Hydrogeochemical study on the cave of Bossea (Cuneo, Piedmont, Italy)
Автор: Rio Lorenzo
Журнал: Антропогенная трансформация природной среды @atps-psu
Рубрика: Сохранение природной среды. Особо охраняемые природные территории
Статья в выпуске: 2, 2016 года.
Бесплатный доступ
In article discusses the hidrogeochemical study of the cave of Bossea in Cuneo, Piedmont, Italy.
Bossea, piedmont''s cave, hydrogeolchemical study, karst system
Короткий адрес: https://sciup.org/147226749
IDR: 147226749 | УДК: 502
Текст научной статьи Hydrogeochemical study on the cave of Bossea (Cuneo, Piedmont, Italy)
Introduction Tliis work concerns the characterization of water circulating in the karst system of hydrogeochemistry Bossea. The main purpose is to understand the origin of the concentrations of Radon in the air and in the water inside tire cave, these measures that are being studied by the underground science laboratory of Bossea. It is known that Radon is a child in the decay of uranium series according to the scheme: 38 U -> 234 Th 234Po 234U 230Th -» 226Rg -» 222Rn
Therefore the characterization performed in tliis study concerned, as well as the analysis of characteristic parameter and tire measure of tire content of uranium in water present in the system, in the main collector and an external springs. Have also carried out tests of metal transfer on various lithotypes present in the Bossea's karst system of water saturated with C02, at x-ray diffraction analysis and finally wet-chemical analysis on rock samples, to determine tire contents of heavy metals and REE. In the cave have been sampled, in different hydrodynamic conditions, tire waters of the main collector (Tonente Mora) and some water supply coming from the drainage pattern of the unsaturated zone above tire cave, which flow into the main collector. Some water are located at the tectonic contact came between the rocks of tire basement metamorphic and the carbonate coverage, others water supplies come directly from tire network of fractures in limestone and dolomite. The flow of these generally is very' low, less than 1 I * s - 1. The karst system of Bossea is located in southern area of Piedmont, Ligurian Alps, at an altitude of between 800 and 1700 m above sea level. The main absorption area is located between the Corsaglia and Maudagna
® Lorenzo Rio, 2016
For the characterization of individual contributions and main collector we proceeded through comparisons of concentrations of major elements by using the diagram. We are also considered other parameters such as the contents of lanthanides. Through the simple assessment of hydrogeochemical facies survey a common footprint bicarbonate-calcic with more or less significant differences of characteristic relationships Mg"" ICa"* andHCO'MSOY• Data about the contents of lanthanides (REE-Rare Earth Elements) normalized using the PAAS (Post-Archean Australian Shale), contribute to the diversification of individual supply that show different anomalies (in particular for cerium and europium) and/or developments in relation to tlte sampling period. The overall analysis of geochemical data obtained emerges a substantial complexity of karst system of Bossea already evidenced by monitoring data of flow, temperature and specific electrical conductivity' of waler from each flow and the main collector [1].
Picture 1. Geography of study area
Hydrogeological Ргатеп’огкЛХж area is characterized by a complex tectonic history' that brought (he ancient carbonate sediments, originally accomodated over an old substrate (represented by porphiroides virtually waterproof and quartzite) created in the permo-carboniferous age, 185
to be rather segmented into large outcrops separated by towering surfaces of dislocation, fractures and faults that border indipendents hydrogeological compartments interconnected so tricky. Bossea system is characterized by a band of limestone and dolomitic limestones, laterally confined by the rocks of the basement metamorphic, polymetamorpliic and quartzite, through a series of tectonic sub-vertical contacts.
The carbonate aquifer is characterized by a relatively high permeability', with an underground movement set to a main collector that receives contributions coming from storage dolomite limestone and rocks of tire basement metamorphic (Picture 3). These rocks are a minor aquifer, set along the discontinuity along the carbonate structure and which. through a series of rackings, feeds the main aquifer (Peano et alii. 2011; Banzato et
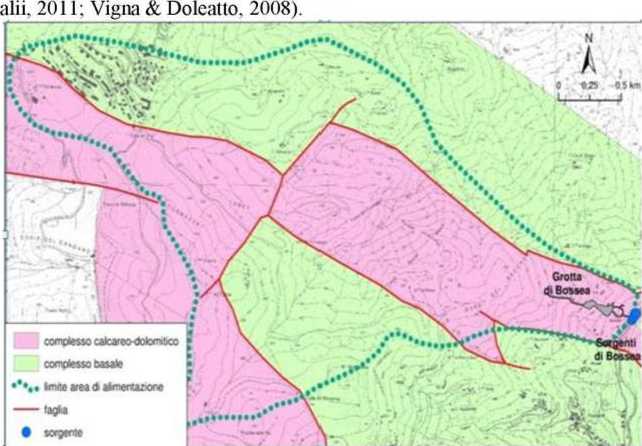
Picture 3. Hydrogeological map of the feeder area of the cave of Bossea [l|
Description of water supply. The Bossea cave is a cave crossed by an important collector, the river Mora, who rims entirely and go back to the light through a series of localized springs near the bed of the stream Corsaglia. The collector receives water from numerous secondary' intake (Picture 4).
The most important flow, called "Polle", are made up of contributions from relatively open fractures and karstified, normally present in tIte contact between carbonate aquifer and the underlying bedrock (Polla.
Polla Orso and Polletta). The secondary flows, called "Stillicidi" and distributed in several sections of the cavity. are located on the ceiling of the cave and typically come from fractures disguised as abundant deposits, producing (Stillicidio Milan. Stillicidio Torre and Stillicidio Sacrestia).
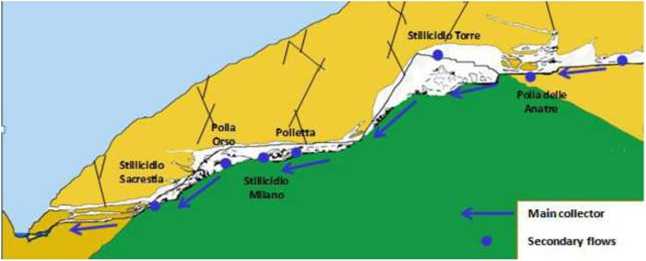
Picture 4. Schematic section of the cave of Bossea [11
They have a ven low flow below 0.5 1/min. with variations closely linked to external weather conditions and become inactive only in particular situations of drought.
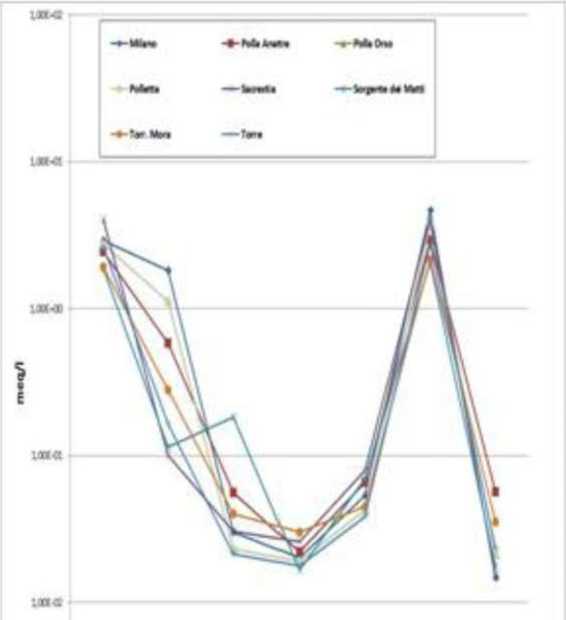
Hydrochemical characterization of contributions. Examining only the parameters characterizing data, using the diagram of Schoeller we recognize essentially two hydrogeochemical facies.
A bicarbonate-calcium-magnesiac relative to Milano and Polletta and anotlier bicarbonate-calcic relative to all other flow. Mora river and Matti spring.
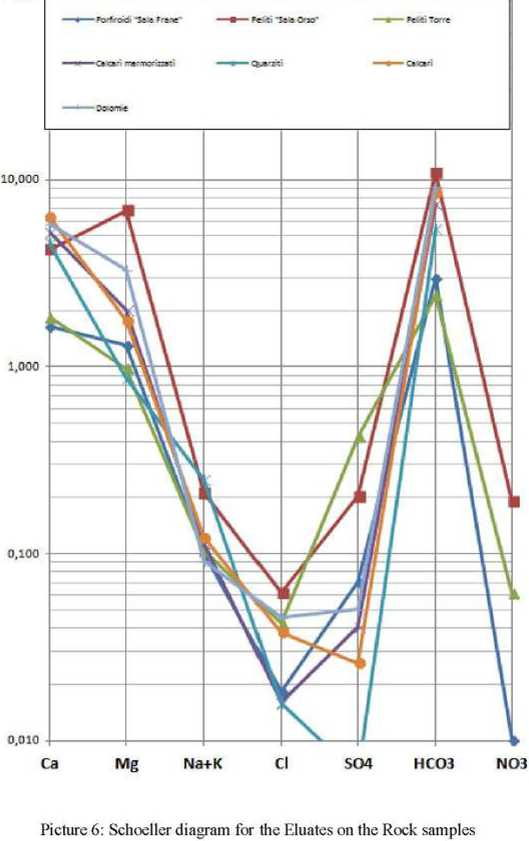
Hydrochemical characterization of lithotypes using Eluates analysLBy examining only the parameters characterizing data, using the diagram of Schoeller recognize different hydrogeochemical facies.
A bicarbonate-calcium-magnesium relative to eluates of Sala Frane Porphiroides Torre Pelites and dolomites: a bicarbonate-calcic on eluates of Metamorphic Limestones, quartzites and Limestones: a bicarbonate-magnesiac-calcic for the eluate of Sala Orso Pelite.
In bicarbonate-magnesiac-calcic facies ratio Co1* ! Mg"+ takes on values between 1.263 and 1.877 (an average of 1.631 ± 0.324). while the ratio ^Ncf + K/^! СГ varies from 2.012 in 5.533 (an average of 3.338 ± 1.914).
ин •
Cl M< K*Ni 0 S04 HC03 N03
Picture 5. Schoeller diagrams of the mean values obtained of analysis of met
Much more varied is the situation regarding both ratios for bicarbonate calcic facies, where values of Ca2tI Mg2* vaty between 2.615 obtained from the eluate of Metamorphic Limestone at 5.200 measured by eluate of quartzites (an average of 3.803 ± 1.304) while ^Na* + K’^/C?
varies between 15.554 in the eluate limestones and 3.214 in the quartzites. In particular, the analysis of these eluates shows that, besides taking the maximum values of the above ratios, they also progress independent of the others flows.
In the eluate of Sala Orso Pelites takes 0.619 value for the ratio calcium-magnesium and alkali -chlorides ratio 3.405.
Those reports take on an average total of 2.417 ± 1.552 for Ccr^ LMg" and 5,621 ±4.738 (Na* +К^/СГ.
Noteworthy is the variability of the values of sulfates and carbonates. The first range from 0.007 meq/1 in the quartzites at 0.423 meq/1 of Pelites of Torre, while the second one vary from 2.402 meq/1 of Pelites Tone 10.870 meq/1 of Pelites of Sala Orso. While the former depends on the sulphate content of the sample, the latter may result both from rock's actual quantity of Carbonates and from CO2 in the eluate.
Thinking in ppm. we can see that tire maximum value of sulfates of 20.299 ppm means a maximum concentration of calcium sulfate di 0.0029%. negligible amount considering that equals 28.768 rng/kg.
An examination of concentrations of metals is observed tliat there are variable values, but > 1 in all specimens.
Exceptions are tire concentrations that remained below tire unit like: aluminium in eluate of Porphiroides, Pelites of Sala Orso, limestone and dolomite; Manganese in eluate of Pelites Torre; iron in Pelites Torre; lead in Pelites Torre, in the metamorphic limestone, quartzite, Limestone and Dolomite Degno di nota ё la presenza del Ferro negli Eluati dei Calcari Marmorizzati, Quarziti e Calcari cite determina valori di Eh significativamente negativi. Inoltre possiamo osservare valori alti di Manganese nei Calcari e nelle Doloniie pud essere spiegato dalla presenza di Ancherite (rilevata dalla diffrattometria).
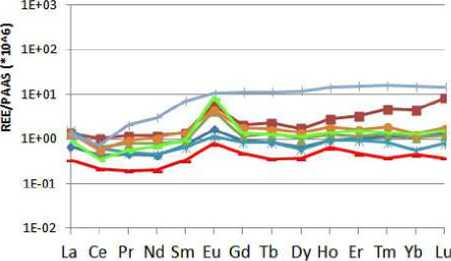
Analysis of lanthanide. An examination of lanthanides (Picture 7) it should be noted tliat in almost all samples the trend is quite similar with a significant peak values of europium (an average of 6,279 ± 6,014).
This value takes a minimum of 0.78 in Torre and a high of 10.50 in the Matti spring, while the other flows remain confined to values 1.16 in Polletta and 8.44 detected in main collector of the cave, tliat is the river Mora.
It is easy' to see as the Matti spring takes on a different behavior than others flows and springs inside the Cave, taking on an upward trend towards the values of lanthanides with higher atomic weight.
100,000
meq/l
♦ Milano
■ Polla Anatre
* Polla Or so
—*-Mlena
—t Sacrestia
Ton. Mora
Picture 7. Trend of average concentrations of lanthanides in the individual water supply
Most notable is the Polla Anatre that from Dysprosium (Z = 66) takes the same behavior of (he abovementioned spring, with values that grow to Lutetium (Z = 71).
An examination of lanthanides (Picture 8) found after the analysis of eluates we can see that there is a peak of europium values ranging from a minimum of 24.92 in dolomite and a maximum of 318.70 in the Porpltiroides of Sala Frane.
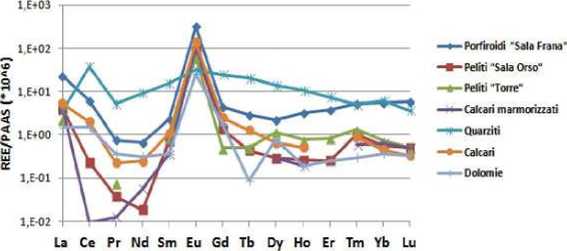
Picture 8. Trend of lanthanides present in Eluates of Rocks samle
As for the Schocller diagram, again, quartzites have a pattern independent from the other analyses that beltave decreasing towards the lanthanides with atomic weight and increased to a maximum in the Cerium.
Other analyses have all a similar trend going towards lutetium, except the Porpltiroides of Sala Frane taking the opposite beltavior.
In fact this sample shows an increase of concentrations as increase the range in atomic weight from Dysprosium (Z = 66) to Lutetium (Z = 71).
The eluate on Dolomite view trends varied with a minimum for terbium and a slight peak in dysprosium, tlien level off in a slight growth from Holmium to Lutetium.
Metal analysis. An analysis of uranium and bismuth concentrations can be seen that there are significant concentrations of uranium in the Matti spring (3.07 ppb).
In the Eluates, we can see that uranium is present in concentrations which take a minimum of 1.383 ppb in Pelites Torre and a maximum of 55.41 ppb of metamorphic Limestones.
■ Al ■ Mn ■ Fe ■ Zn ■ Sr ■ Ba e Pb ■ U
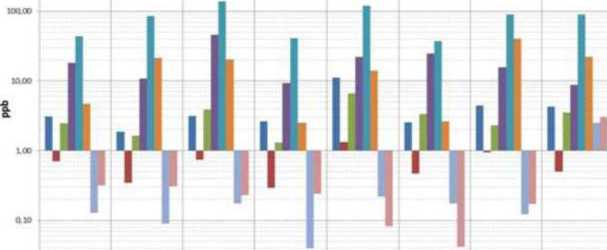
Maleno Polia Anetre Polla Ono Pcilatta Secreatle Torr* Torr More kx, del Matti
Picture 9. Concentrations of the heavy metals in the flows (above) and the eluate (below)
Also in recent Bismuth concentrations are found in all analyses, except for the Porphiroides of Sala Frane and Pelites Torre. While the latters show no cliange in values after the acid attack, the Porphiroides show a 1.22 ppm concentrations of bismuth.
This could mean that this quantity is present, but it's not soluble. At (lie end after the acid attack uranium concentrations remain measured in ppm and therefore not ven meaningful for all samples (Picture 10).
However, from data obtained, there is a presence of uranium in all lithotypes analyzed though not in high concentrations, but still not negligible. Tlie presence of uranium inside the Rock structure is reflected in the eluates to become negligible in the water.
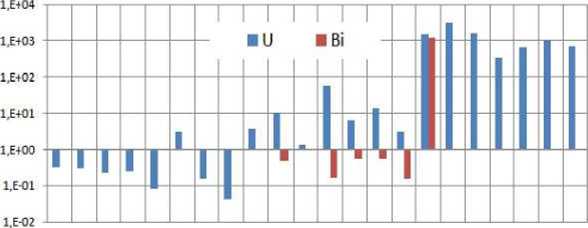
Milano
Poll» An at re
PolliOrso
Folletts
Picture 10. Concentrations of uranium and Bismuth in analyses; the latest analysis will relate to valuesobtained as a result of acid attack
The greater presence of uranium in the eluates, compared to water, it must be connected to the fact that the attack was run with CO2-saturated water (pH = 3.9). therefore much more aggressive tlian a normal rainwater that is not saturated with CO2 and has a pH of about 5.7.
Also the transfer test was performed on granular sample, which may not be representative of tlie true extent of the contact surface water-rock. This is the main parameter for putting minerals in solution along with the contact times.
Therefore the scarce presence of uranium in (he water may not be connected w ith (he scarce presence in the rocks.
The geochemical characterization of water through the main parameters shows substantial differences facies between the various flows, which obviously have an imprint bicarbonate-calcic or bicarbonate-megnisiac-cancic or by virtue of tlie fact (hat they are waters that flow within carbonate clusters. More diversity emerge from the data coming from the concentration of Rare earths.
Therefore Bossea proves, once again, be a Karst system as beautiful as it is complex, but, despite of a long series of measurements for thirty years, still capable of providing new ideas for research.
Список литературы Hydrogeochemical study on the cave of Bossea (Cuneo, Piedmont, Italy)
- "Hydrogeochemical study of karst system of Bossea", Fiorucci Moitre Vigna, 2015;
- Peano et alii, 2011; Banzato et alii, 2011; Vigna & Doleatto, 2008
- "Fundamentals of analytical chemistry", Guido Saini e Edoardo Mentasti, UTET, 1995;


