Hydrogen sulfide surface adsorption study on composite materials from waste water of thermal power plants
Автор: Filimonova A.A., Vlasova A.Y., Chichirova N.D., Kamalieva R.F.
Журнал: Журнал Сибирского федерального университета. Серия: Техника и технологии @technologies-sfu
Рубрика: Исследования. Проектирование. Опыт эксплуатации
Статья в выпуске: 3 т.18, 2025 года.
Бесплатный доступ
Environmental safety of industrial enterprises is one of the most important tasks, ensuring both high-quality preliminary preparation of gaseous fuel and purification of smoke emissions. Purification from sulfur compounds, such as hydrogen sulfide and mercaptans is most often carried out by the adsorption method using multicomponent materials. The authors have developed compositions of adsorption materials with the inclusion of solid waste from water treatment plants. Such compositions are distinguished by economic availability, increased resource conservation, and are also an ecological alternative to the disposal of industrial waste. As part of the work, the sulfur capacity of the developed compositions in laboratory conditions was determined, Langmuir and Freundlich adsorption isotherms were built for each composition, and the equilibrium constant. Based on the analysis of the data obtained, conclusions were made on the adsorption mechanisms for adsorbents.
Hydrogen sulfide, adsorbents, sludge from water treatment plants, gas purification, adsorption isotherms
Короткий адрес: https://sciup.org/146283162
IDR: 146283162 | УДК: 544.723.21
Текст научной статьи Hydrogen sulfide surface adsorption study on composite materials from waste water of thermal power plants
Цитирование: Филимонова А. А. Изучение поверхностной адсорбции сероводорода на композитных материалах из отходов водоподготовительной установки ТЭС / А. А. Филимонова, А. Ю. Власова, Н. Д. Чичирова, Р. Ф. Камалиева // Журн. Сиб. федер. ун-та. Техника и технологии, 2025, 18(3). С. 369–379. EDN: CLUBKB scheme is determined by economic considerations, fuel gas characteristics, and the characteristics of the sorption materials.
Currently, industrial enterprises use gaseous petrochemical waste in addition to natural gas. Multi-component gaseous waste is formed during the catalytic and thermal processing of oil and is a valuable energy resource, and its use significantly increases the economic profit of the enterprise. In addition to resource conservation, the use of gaseous waste allows for energy autonomy, reducing the negative impact on the environment. But for the possibility of safe use of multi-component gaseous waste, preliminary adsorption purification is required, which will clean the gas from negative impurities (hydrogen sulfide, ethyl / methyl mercaptans) [1].
Literature review
Today, there is a huge range of adsorption materials on the world market, which differ in composition and performance characteristics, and therefore have different efficiency. Materials of various actions are used as adsorbents: physical adsorption, chemisorption, combined. It is also worth noting that the adsorption materials presented on the market are very expensive. Therefore, materials made from industrial waste are of practical importance for enterprises, which significantly reduces their cost [2]. Therefore, the authors of this work have developed complex-component adsorption compositions aimed at desulfurization of gaseous fuel.
It is known that adsorbents based on iron compounds (salts, hydroxides, oxides) are used to purify gases from sulfur compounds. Thus, the enterprise “Polieks” has obtained a series of sorption materials based on iron oxides, intended for purifying gas emissions from hydrogen sulfide and its compounds. The authors present sorbent compositions consisting mainly of iron oxide, and oxides of aluminum, calcium, magnesium, potassium, sodium, silicon, manganese, and titanium are used as active additives [3].
The authors [4] propose the use of inexpensive and safe natural sorbents based on oxide and hydroxy-oxide compounds of metals. The authors refer to works where iron-manganese ores are used as sorbents of toxic sulfur-containing gases. According to literary sources [5–8], the ores have a good binding capacity for sulfur compounds in the gas flow. The sulfur adsorption of this sorbent is not high, only 25 ppm for hydrogen sulfide, so this material will require frequent regeneration. The advantages of this sorbent include simple regeneration, which is carried out by washing with water.
Foreign researchers considered the possibility of using sewage sludge after preliminary treatment. Preliminary treatment consisted of solid waste pyrolysis at a temperature of 400–1000 °C. Spectral analysis showed that these sorbents consist of 20–40 % carbon, up to 3–5 % iron, 4–5 % calcium in the form of oxides, as well as oxides of other metals Mg, Cu, Zn, Cr, Al in a smaller percentage content. Preliminary thermal treatment of waste allows obtaining a porous structure with a fairly large specific surface [9–11].
The authors [12] proposed an adsorbent for cleaning hydrogen-containing gas from sulfur compounds based on heavy metal oxides (cobalt, nickel, molybdenum) applied to an inorganic carrier. To increase the absorption efficiency, the adsorbent is pre-saturated with 3–10 % moisture. The sorbent is regenerated at a temperature of 180–350 °C.
The efficiency of adsorption processes directly depends on the component composition of the adsorbent and adsorbate, as well as on the process conditions: gas pressure (or concentration of the dissolved substance) and temperature. Based on the obtained data, the dependence of the adsorbed amount of gas on pressure is constructed – the adsorption isotherm. During the adsorption process, the adsorbent – 371 – surface is successively coated with particles of the adsorbed substance. First, the areas with the highest activity are filled, and then the coating spreads to the entire available area. After complete saturation of the first layer, the molecules are able to form subsequent layers, forming a multilayer structure. In this regard, two types of adsorption are distinguished: monomolecular, in which only one layer is formed, and polymolecular, characterized by the formation of several layers of the adsorbed substance.
There are different types of isotherms, including Langmuir and Freundlich isotherms, each of which describes different mechanisms of adsorption and the interaction of the adsorbate with the adsorbent surface. The type of experimental adsorption isotherms depends on many factors, so D. Langmuir introduced a number of simplifications in order to derive the adsorption isotherm equation [13]. Analysis of isotherms allows us to determine adsorption parameters, such as the maximum adsorption capacity and the adsorption equilibrium constant, which is necessary for optimizing adsorption processes. It also follows that a comparison of experimentally obtained data with the shape of the isotherm allows us to make an assumption about the adsorption mechanism and the pore size in the material under study [14].
There are five key types of adsorption isotherms to describe the process of absorption of gaseous medium by a solid material (Fig. 1). The first type is usually observed in microporous adsorbents. The initial convex segments of the isotherms of the second and fourth types are due to the presence of micropores.
The subsequent form of isotherms is determined by multilayer adsorption and capillary condensation. Concave sections observed in isotherms of the third and fifth types are characteristic of «adsorbent– adsorbate» systems, where the force of interaction between the adsorbate molecules and the adsorbent is inferior to the force of intermolecular interaction between the adsorbate molecules.
There are various mathematical models of adsorption, such as monomolecular adsorption, multilayer adsorption and capillary condensation. Each of them adequately describes the experimental data obtained under specific conditions [15].
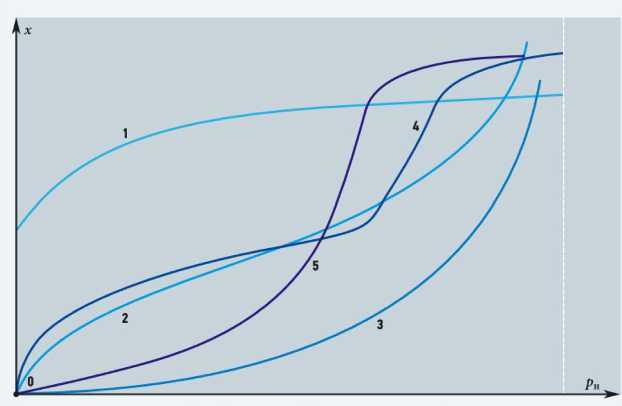
Fig. 1. Main types of adsorption isotherms (type 1 – microporous adsorbents; type 2 – polymolecular adsorption on non-porous or macroporous adsorbents; type 3 – non-porous sorbents with low energy of «adsorbent–adsor-bate» interaction; types 4 and 5 are similar to types 2 and 3, but for porous adsorbents)
Each adsorbent-adsorbate system has its own isotherm. If the theoretical equation of the adsorption isotherm converges within the permissible error with the results of experimental studies, then it is possible to calculate unknown adsorption values under different conditions ( p and T ) and determine various geometric parameters of solids.
In this work, the authors developed and presented adsorption compositions based on solid waste from water treatment plant of TPP. Sludge as one of the components of sorbents contains mainly metal oxides, active with respect to hydrogen sulfide. Composite materials are complex adsorbents in which the percentage of components was selected taking into account the chemical and physical properties of the materials. The main task of the authors was to develop an effective adsorbent of sulfur compounds from an accessible and free waste – sludge from a thermal power plant. The efficiency of adsorption compositions in binding sulfur compounds was determined on a laboratory setup [16].
Methods and materials
The efficiency of the developed adsorbents was tested in laboratory conditions by passing hydrogen sulfide through an adsorption column. Hydrogen sulfide was generated by a chemical reaction between sodium sulfide and hydrochloric acid. The gas mixture was directed by an air flow into an adsorber with a sorption material. At the outlet of the adsorber, the purified gases were directed into a flask with an absorbing solution to capture residual sulfur-containing components. The concentration of these compounds in the absorbing solution was determined according to GOST 22387.2–2021 «Natural gas». Methods for determination of hydrogen sulfide and mercaptan sulfur”. When the concentration of sulfur compounds in the absorbing solution was more than 100 mcg, the experiment was stopped, since the sorption material needed to be regenerated. The concentration of sulfur compounds in the absorbing solution was determined using a spectrophotometer at a wavelength of 665 nm. The sulfur capacity of the studied compositions was calculated as the ratio of the mass of sulfur compounds retained by the adsorbent to the mass of the adsorbent. The results are presented in Table 1.
Table 1. Compositions of the developed adsorbents and their sulfur capacity
|
No. |
Sorbent composition |
Sulfur adsorption capacity mg/g (%) |
|
1 |
Calcined sludge 50 %, NaOH 8.5 %, red clay 41.5 % |
312.1 (31.2) |
|
2 |
Calcined sludge 70 %, NaOH 8.5 %, ZnO 21.5 % |
387.6 (38.7) |
|
3 |
Non-calcined sludge 10 %, NaOH 8.5 %, ZnO 50 %, MnO 10 %, Fe 2 O 3 21.5 % |
388.96 (38.89) |
|
4 |
Calcined sludge 50 %, non-calcined sludge 10 %, NaOH 8.5 %, Fe2O3 31.5 % |
413.3 (41.3) |
|
5 |
Calcined sludge 31.5 %, non-calcined sludge 10 %, NaOH 17 %, Fe2O3 41.5 % |
452 (45.2) |
|
6 |
Non-calcined sludge 10 %, NaOH 15.3 %, ZnO 74.7 % |
529.6 (52.96) |
|
7 |
Calcined sludge 40 %, non-calcined sludge 10 %, NaOH 10 %, Fe2O3 10 %, MnO 10 %, ZnO 20 % |
600 (60) |
|
8 |
Calcined sludge 50 %, NaOH 8.5 %, ZnO 41.5 % |
686 (68) |
|
9 |
Calcined sludge 50 %, NaOH 8.5 %, ZnO 31.5 %, CuO 10 % |
967.3 (96.7) |
|
10 |
Calcined sludge 50 %, NaOH 8.5 %, Fe 2 O 3 41.5 % |
980 (98) |
Results
Langmuir and Freundlich adsorption isotherms were constructed based on the obtained experimental data. The type of the obtained isotherm forms confirms the possibility of using them to describe the process of sulfur compounds adsorption on the developed compositions. The adsorption equilibrium constant K was determined for each composition individually as the ratio of the equilibrium adsorption value to the equilibrium concentration of sulfur compounds in the solution. The constants were determined graphically, using the linearization of the Langmuir (1) and Freundlich (2) isotherms:
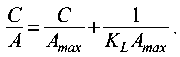
In Л = lnA'F + ^-InC, п
where – C is the residual concentration, mg/l; A is the sulfur capacity, mg/g; Amax is the maximum sulfur capacity, mg/g.
The constants for the Langmuir and Freundlich isotherms are presented in Table 2.
Table 2. Constants and correlation coefficients for Langmuir and Freundlich isotherms
|
No. |
Langmuir isotherm |
Freundlich isotherm |
||||
|
А max , mg/g |
K L , l/mg |
r |
n |
K F |
r |
|
|
1 |
9.0 |
0.01400 |
0.98479 |
1.4 |
0.1650 |
0.85565 |
|
2 |
12.4 |
0.00460 |
0.99694 |
2.0 |
0.4070 |
0.96807 |
|
3 |
14.0 |
0.00420 |
0.95698 |
1.0 |
0.0301 |
0.91712 |
|
4 |
32.0 |
0.00034 |
0.94443 |
0.9 |
0.0041 |
0.89051 |
|
5 |
9.0 |
0.01100 |
0.99371 |
2.0 |
0.3680 |
0.91522 |
|
6 |
2.5 |
0.00093 |
0.99543 |
1.2 |
0.0041 |
0.98095 |
|
7 |
20.0 |
0.00140 |
0.98609 |
1.8 |
0.2470 |
0.96628 |
|
8 |
2.4 |
0.00690 |
0.98822 |
1.6 |
0.0500 |
0.89787 |
|
9 |
20.0 |
0.00670 |
0.97483 |
1.8 |
0.4490 |
0.89851 |
|
10 |
39.8 |
0.00500 |
0.98108 |
1.5 |
0.4070 |
0.88601 |
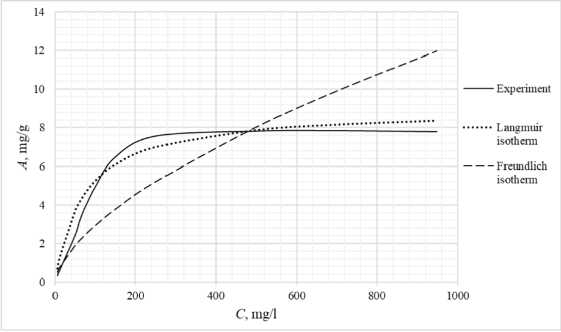
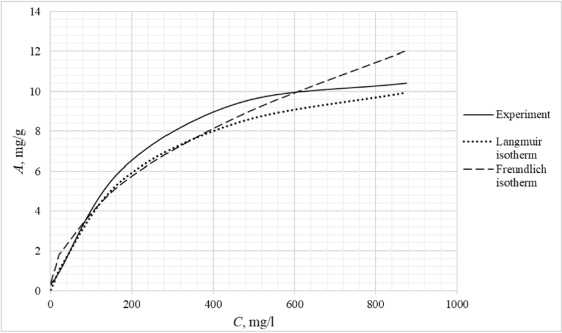
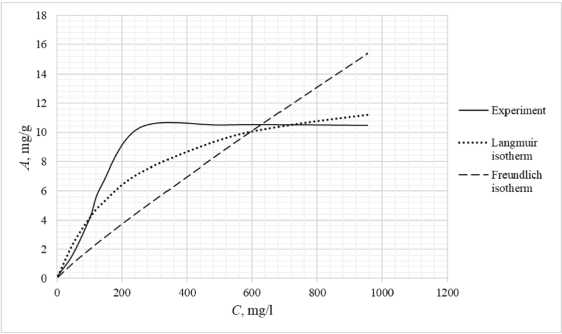
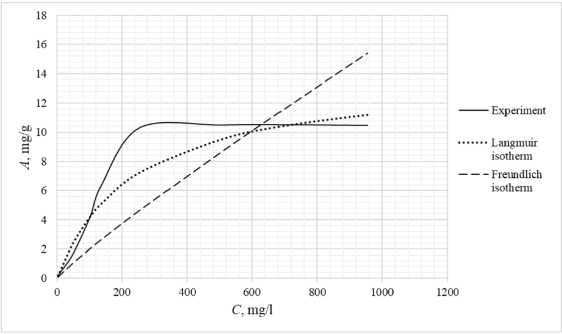
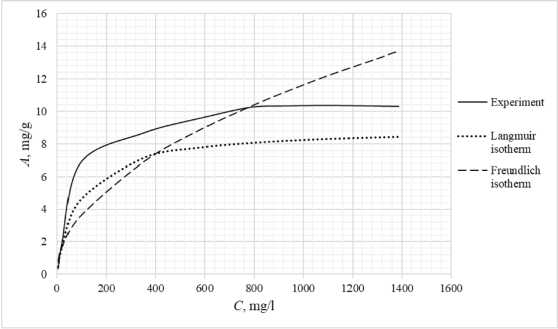
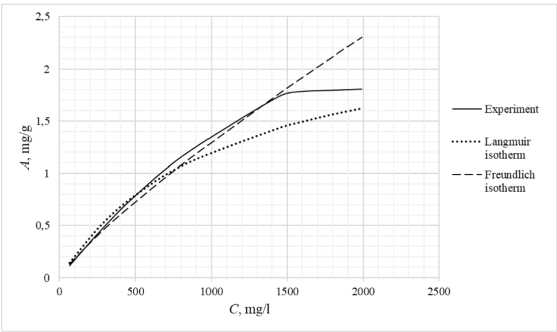
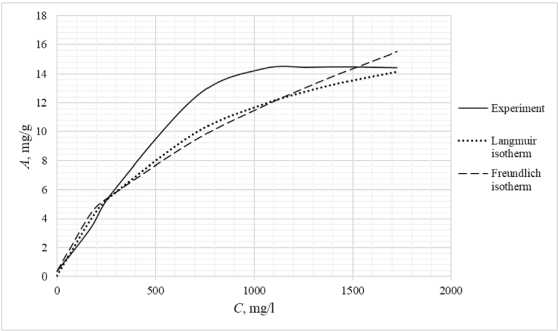
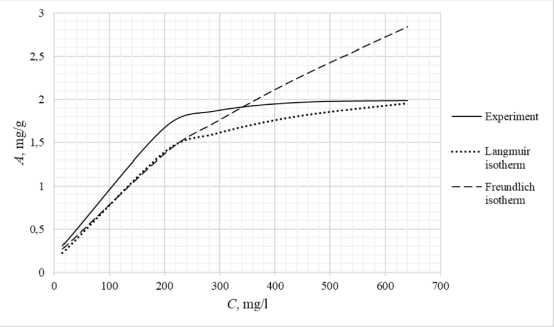
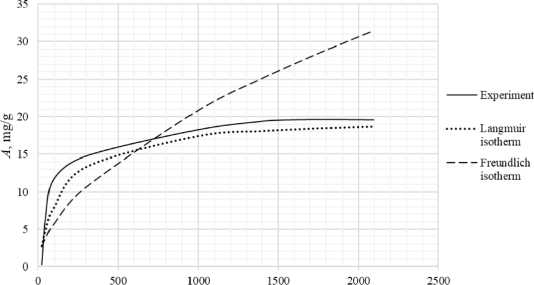
Table 3 shows the appearance of the developed sorption materials, as well as the calculated curves of the Langmuir isotherm, constructed using the equation and the Freundlich isotherm. Considering that the correlation coefficient from Table 2 for the Langmuir and Freundlich isotherms lie in the confidence interval, the experimental data obtained empirically agree with the theoretical data. A correlation coefficient close to one indicates a complex nature of sorption, physical and chemical, this can be explained by the heterogeneity of the location of the adsorption centers. The greatest proximity of the correlation coefficient is in the Langmuir isotherm, therefore it describes the sorption process most correctly.
Based on the obtained results, it follows that all the studied sorbents have the first type of sorption isotherm and are characterized by a monomolecular adsorption mechanism. This type of isotherm – 374 –
Table 3. Appearance of sorbents. Langmuir and Freundlich isotherms, experimental curve
No.
Appearance
Adsorption isotherm



No.
Appearance
Adsorption isotherm



No.
Appearance
Adsorption isotherm



No.
Appearance
Adsorption isotherm

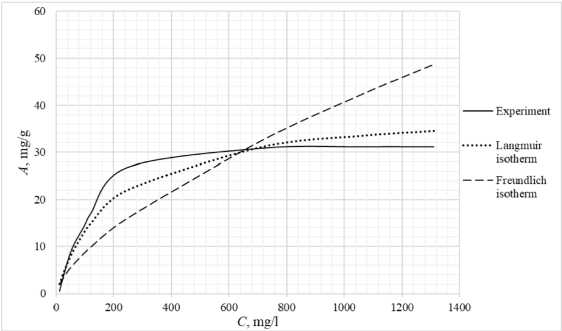
assumes the formation of one layer of adsorbate on the adsorbent surface. At the beginning of the process, a sharp increase in adsorption is observed, due to the high availability of active centers, and therefore a high degree of interaction. The curved section of the isotherm indicates the saturation of the monomolecular layer.
As the surface is filled, the rate of adsorption decreases until a state of saturation is reached, when all available sites are occupied. The first type of isotherm has a convex shape, indicating a strong interaction between the adsorbate and the adsorbent. This type of isotherm is often found in systems where the adsorbate has a high affinity for the adsorbent surface. The first type of isotherm is typical for adsorbents with a limited porous structure, where the formation of multilayer films is impossible.
Conclusion
The paper presents the results of the study of the adsorbents developed compositions for hydrogen sulfide. The uniqueness of these adsorbents was that their composition includes waste from the water treatment plant of the TPP up to 50 %, which significantly reduces the cost of the sorbent and makes it as accessible as possible. In addition to the sludge from the thermal power plant, reagents such as sodium alkali, zinc oxide, iron oxide, manganese oxide and copper oxide were used as additives providing chemical binding of hydrogen sulfide.
These compositions were analyzed and the main parameters of adsorption equilibrium were established: adsorption capacity of the sorbent, equilibrium constant, correlation coefficient between experimental data and Langmuir and Freundlich models. Based on the obtained data, the applicability of Langmuir and Freundlich isotherm models was estimated. Statistical processing of the experimental data results allowed correctly describe the sorption process.
The results of modeling show that the developed adsorbent samples have a microporous structure. Also, the predominant adsorption mechanism is chemisorption due to the interaction of hydrogen sulfide adsorbate with metal oxides (alkaline, alkaline earth), which are part of the developed adsorbents.


