Hydrogen peroxide and phenylalanine ammonialyase as signalling molecules in barley leaves challenged with Cochliobolus sativus
Автор: Al-Daoude Antonious, Jawhar Mohammed, Al-Shehadah Eyad, Shoaib Amina, Arabi Mohammed Imad Eddin
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 2 т.18, 2022 года.
Бесплатный доступ
Hydrogen peroxide (H2O2) and phenylalanine ammonia-lyase ( PAL ) have been reported as important signaling molecules during plant resistance against many fungal pathogens. In this study, the relative contributions of H2O2 and PAL were investigated at early time periods of barley infection with Cochliobolus sativus , the causal agent of spot blotch disease. H2O2 activity was observed in leaf tissues 24 hours post inoculation (hpi) and was accompanied with an increase in PAL expression in resistant and susceptible genotypes. However, the resistant genotype ‘Banteng’ contained higher levels of H2O2 and PAL , as compared with the susceptible one ‘WI 2291’. Results demonstrated that the cooperative function of H2O2 and PAL in barley responses to C. sativus appeared to be dependent on the plant genotype, and it is hypothesized that the peak of activity of PAL at 48h and 72 h, and the rapid increase in H2O2 24 h in resistant and susceptible genotypes are considered general defense responses.
Hordeum vulgare, cochliobolus sativus, h2o2, pal expression, real time pcr
Короткий адрес: https://sciup.org/143178805
IDR: 143178805
Текст научной статьи Hydrogen peroxide and phenylalanine ammonialyase as signalling molecules in barley leaves challenged with Cochliobolus sativus
Spot blotch (SB) caused by the necrotrophic [ Cochliobolus sativus ( Cs ) Drechs. ex Dastur] is a globally distributed disease of barley ( Hordeum vulgare L.) causing significant yield losses (Rehman et al., 2020). Typically, SB is characterized by discrete necrotrophic spots that extend beyond vascular bundles (Al-Sadi, 2021). Yield loss is mainly due to reductions in kernel size and weight (Kumar et al., 2002). The development of new barley resistant cultivars is the best method for the successful control of this disease (Gupta et al., 2001; Leng et al., 2016), however, the basis of fungal resistance strategies and the early barley responses towards SB are still not clear.
Barley plants respond to SB by activating different mechanisms that are regulated through various plant signaling pathways. Hydrogen peroxide (H 2 O 2 ) is an important reactive oxygen species (ROS) molecule that serves as a signal of oxidative stress and activation of signaling pathways as a result of the early response of the plant towards fungal pathogens attack (Juan et al., 2021). H 2 O 2 is engaged in genes encoding to phenylalanine lyase ( PAL ), which are extensively used as indicators of ROS responsive and oxidative stress specific signaling (Sahebani et al., 2009).
The activation of phenylpropanoids under different stress factors has lead to be used as genetic markers for the initiation of plant defense responses. PAL catalyzes the first step in phenylpropanoid biosynthetic pathway (Yadav et al., 2020), and is involved in the synthesis of plant secondary antimicrobial substances, that are essential for plant defense responses (Kim et l., 2014) and also in the biosynthesis of salicylic acid which is an essential signal engaged in plant systemic resistance (Chaman et al. , 2003).
Reverse transcription-polymerase chain reaction (RT-PCR) is the most reliable technique for measuring the relative expression level of a particular transcript and determining its expression after exposure to a specific alteration, such as infection by a fungal pathogen (Derveaux et al., 2010). In the current work, we studied defense responses of two barley genotypes Banteng and WI 2291, which are integrated in international breeding programs aimed at developing C. sativus resistant barley genotypes. Banteng was described as highly resistant to SB (Arabi and Jawhar 2003; 2004), i.e. exhibited a lower level of symptom development as compared with WI2291.
To complete the picture of barley defense responses drawn (Al-Daoude et al., 2013), the present work investigates possible changes of endogenous H 2 O 2 and PAL expression after barley infection with C. sativus at different time intervals.
MATERIALS AND METHODS
Plant material
The most resistant German cv. Banteng and the universal Australian susceptible control cv. WI2291 to SB (Arabi and Jawhar, 2003) were used in the experiments. Plants were grown in flats filled with sterilized peatmoss with three replicates, and 10 seedlings per experimental unit. They were placed at temperatures 22°C (day) and 18°C (night) with in a 12-h light / 12-h dark cycle and relative humidity (RH > 90%).
Infection with C. sativus
The highly C. sativus virulent pathotype Pt4 described by Arabi and Jawhar (2004) was used. The fungus was grown in Petri dishes containing potato dextrose agar (PDA, DIFCO) under 20±°C in the dark for 10 days. Seedlings were inoculated with suspension of conidia 2 x 104 conidia/mL using a hand-held sprayer. C. sativus inoculum preparation, inoculation, postinoculation and disease records were similar to those described by Fetch and Steffenson (1999).
Detection of H2O2
H2O2 was detected in barley leaves using 3,3-diaminobenzidine (DAB) according to the protocol described by Thordal-Christenssen et al. (1997). The stained samples were examined using a fluorescence microscope (Olympus-ix21 station, X400, Japan). H2O2 was localized due to dark blue coloration in the periplasmic space of the cells. Observations were made for 25 infection sites per leaf sample collected from four to six inoculated plants. Dark-brown zones indicated the presence of H2O2. Hydrogen peroxide was measured using the titanium tetrachloride precipitation method as described by Brennan and Frenkel (1977) at 0, 24, 48 and 72 hpi.
RNA isolation and PAL expression
Barley primary leaves were collected 0, 24, 48 and 72 hpi and homogenized with a tube pestle in liquid nitrogen. mRNA was extracted from frozen samples using Nucleotrap mRNA mini kit (Macherey-Nagel, MN, Germany). The QuantiTect Reverse Transcription Kit (Qiagen) was used for cDNA synthesis. The control samples were collected from the non- inoculated plants at the same time points. PAL expression was evaluated using SYBR Green Master kit (Roche, USA) in Step One system. PAL primers (Table 1) were designed based on NCBI database. The threshold cycle (Ct) value was determined by the real time PCR system. Seedlings inoculated with distilled water served as a control. Average Ct values were calculated from the three replications, with the ΔCT value determined by subtracting the average Ct value of genes from the Ct value of the EF1α gene and the equation 2-ΔΔCT was used to determine the expression levels (Livak and Schmittgen, 2001). Statistical analysis was conducted by Tukey's test at the 0.05 significance level. Values were presented as mean ± standard deviation.
RESULTS AND DISCUSSION
In this study, two barley genotypes with different reaction levels to C. sativus were used. As shown in Figure 1, SB severity was more in the highly susceptible genotype WI9921 compared with the resistant cv. Banteng. The results are in line with our previous observations under natural field conditions (Arabi and Jawhar, 2004).
Data showed that H2O2 production rate increased sharply in the resistant cv. Banteng, which got the high level of 04.5 µmol g-1 FW min-1 at 24 hpi, then reached to maximum level 09.7 µmol g-1 FW min-1 at 72 hpi. By contrast, in the susceptible cv. WI 2291 , H2O2 increased slowly within the first 24 h and then increased gently to the peak of 0.3.3 µmol g-1 FW min-1 at 72 hpi (Fig. 2). This might explain the high resistance level of cv. Banteng against SB disease, since it exhibited an early and quick “first oxidative burst”, likely inducing disease resistance mechanisms to set in. To further confirm this event, we examined the H2O2 accumulation in the two barley genotypes. Interestingly, early H2O2 accumulation in leaf tissues was detected in both genotypes, however, the resistant cultivar constitutively contained higher levels of H2O2 thanthe susceptible one (Fig. 2).
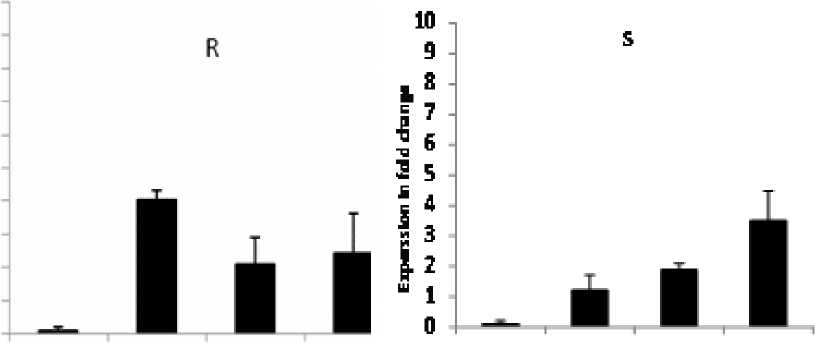
On the other hand, results demonstrated that PAL expression in the resistant cv. Banteng was higher during different time points post inoculation, and its expression increased sharply 24 hpi, by 4.2 fold increases, then decreased to basal levels 72 hpi. In the case of cv. WI 2291, there was a slower increase in PAL expression until 72 hpi (Fig. 3). This might be due to the formation of a rapid signaling from hydrogen peroxide for activating PAL , which catalyzes the first step in phenylpropanoid biosynthetic pathway against C. sativus infection. This step is considered to be very important as regulation point between primary and secondary metabolism ( Vogt, 2010 ) which may be the cause of barley cell wall leakage during C. sativus infestation.
However, there have been contradictory results in relation to the association between H 2 O 2 and PAL gene induction. Delledonne et al. (1998) reported that H 2 O 2 was not a signal for PAL activation in soybean cell suspensions, while in Arabidopsis and tobacco cultured cells, H 2 O 2 prompted PAL gene expression (Desikan et al., 1998). Our results might hypothesize that the peak of activity of PAL at 48h and 72 h, and the rapid increase in H 2 O 2 at 24 h in both resistant and susceptible genotypes are general defense responses.
It is well documented that in barley leaves, C. sativus spores that contact the leaves surface germinate within few hours and produce appressoria from which penetration hyphae breach the leaf cuticle. We hypothesize that the C. sativus infection point will subsequently become the local source for ROS production that will activate the phenylpropanoid pathway, preserve or increase the levels of the antifungal compounds, which inhibit fungal development (Kumar et al., 2002).
Table-1 Sequences of used primers
|
Gene Gene description |
Accession Amplified Sequence No. fragment (bp) |
|
PAL Phenyl alanine amino lyase |
CCATTGATGAAGCCAAAGCAAG AT2G14610 123 ATGAGTGGGTTATCGTTGACGG |
|
Elongation foctor-1 EF1α Alapha |
GGCTGATTGTGCTGTGCTTA CV066174 153 TGGTGGCATCCATCTTGTTA |
10 9
№ j 7 з б e = 5 i 4
S 2
1 0
Oh ?4h JRh 7>h un "’’ 40,1 ^п
IbnefHcu^
Figure 3. Relative PAL expression profiles in the resistant cv. Banteng (R) and in the susceptible cv. WI2291(S) during the time course following C. sativus infection. Error bars are representative of the standard error (Mean ± SD, n = 3). Data are normalized to Elongation factor 1α (EF-1α) gene expression level (to the calibrator, Control 0 h, taken as 1.00).
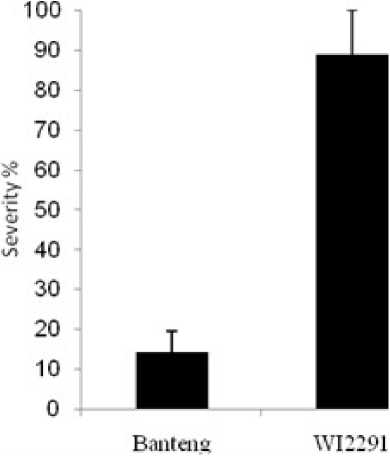
Figure 1. Disease reactions of barley (a) resistant cv. Banteng and (b) susceptible cv. WI2291 towards C. sativus pathogen
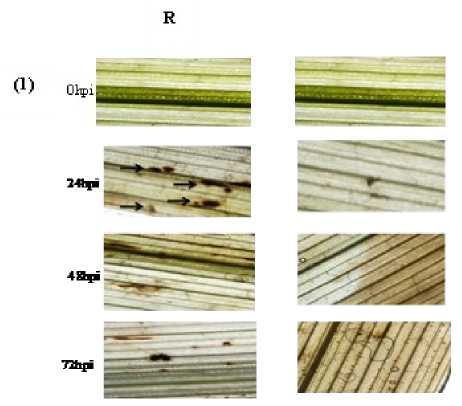
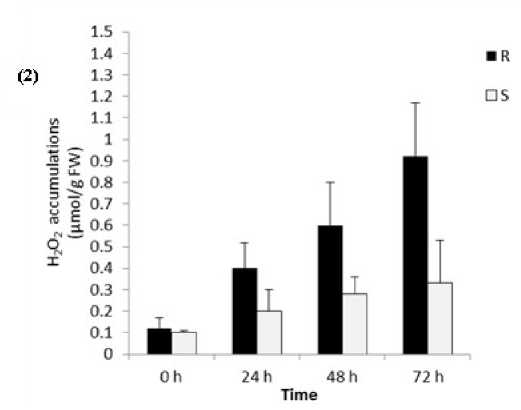
Figure 2. (1) Localization of H2O2 and (2) its changes in tissues of barley leaves; (R) resistant cv. Banteng and (S) susceptible cv. WI2291 after different time points of inoculation with C. sativus .
CONCLUSIONS
This work sheds some light on the relative contributions of H 2 O 2 and PAL during C. sativus – barley interactions. Results showed that their contribution to the resistance response appears to depend on the plant genotype. We can hypothesize that the peak of activity of PAL at 48h and 72 h, and the rapid increase in H 2 O 2 at 24 h in both resistant and susceptible genotypes are general defence responses. However, since SB-infection caused spreading cellular H2O2accumulation and increased PAL expression, thus, H2O2 could be the common inductive factor regulating this gene through still unidentified signal transduction pathway.
ACKNOWLEDGEMENTS
The authors thank the Director General of AECS and the Head of Molecular biology and Biotechnology Department for their much appreciated help throughout the period of this research.
CONFLICTS OF INTEREST
The authors declare that they have no potential conflicts of interest.
Список литературы Hydrogen peroxide and phenylalanine ammonialyase as signalling molecules in barley leaves challenged with Cochliobolus sativus
- Al-Daoude A, Jawhar M, Arabi MIE. 2013. Hydrogen peroxide induction in barley-Cochliobolus sativus interaction. J. Plant Pathol., 95: 197-199.
- Arabi MIE, Jawhar M. 2003. Pathotypes of Cochliobolus sativus (spot blotch) on barley in Syria. J. Plant Pathol., 85: 193-196
- Arabi MIE, Jawhar M. 2004. Identification of Cochliobolus sativus (spot blotch) isolates expressing differential virulence on barley genotypes in Syria. J. Phytopathol., 152: 461-464.
- Al-Sadi AM. 2021. Bipolaris sorokiniana-induced black point, common root rot and spot blotch diseases of wheat: A review. Front. Cell. Infect. Microbiol., 11: 584899.
- Brennan T, Frenkel C. 1977. Involvement of hydrogen peroxide in the regulation of senescence in pear. Plant Physiology 59: 411-416.
- Chaman ME, Copaja SV, Argandona VH. 2003. Relationships between salicylic acid content, phenylalanine ammonia-lyase (PAL) activity, and resistance of barley to aphid infestation. J. Agri. Food Chem., 51: 2227-2231.
- Delledonne M, Xia YJ, Dixon RA, Lamb C. 1998. Nitric oxide functions as a signal in plant disease resistance. Nature, 394: 585-588.
- Derveaux S, Vandesompele J, Hellemans J. 2010. How to do successful gene expression analysis using real-time PCR. Methods, 50: 227-230.
- Desikan R, Reynolds A, Hancock JT, Neill SJ. 1998. Harpin and hydrogen peroxide both initiate programmed cell death but have differential effects on defence gene expression in Arabidopsis suspension cultures. Biochem. J., 330: 115-120.
- Fetch TG, Steffenson BJ. 1999. Rating scales for assessing infection responses of barley infected with Cochliobolus sativus. Plant Dis., 83: 213-217.
- Juan CA, Pérez de la Lastra, JM, Plou FJ, Pérez-Lebeña E. 2021. The chemistry of reactive oxygen species (ROS) revisited: Outlining their role in biological macromolecules (DNA, lipids and proteins) and induced pathologies. Int. J. Mol. Sci. 22: 4642.
- Gupta S, Loughman R, Platz GJ, Lance R, Jones M. 2001. Journey of net blotch from pathotype diversity to useful resistance in barley. Proceedings of the 9th Australian Barley Technical Symposium, Melbourne, Australia.
- Kim DS, Hwang BK. 2014. An important role of the pepper phenylalanine ammonia-lyase gene (PAL1) in salicylic acid-dependent signalling of the defence response to microbial pathogens. J. Exp. Bot., 65: 2295-2306.
- Kumar J, Schafer P, Huckelhoven R, Langen G, Baltruschat H, Stein E, Nagarajan S, Kogel HK. 2002. Bipolaris sorokiniana, a cereal pathogen of global concern: cytological and molecular approaches towards better control. Mol. Plant Pathol. 3: 185-195.
- Leng Y, Wang R, Ali S, Zhao M, Zhong S. 2016. Sources and genetics of spot blotch resistance to a new pathotype of Cochliobolus sativus in the USDA small grains collection. Plant Dis., 100: 1988-1993.
- Livak K J, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (-Delta Delta C (T)) Method. Methods, 25: 402-408.
- Rehman S, Gyawali S, Amri A, Verma RPS. 2020. First report of spot blotch of barley caused by Cochliobolus sativus in Morocco. Plant Dis., 104: 3.
- Sahebani N, Hadavi N. 2009. Induction of H2O2 and related enzymes in tomato roots infected with root knot nematode (M. javanica) by several chemical and microbial elicitors. Biocontrol. Sci. Technol., 19: 301-313.
- Thordal-Christensen H, Zhang Z, Wei Y, Collinge D B. 1997. Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley—powdery mildew interaction. Plant J., 11: 1187-94.
- Vogt T. 2010. Phenylpropanoid biosynthesis. Mol. Plant, 3: 2-20.
- Yadav V, Wang Z, Wei C, et al. 2020. Phenylpropanoid pathway engineering: An emerging approach towards plant defense. Pathogens. 9: 312.


