare the significance between the groups. The results of comparison between the groups are significant if P < 0.05.
Results of the Histological Study
Effect of mercury chloride on variations in the ACTH level in male rats of the Wistar strain. The administration of mercury chloride intraperitoneally at 0.20 mg / kg bodyweight over a 20-day period caused a decrease in the secretion of the hormone ACTH in the group of male rats. According to our results, chloride has been found to cause a decrease in ACTH secretion in male rats. Our results have demonstrated that there is significant difference between the batches of rats treated with mercury chloride, the plant, Plant + mercury, Mercury + Plant. The p-values 0.006092. The result is significant at p < 0.05 (see Fig. 3).
Effect of mercury chloride on variations in the ACTH level in female rats of the Wistar strain. The administration of mercury chloride intraperitoneally at 0.20 mg / kg bodyweight over a 20-day period caused a decrease in the secretion of the hormone ACTH in the group of female rats. ACTH levels have been found to increase in groups treated with Calotropis procera, Calotropis procera + mercury, mercury + Calotropis procera. Our results have shown that there is an insignificant difference between the batches treated with mercury chloride. The p-value is 0.00001. The result is significant at p < 0.05 (see Fig. 3).
Discussion. The microscopic study of the kidneys of female rats treated with mercury chloride demonstrated a normal renal parenchyma with slight increase in glomerular cellularity (see Fig. 1). This observation shows that the mercury chloride caused a discreet lesion in the glomerulus and for this reason, the renal parenchyma appeared normal. Studies have found the same observations as our study in malaria infestations
Experimental animal design
|
Groups of Rats |
Number of rats |
Treatment |
|
Group 1 |
6 |
Healthy control group received distilled water for 20 days |
|
Group 2 |
6 |
The animals received 10 ml of the Calotropis procera plant by intra-peritoneal injection |
|
Group 3 |
6 |
The animals were treated by intraperitoneal injection with 10 ml of the plant Calotropis procera and 0.2 mg of mercury chloride (Plant + mercury chloride) |
|
Group 4 |
6 |
The animals were treated intra-peritoneal injection with 0.2 mg of mercury |
|
Group 5 |
6 |
The animals treated intra-peritoneal injection with 0.2 mg of mercury chloride and 10 ml of the plant Calotropis procera |
in mice. They observed that the kidneys are of normal structure. In parallel, they observed discreet histological signs of glomerular involvement (slight increase in cellularitis) and discreet infiltration of inflammatory lymphocytic cells [6]. Mice infection with malaria leads to an increase in cellularity and the number of macrophages [1].
The cellularity observed during malaria infection is due to the accumulation of erythrocytes infected by the parasite plasmodium chabaudi in macrophage [18] these macrophages release the factor inhibiting the migration of macrophages (MIF)involved in the onset of chronic inflammatory diseases.
The catabolism of erythrocyte hemoglobin by malaria produce an insoluble pigment, “hemozoin that increase cellularity and the number of macrophages [19]. The accumulation of hemozoin has consequences for the activities and functions of macrophages. The latter makes the macrophages unable to degrade the ingested material and repeat the phagocytic cycle. This action seriously and permanently damages the macrophages [3].
Based on our result the toxicity of mercury chloride is manifested by a discrete lesion in the glomerulus, normal renal parenchyma and increased glomerular cellularity. Our finding are consistent with study in malaria infestations in mice how observed that the kidneys are of normal structure. In parallel, they observed discreet histological signs of glomerular cellularity. There are also studies, which have found that heavy metals act by a mechanism similar to that observed during malaria infection. For example, Margot Christensen et all [3] how observed that mercuric chloride accumulates in macrophages and causes increased cellularity. It causes from low concentration the death of macrophages from
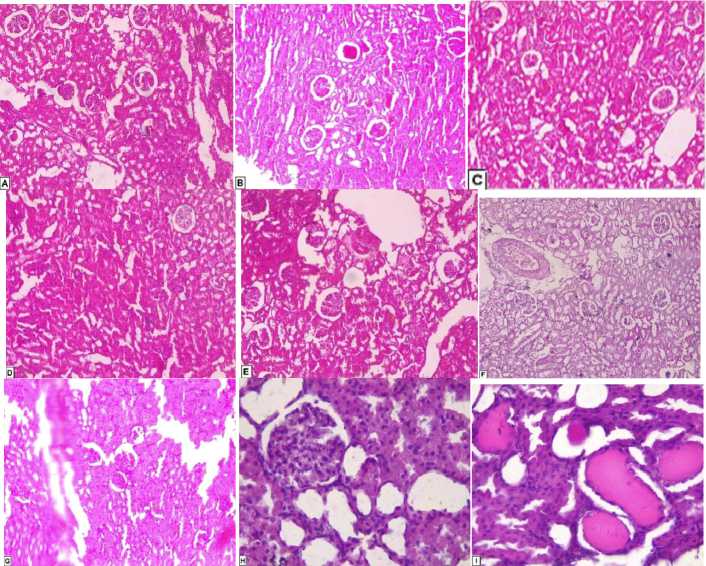
Fig. 1. Rat Histological Study of Kidney:
A – Histological section of the female rat kidney control, normal renal parenchyma; B – Histological section of the rat kidney mercury, normal renal parenchyma and slight increase in glomerular cellularity; C – Histological section of the female rat kidney plant + mercury, normal renal parenchyma; D – Histological Section of the Female Rat Kidney Mercury + Plant, Normal renal parenchyma; E – Histological section of the female rat kidney plant, normal renal parenchyma;
F – Histological Section of the Rat Male Control Kidney, Normal renal parenchyma; G – Histological Section of the Male Rat Mercury, Slight increase in glomerular cellularitis, renal vascular congestion; H – Histological Section Rat Male Mercury + Plant, Normal renal parenchyma. Slightly ischemic glomeruli. Very focal (limited) pseudo-thyroid aspects;
I – Histological section male rat mercury, slight increase in glomerular cellularitis, renal vascular congestion
these low levels of mercury chloride and that it causes the death of macrophages by its action on their migration and their phagocytosis capacity.
There are also studies, which have also found that heavy metals act by mechanism similar to that observed during malaria infection. For example, the aluminum poisoning causes an increase in the number of macrophages and cellularity in the brain and causes the macrophagicmyophac it is MEC [21]. Gérardie and Authier [9] have found that rats intoxicated by aluminum accumulate insoluble pigment osmiophilic crystals in their macrophages.
Microscopic analysis of kidney tissue of male rats treated with mercury chloride and the plant Calotropis procera, demonstrated a normal renal parenchyma. Slightly ischemic glomeruli with a focal pseudo-thyroid aspect (limited) (see Fig. 2).
These observations show that the glomeruli have taken on the structure of the thyroid gland. It can be explained by saying that mercury chloride accumulates in the thyroid gland and the vocal cords and by this action; it blocks the thyroid gland and the vocal cords (the sense). Calotropis procera released the vocal cords and sound. By this action, it created a cynicism or vibration, which caused the displacement of the cells of the thyroid gland with mercury chloride towards the renal glomeruli. Calotropis procera
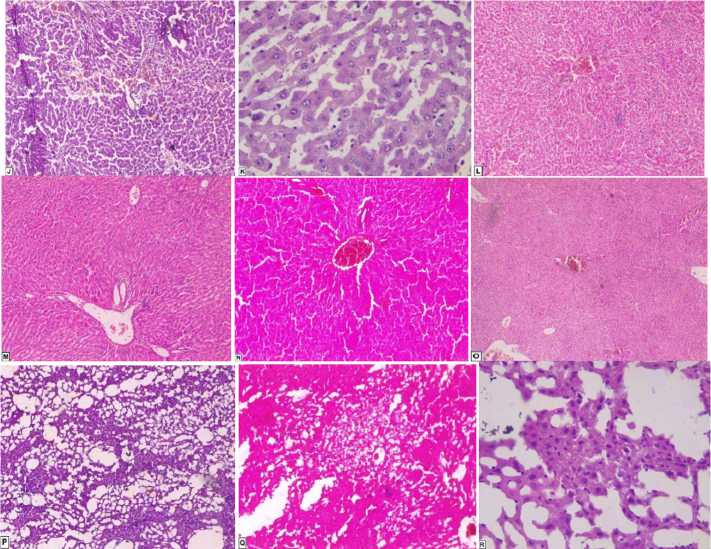
Fig. 2. Rat histological study of liver:
J – H+istological section of female rat control, hepatic parenchyma of subnormal morphology. Vascular congestion. Photo centered on a door space; K – Histological section of female rat treated with mercury chloride. Hepatic parenchyma of subnormal morphology. Dilation of the hepaticsinusoids + Vascular congestion (including for the door spaces);
L – Histological Section Female Rat Treated with Mercury Chloride and the Plant, Hepatic parenchyma of subnormal morphology. Vascular congestion. Artifacts related to a fixation deficit; M – Female rat treated with the plant and mercury chloride: hepatic parenchyma of subnormal morphology. Vascular congestion. Artifact srelated to a fixation deficit;
N – Rat Female Treated with the Plant: Hepatic parenchyma of subnormal morphology. Vascular congestion;
O – Histological section male rat control (liver), hepatic parenchyma of subnormal morphology; P – Histological section of male rat treated with mercury chloride, the study of a histological section of the liver in rats treated with mercury chloride shows a diffuse macro-vacuolar steatosis; Q – Histological section male rat treated with the plant+mercury chloride, Foci of peri-centro-lobular medio-vacuolar steatosis. Dilation of the hepatic sinusoids + congestive aspects.
We note the presence of foci of medio vacular steatosis localized pericentrolobulaire. Discreet peri-centro-lobular mid-vacuolar steatosis. The plant reduced the steatosis in foyes located in the center. Besides, the plant protected the male rat against steatosis; R – Histological section male rat treated with mercury chloride + plant, macro-vacuolar and somewhat micro-vacuolar, diffuse steatosis
has caused the displacement and detachment of mercury chloride. Recent studies [12] have shown that mercuric chloride has a great affinity for thiol groups. Calotropis procera is a source of thiols protease which are known for their chelating action of heavy metals thanks to their richness in sulfur component. It is thanks to these molecules that Calotropis procera was able to fix and displace mercury chloride [11].
The thyroid-like aspect which appeared in the glomeruli of rats treated with Calotropis procera shows that the plant Calotropis procera has an activity which resembles to the activity of the thyroid gland. The Calotropis procera plant contains molecules that have a relationship with the thyroid gland. Rutinis one of the constituents of the Calotropis procera plant and which seems to stimulate thyroid function by absorption of thyroid iodide and the synthesis of T3, T4 hormones [10]. Stigmasterol is also a major constituent of the Calotropis procera plant, which has a relationship with the thyroid gland. For example, studies have shown that stigmasterol are duce serum level so triiodothyronine (T3), thyroxine (T4) indicating its thyroid-inhibiting properties. This shows that this molecule Stigmasterol from Calotropis procera has similar activity in the thyroid gland [15].
Histopathological investigation of kidney tissue sections of male Wistar rats treated with the plant Calotropis procera and mercury chloride shows that the renal glomeruli are of normal structure. Our results show that the Calotropis procera plant protected the kidneys of male rats against the toxicity of mercury chloride. The protective effect of the plant Calotropis procera is due to its richness in polyphenol components. The latter are known by its protective action of the kidneys [24].
Microscopic examination of section of the liver of male rats treated with mercury chloride shows diffuse macro-vacuolar steatosis. Our results have shown that the toxicity of mercury chloride manifests itself in the liver through diffuse macro-vacuolar steatosis. This shows that mercury chloride acts by a mechanism, which increases the release of lipids. These results agreement with research published, who notify hat mercury chloride causes fatty liver disease in adults [2]. The toxic effects of mercury chloride on the liver have been explained by other studies [8] that mercury chloride have an action on the metabolism of thiols. It binds with free sulfhydryl groups, which displaces it towards plasma membranes and causes lipid solubility. Eusebio Mayz, J.W. Daniel [7] have found also that mercury chloride causes fatty liver disease through a mechanism that involves the hormone ACTH. It promotes lipolysis through the inhibition of the hormone ACTH activity.
Microscopic investigation of section liver tissue of male rats treated with the plant Calotropis procera and the mercury chloride upstream that the plant Calotrpois procera hid the steatoses and centralized them in the center under the aspect of mid-vacuolar steatosis periCentro- lobular.
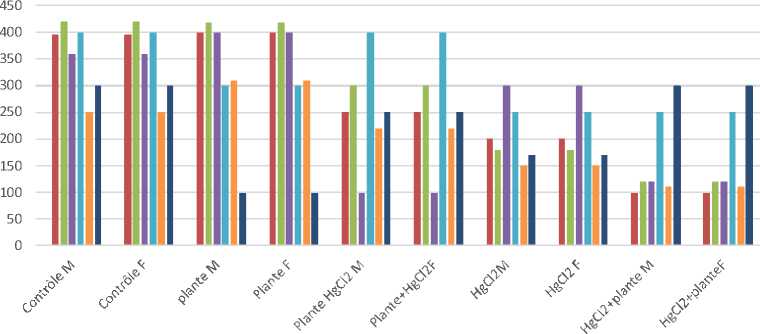
Fig. 3. Variation in the Adreno Cortico-Trophic Hormone ACTH in male and female rats treated with the plant Calotropis procera; Calotropis procera plant + mercury chloride, mercury chloride + Calotropis procera plant. NS (significant difference P < 0, 05)
Our results show that the plant hides the lesions of mercury chloride which appeared in the liver in the form of diffuse macro-vascular steatosis. On the other hand, it has reduced the extent of steatosis. According to histological sections, it has been found that in the groups of rats treated only with mercury steatosis is diffuse while in the group treated with plant and mercury, the steatosis is discreet and centro-lobular. Our results have shown that the plant reduced steatosis in foci located in the center. The plant hides these steatoses and could protect the male rat against fatty liver. Our results have shown that the treatment of male rats with mercury chloride as first treatment and afterwards by the plant caused macro-vacuolar steatosis and a little micro-vacuolar, diffuse. This shows that the plant protects the liver of male rats when it was injected as the first treatment. Our results then demonstrate that the Calotropis plant will have a protective effect on the liver against the toxicity of mercury chloride. A study also reported that the plant Calotropis procera contains flavonoids, which can decrease liver damage due to paracetamol [17].
The histological study of the liver of female rats treated with mercury chloride and the plant Calotropis procera showed a liver parenchyma of subnormal morphology. This shows that the Calotropis plant will fully protect the liver of female rats. “The results of this study show that the plant Calotropis procera protected the kidneys of male rats and protected the liver of female rats.”
Results of the analysis of the hormone ACTH have shown that the plant Calotropis procera causes an increase in the level of ACTH in female rats. Eusebio Mayz, J.W. Daniel [7] have found that mercury chloride causes the appearance of fatty liver disease by a mechanism that involves the hormone ACTH. It promotes lipolysis through the inhibition of the hormone ACTH activity. Other studies have found a significant association between exposure to heavy metals (arsenic, mercury, cadmium, chromium, copper, nickel, lead, and zinc) of soil and fatty liver disease in men. They found that men were significantly more likely to have severe fatty liver disease than women [13]. The results of our work show that the liver of female rats is of subnormal structure and that the plant increased the rate of ACTH in female rats. This shows that the plant Calotropis procera has molecules, which have increased in female rats their ability to resist the toxicity of mercury chloride via the hormone ACTH. Cardiac glycosides are among the main components that characterize the plant Calotropis procera. Two cardiac glucosides, cycloartans: Neocimicigenosides A (1) and B (2) have been isolated from the plant Cimicifuga racemose that it increases the secretion of the hormone ACTH [22].
Conclusion
This study sheds light on a plant called Calotropis procera and its detoxifying effect on mercury chloride. Our study unveiled a new mechanism for detoxifying a metal as strong and toxic as mercury chloride. This study brings a new understanding in the detoxification of mercury chloride using the plant Calotropis procera. The salts obtained in the is study showed that the plant Calotropis procera imprinted a new path of detoxification of mercury chloride; Calotropis procera caused the detachment of the mercury chloride to shift. It moved the mercury chloride from the thyroid gland to the kidneys. At this level, it changed the shape of the glomeruli to the shape of the thyroid gland. By this form, it hides the lesions with mercury chlorides to stop the inflammatory reactions of female rats. The plant protected the kidneys of male rats. In the liver the Calotropis, the plant also hides the steatosis of the liver of male rats. The plant protected the liver of female rats. This plant protected the kidneys of male rats and it protected the liver from female rats.
Список литературы Histological and hormonal study of the protective effect of the Calotropis procera against the toxicity of mercury chloride
- Akinosoglo K.S., Solomou E.E., Gogos C.A. Malaria: a Hematological Disease. Hematology, 2012, vol. 17, no. 2, pp. 106-114. DOI: https://doi.org/10.1179/ 102453312X13221316477336.
- Cave M., Appana S., Patel M., et al. Polychlorinated Biphenyls, Lead, and Mercury are Associated with Liver Disease in American Adults: NHANES 2003-2004. Environ Health Perspect, 2010, vol. 118, no. 12, pp. 1735-1742. DOI: https://doi.org/ 10.1289/ehp. 1002720.
- Christensen, M., Mogensen, S.C., Rungby, J. Toxicity and Ultrastructural Localization of Mercuric Chloride in Cultured Murine Macrophages. Arch Toxicol, 1988, vol. 62, no. 6, pp. 440-446. DOI: https:// doi.org/10.1007/BF00288347.
- Dardouri K., Haouem S., Gharbi I., et al. Combined Effects of Cd and Hg on Liver and Kidney Histology and Function in Wistar Rats. Journal of Agricultural Chemistry and Environment, 2016, vol. 5, no. 5, pp. 159-169. DOI: http://dx.doi.org/10.4236/ jacen.2016.54017.
- Deepmala J., Sangeeta S., Srivastav A.K., Mercury Toxicity and its Treatment Options. Lambert Academic Publishing, 2013. 64 p.
- Dûment M.E., Maurois P., Annales de Parasitologie Humaine et Compare. Masson, 1988.
- Eusebio M., Daniel J.W. Mercury Poisoning: II. New York, MSS Information Corporation, 1973. 293 p.
- George J.M. Effect of Mercury on Response of Isolated Fat Cells to Insulin and Lipolytic Hormones. Endocrinology,1971, vol. 89, no. 6, pp. 1489-1498.
- Gérardie R., Authier F. J. Caractérisation et Nouvelles Pistes Physiopathologiques. Kinésithérapie la Revue, 2008, vol. 8, no. 79, pp. 22-30. DOI: https:// doi.org/10.1016/S1779-0123(08)70606-5.
- Gonçalves C.F., Santos M.C., Ginabreda M.G., et al. Flavonoid Rutin Increases Thyroid Iodide Uptake in Rats. PLoSOne, 2014, vol. 9, no. 1, p. e73908. DOI: https://doi.org/10.1371/journal.pone.0073908.
- Kwon C.W., Park K-M., Kang B-Ch., et al. Cysteine Protease Profiles of the Medicinal Plant Calotropis Procera R. Br. Revealed by De Novo Transcriptome Analysis. PLoS One, 2015, vol. 10, no. 3, p. e0119328. DOI: https://doi.org/10.1371/ journal.pone.0119328.
- LaVoie S.P., Mapolelo D.T., Cowart D.M., et al. Organic and Inorganic Mercurials Have Distinct Effects on Cellular Thiols, Metal Homeostasis, and Fe-Binding Proteins in Escherichia Coli. J. Biol. Inorg Chem, 2015, vol. 20, no. 8, pp. 1239-1251. DOI: https:// doi.org/10.1007/s00775-015-1303-1.
- Lin Y.C., Lian I.B., Kor C.T., et al. Association Between Soil Heavy Metals and Fatty Liver Disease in Men in Taiwan: a Cross Sectional Study. BMJ Open, 2017, vol. 7, no. 1, p. e014215. DOI: https://doi.org/ 10.1136/bmjopen-2016-014215.
- Mieiro C.L., Pardal A.M., Duarte A.S. Impairment of Mitochondrial Energy Metabolism of Two Marine Fish by in Vitro Mercuric Chloride Exposure. Mar Pollut Bull, vol. 15, no. 97 (1-2), pp. 488-493. DOI: https://doi.org/10.1016/j.marpolbul.2015.05.054.
- Panda S., Jafri M., Kar A., Meheta B.K. Thyroid Inhibitory, Antiperoxidative and Hypoglycemic Effects of Stigmasterol Isolated from Butea Monosperma. Fitoterapia, 2009, vol. 80, no. 2, pp. 123-126. DOI: https://doi.org/10.1016/ j.fitote.2008.12.002.
- Rahul K.S. Patient Safety and Quality Improvement Otolaryngologic Clinics of North America. The Clinics: Surgery 52-1. Amsterdam, Elsevier Health Science, 2018. 240 p.
- Ramachandra Setty S., Quereshi A.A., Viswanath Swamy A.H., et al. Hepatoprotective Activity of Calotropis Procera Flowers Against Paracetamol-Induced Hepatic Injury in Rats. Fitoterapia, 2007, vol. 78, no. 7-8, pp. 451-454. DOI: https://doi.org/10.1016/j.fitote.2006.11.022.
- Rosado Jde. D. Rodriguez-Sosa M. Macrophage Migration Inhibitory Factor (MIF): a Key Player in Protozoan Infections. Int J Biol Sci, 2011, vol. 7, no. 9, pp. 1239-1256. DOI: https://doi.org/ 10.7150/ijbs.7.1239.
- Schwarzer E., Turrini F., Ulliers D., et al. Impairment of Macrophage Functions After Ingestion of Plasmodium Falciparum-Infected Erythrocytes or Isolated Malarial Pigment. J ExpMed, 1992, vol. 176, no. 4, pp. 1033-1041.
- Shaw C. A., Li Y., Tomljenovic L. Administration of Aluminium to Neonatal Mice in Vaccine Relevant Amounts is Associated with Adverse Long Term Neurological Outcomes. J Inorg Biochem, 2013, no. 128, pp. 237-244. DOI: https://doi.org/10.1016/ jjinorgbio.2013.07.022.
- Guria S., Alteration of Cytomorphology of Peritoneal Macrophages of Albino Rat Exposed to Mercuric Compound. World scientific News, 2018, vol. 92, no. 2, pp. 392-399.
- Su Y., Chi W.C., Wu L., et al. Photochemistry and Pharmacology of 9, 19-Cyclolanostane Glycosides Isolated from Genus Cimicifuga. Chin J Nat Med, 2016, vol. 14, no. 10, pp. 721-731. DOI: https://doi.org/ 10.1016/s1875-5364(16)30087-5.
- Uzunhisarcikli M., Aslanturk A., Kalender S., et al. Mercuric Chloride Induced Hepatotoxic and Hematologic Changes in Rats: The Protective Effects of Sodium Selenite and Vitamin E. Toxicol and Health, 2016, vol. 32, no. 9, pp. 1651-1662.
- Wongmekiat O. , Leelarugrayub N. , Thamprasert K. Beneficial Effect of Shallot (Allium ascalonicum L.) Extract on Cyclosporine Nephrotoxicity in Rats. Food Chem Toxicol, 2008, vol. 46, no. 5, pp. 1844-1850. DOI: https://doi.org/ 10.1016/j.fct.2008.01.029.
- Zehra R., Carpenter D.O., Fatmi S.S. Pathophysiological Mechanisms of Mercury's Effect on Thyroid Gland. International Journal of Thyroid Disorder and Therapy, 2018, vol. 1, no. 1, pp. 1-6.


