Иммуноморфологическая специфичность HER2-low рака молочной железы
Автор: Михайлов И.В., Еремеева Е.Р., Глазков А.А., Тележникова И.М., Сетдикова Г.Р., Балканов А.С.
Журнал: Сибирский онкологический журнал @siboncoj
Рубрика: Лабораторные и экспериментальные исследования
Статья в выпуске: 5 т.23, 2024 года.
Бесплатный доступ
Оценка уровня инфильтрации опухоль-ассоциированными лимфоцитами (TiLs) уже хорошо зарекомендовала себя как дополнительный инструмент прогнозирования выживаемости при триплнегативном (ТПн) и HER2/neu позитивном (HER2+) подтипах рака молочной железы (РМЖ). Последнее время РМЖ, в том числе вышеобозначенных подтипов, характеризующийся низкой/неопределенной экспрессией HER2/neu, выделяют в отдельную группу, получившую обозначение HER2-low РМЖ. Взаимозависимость между клинико-морфологическими параметрами и инфильтрацией иммунокомпетентными клетками, включающими опухоль-ассоциированные макрофаги (ОАМ), и HER2-low РМЖ, на данный момент не изучена. Цель исследования - выявление значимых взаимосвязей между уровнем субпопуляций иммунокомпетентных клеток (TiLs + ОАМ) и клинико-морфологическими параметрами HER2-low (экспрессия HER2/neu +1 или +2) РМЖ. Материал и методы. В рамках исследования изучен операционный материал (мастэктомия/резекция) 33 пациенток с HER2-low РМЖ. Подсчет TiLs и оценку уровня субпопуляций иммунокомпетентных клеток (ИКК: Т-хелперов, Т-киллеров, М1 и М2 макрофагов) методом ИГХ осуществляли во внутриопухолевых участках и в инвазивном крае первичной опухоли.
Рак молочной железы, негативный her2-neu статус, her2-low статус, опухоль-ассоциированные макрофаги, периневральная инвазия
Короткий адрес: https://sciup.org/140307921
IDR: 140307921 | УДК: 618.19-006.6-092.18 | DOI: 10.21294/1814-4861-2024-23-5-47-58
Текст научной статьи Иммуноморфологическая специфичность HER2-low рака молочной железы
Рак молочной железы (РМЖ) характеризуется гетерогенным фенотипом, обусловливающим вариабельность клинического течения данной опухолевой патологии. В этой связи для определения тактики лечения, кроме клинической характеристики опухоли, рекомендуется также ориентироваться на молекулярный подтип РМЖ. Выделяют 4 подтипа – люминальный А и В (лА/В), HER2/neu позитивный (HER2+) и триплнегативный (ТПн). Только в случае HER2+ РМЖ, диагностируемого в соответствии с требованиями American Society of Clinical Oncology (ASCO)/College of American Pathologists (CAP) при выявлении в рамках иммуногистохимического исследования (ИГХ) позитивной (+3) экспрессии HER2/neu или в случае неопределенной экспрессии HER2/neu (+2) при амплификации гена HER2/neu, прогнозируется высокая выживаемость в ответ на применение таргетной терапии ингибиторами HER2/neu [1].
Публикуются данные, свидетельствующие о том, что HER2/neu негативный (HER2-) РМЖ представляет собой неоднородную группу по уровню экспрессии HER2/neu. Так, у 47,5 % пациенток выявляется низкая (+1) или неопределенная (+2)
экспрессия HER2/neu и только в 52,5 % случаев экспрессия HER2/neu отсутствует (0). Гормонопозитивный статус (лА/В) диагностируется у 64 % пациенток с низкой/неопределенной экспрессией HER2/neu и только у 36,7 % пациенток – при отсутствии экспрессии HER2/neu (лА/В диагностируется у 28 % пациенток с HER2+ РМЖ) [2–4]. Некоторые авторы предлагают обозначать РМЖ с низкой/неопределенной экспрессией HER2/neu как HER2-low подтип, а при отсутствии экспрессии HER2/neu – как HER2-zero подтип [2].
В зависимости от уровня экспрессии HER2/ neu в клетках HER2- РМЖ наблюдаются отличия как клинического течения опухолевого процесса, так и его ответа на проводимое лечение. Среди пациенток с первично выявленным метастатическим РМЖ число HER2-low случаев значительно выше, чем с HER2-zero [3]. В ответ на проведение неоадъювантной химиотерапии (neoХТ) RCB-0 (pathological complete response – pCR) фиксируется существенно чаще среди пациенток с HER2-zero РМЖ, чем с HER2-low РМЖ [2]. Появились сообщения об эффективности терапии трастузумабом дерукстеканом (конъюгат анти-HER2/neu антитела с химиопрепаратом) у пациенток с метастатическим HER2- РМЖ, но только с HER2-low подтипом [5, 6]. Вышеприведенные факты стали основанием для пристального изучения особенностей течения HER2- РМЖ в зависимости от уровня экспрессии HER2/neu.
Оценка уровня инфильтрации РМЖ лимфоцитами (TiLs) уже зарекомендовала себя как дополнительный инструмент прогнозирования эффективности лечения, особенно при ТРн РМЖ (существенная доля ТРн РМЖ характеризуются низкой/неопределенной экспрессией HER2/ neu) [7]. Наиболее часто при РМЖ выявляются Т-лимфоциты – хелперы (CD4+ лимфоциты), наименее часто – Т-лимфоциты – киллеры (CD8+ лимфоциты). Уровень инфильтрации иными иммунокомпетентными клетками (ИКК), например опухоль-ассоциированными макрофагами (ОАМ: CD68+/CD163+), характеризуется промежуточными значениями [8].
Характер инфильтрации ИИК коррелирует с молекулярным подтипом опухоли. Так, при лА/В, HER2+ и ТРн РМЖ уровень TiLs составляет 5 % [9], 12,5–19 % [10, 11] и 20 % соответственно [12, 13]. Высокий уровень TiLs ассоциирован с подтипом РМЖ (лА/В – у 6 %, HER2+ – у 10–24 %, ТРн – у 35 % пациенток) [14], молодым возрастом, G3, высоким уровнем Ki67, лимфогенным метастазированием и выживаемостью [7, 10, 11, 13, 15–17].
Принимая во внимание отсутствие информации, посвященной специфичности субпопуляций ИИК в ткани HER2-low РМЖ, мы провели ретроспективный сравнительный анализ клиникоморфологических и иммунных параметров у таких пациенток.
Цель исследования – выявление значимых взаимосвязей между уровнем субпопуляций иммунокомпетентных клеток (TiLs + ОАМ) и клинико-морфологическими параметрами HER2-low (экспрессия HER2/neu +1 или +2) РМЖ.
Материал и методы
В период с 01.01.21 по 31.12.21 (12 мес) у 41 (19 %) из 217 пациенток, получивших хирургическое лечение в условиях стационара ГБУЗ МО МОНИКИ, при исследовании операционного материала установлен низкий (+1) или неопределенный (+2) статус HER2/neu. Такие опухоли в нашем исследовании получили обозначение HER2-low РМЖ. Состояние морфологического материала признано удовлетворительным лишь у 33 (медиана возраста – 66 [53; 75] лет) из 41 пациентки. Из них и была сформирована группа для исследования.
Операционный материал первичной опухоли молочной железы фиксировали в 10 % забуферен-ном растворе формалина, затем заливали в парафин и готовили серийные срезы. После депарафинизации препараты окрашивали гематоксилином и эозином. Иммуногистохимическое исследование (ИГХ) проводили на серийных парафиновых срезах толщиной 2 мкм с использованием иммуностейнера закрытого типа Ventana Bench Mark Ultra. Для определения молекулярного типа опухоли (рецепторы эстрогена – ER, рецепторы прогестерона – PR, HER2/new, Ki67) и субпопуляций ИКК (CD4 – лимфоцитов, CD8 – лимфоцитов, CD68 – М1 макрофагов, CD163 – М2 макрофагов) использовали антитела производства «Roche Ventana» (США). Анализ субпопуляций ИКК проводили во внутриопухолевых участках и в инвазивном крае (рис. 1). При неопределенном HER2/neu статусе (2+) выполнялось дополнительное исследование методом двухцветной хромогенной in situ гибридизации (набором Inform Her2 Dual ISH DNA Probe Cjctail Assay RocheVentana).
Интенсивность экспрессии биомаркеров оценивали полуколичественным методом. Учитывалась также их локализация. При ядерной локализации оценивали процент окрашенных ядер на 100 исследованных ядер. Подсчет TiLs проводили в соответствии с рекомендациями International Immunooncology Biomarkers Working Group [14, 18]. Для повышения точности измерения стекло-препараты были отсканированы с помощью сканирующего микроскопа Leica Aperio AT2 (Leica, Германия) и программного обеспечения ScanScope (Aperio ScanScope XT Leica).
Статистический анализ выполнен в среде R Studio (v. 2023.06.0+421) с помощью языка R (v. 4.3.0). Для количественных переменных рассчитывали относительные частоты (n (%)). Сравнение частот качественных признаков в двух независимых группах проводили с помощью критерия χ2 или точного критерия Фишера (при значениях ожидаемых частот менее 5). Сравнение количественных переменных в двух независимых выборках проводили с помощью критерия Манна–Уитни. Уровень ошибки первого рода (α) был установлен равным 0,05, нулевые гипотезы отвергали при p<0,05.
Результаты
Наиболее часто – у 31 (94 %) из 33 пациенток – диагностирован лА/В подтип РМЖ (табл. 1). Размер первичной опухоли у подавляющего большинства пациенток (55 %) на момент выполнения хирургического лечения соответствовал рТ2. Лимфогенные метастазы (pN+) имели место лишь у 33 % пациенток. Лимфоваскулярная (LV+) и периневральная (Pn+) инвазия установлены у 15 и 27 % пациенток соответственно. Проведение neoХТ имело место у 7 (21 %) пациенток. У 33 пациенток с HER2-low РМЖ обнаружены Tils (Ме – 5 [5; 10] %) (рис. 2). Высокий (≥20 %) уровень Tils выявлен только у 4 (12 %) пациенток.
При исследовании субпопуляций ИКК установлено, что в инвазивном крае и во внутриопу-холевых участках наиболее высокой оказалась
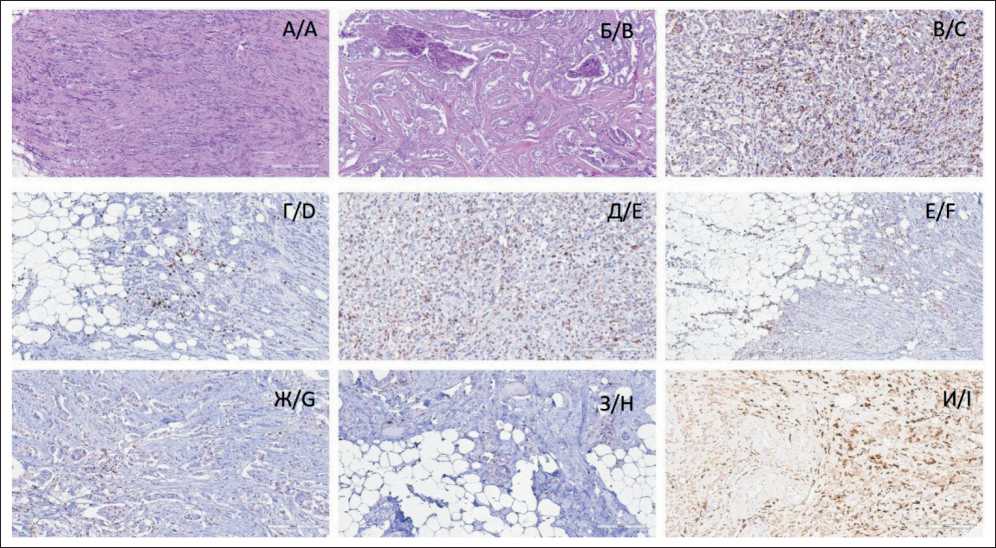
Рис. 1. Микрофото. Специфичность инфильтрации HER2-low рака молочной железы иммунокомпетентными клетками, ×400: А – высокий уровень TiLs (окраска гематоксилином и эозином); Б – низкий уровень TiLs (окраска гематоксилином и эозином); В – внутриопухолевая инфильтрация CD4+ лимфоцитами (ИГХ); Г – инфильтрация СD4+ лимфоцитами инвазивного края (ИГХ);
Д – внутриопухолевая инфильтрация CD8+ лимфоцитами (ИГХ); Е – инфильтрация СD8+ лимфоцитами инвазивного края (ИГХ); Ж – внутриопухолевая инфильтрация CD68+ макрофагами (ИГХ); З – инфильтрация СD68+ макрофагами инвазивного края (ИГХ); И – внутриопухолевая инфильтрация CD163+ макрофагами (ИГХ). Примечание: рисунок выполнен авторами Fig. 1. Microphoto. Specificity infiltration of HER2-low breast cancer by immunocompetent cells, ×400: A – high level of TILs (hematoxylin and eosin staining); B – low level of TILs (hematoxylin and eosin staining); C – intra-tumor infiltration by CD4 lymphocytes (IHC); D – CD4 infiltration lymphocytes, invasive margin (IHC); E – intra-tumor infiltration by CD8 lymphocytes (IHC); F – infiltration by CD8 lymphocytes, invasive margin (IHC); G – intra-tumor infiltration by CD68 (IHC); H – infiltration by CD68 macrophages, invasive margin (IHC); I – intra-tumor infiltration by CD163 macrophages (IHC). Note: created by the authors
Таблица 1/table 1
Характеристика пациенток, включенных в исследованиеCharacteristics of patients included in the study
|
Параметры/Parameters |
Значение/Values n=33 |
|
|
Возраст, годы/Age, years Me [LQ; UQ]/min-max |
66 [53; 75] 33–84 |
|
|
І |
12 (36 %) |
|
|
рТ |
ІІ |
18 (55 %) |
|
ІІІ |
3 (9 %) |
|
|
pN+ |
11 (33 %) |
|
|
Люминальный/Luminal A, |
15 (45 %) |
|
|
Молекулярный подтип/ Люминальный/Luminal B |
16 (49 %) |
|
|
Триплнегативный/Triple negative |
2 (6 %) |
|
|
Протоковая/Ductal Гистологический подтип/ |
25 (76 %) |
|
|
Histological subtype |
Дольковая/Lobular |
3 (9 %) |
|
Муцинозная/Mucinous |
5 (15 %) |
|
|
LV+ |
5 (15 %) |
|
|
Pn+ |
9 (27 %) |
|
|
Ki67, Me [LQ; UQ] % |
29 [12; 36] |
|
|
min-max |
1–57 |
|
|
neoХТ/neoCT + |
7 (21 %) |
|
Примечание: таблица составлена авторами.
Note: created by the authors.
Таблица 2/table 2
Инфильтрация tils внутриопухолевых участков и инвазивного края HeR2-low рака молочной железы infiltration of tils of intratumor sites and the invasive margin of HeR2-low breast cancer
|
Параметры/Parameters |
Значение/Values n=33 |
|
Tils, Me [LQ; UQ] % |
5 [5; 10] |
|
Внутриопухолевая субпопуляция Tils/Tils subpopulation in intra-tumor sites |
|
|
CD68с, Me [LQ; UQ] % |
5 [2; 7] |
|
CD163с, Me [LQ; UQ] % |
15 [7; 30]* |
|
CD4с, Me [LQ; UQ] % |
5 [5; 7] |
|
CD8с, Me [LQ; UQ] % |
2 [2; 5] |
|
Субпопуляции Tils в инвазивном крае/Tils subpopulations in |
the invasive margin |
|
CD68inv, Me [LQ; UQ] % |
5 [2; 10] |
|
CD163 inv, Me [LQ; UQ] % |
20 [10; 40]* |
|
CD4 inv, Me [LQ; UQ] % |
5 [5; 10] |
|
CD8 inv, Me [LQ; UQ] % |
5 [2; 5] |
Примечания: * – р<0,001–0,005; таблица составлена авторами.
Notes: * – р<0.001–0.005; created by the authors.
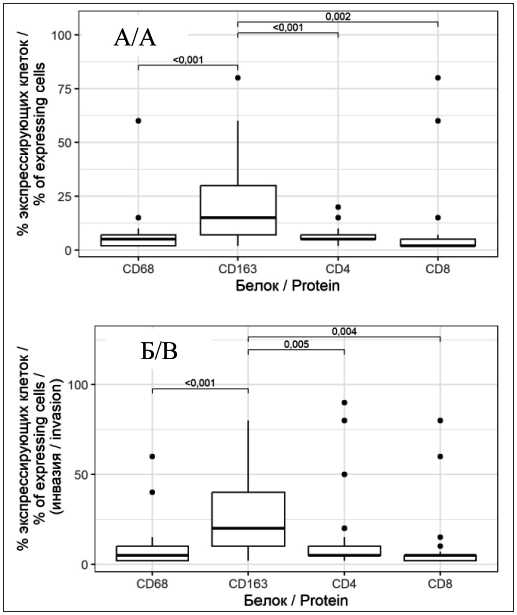
Рис. 2. Специфичность субпопуляций
TiLs HER2-low рака молочной железы:
А – внутриопухолевый участок; Б – инвазивный край.
Примечание: рисунок выполнен авторами
Fig. 2. Specificity of TILs subpopulations in HER2-low breast cancer: А – intratumoral site; B – invasive margin.
Note: created by the authors
экспрессия CD163: CD163inv: Ме=20 [10; 40] % и CD163с: Ме=15 [7; 30] % соответственно (табл. 2, рис. 2). Параметры экспрессии CD4с, CD8с, CD68c, CD4inv, CD8inv и CD68inv оказались существенно ниже и достоверно не отличались друг от друга.
Более высокий уровень экспрессии CD163inv по сравнению с CD163с обнаружен только у 3(9 %) пациенток. Высокий уровень экспрессии CD4inv, CD8inv и CD68inv (по сравнению с внутриопухоле-выми участками) также выявлялся редко – у 2 (6 %), 4 (12 %) и 3 (9 %) пациенток соответственно. Не установлено факта более высокой экспрессии маркеров субпопуляций ИКК во внутриопухолевых участках по сравнению с инвазивным краем.
Исследование экспрессии субпопуляций ИКК в нативных образцах HER2-low РМЖ и образцах, подвергшихся воздействию neoХТ, осуществлялось в двух группах: 1-я группа состояла из пациенток, получивших neoХТ (7 пациенток), во 2-ю группу включены пациентки, у которых neoХТ не проводилась (22 пациентки). Установлено, что уровни TiLs и отдельно субпопуляций ИКК у пациенток из 1-й и 2-й групп достоверно не отличались (табл. 3). По нашему мнению, это может косвенно свидетельствовать об отсутствии влияния neoХТ на уровень экспрессии субпопуляций ИКК в HER2-low РМЖ. Вместе с тем, стоит отметить, что после проведения neoХТ все же имела место тенденция (р=0,066) увеличения количества клеток, экспрессирующих CD8c.
Далее, на основании уровня М2 макрофагов в инвазивном крае (экспрессия CD163inv), являющегося, как считают многие [19], ключевым звеном в прогрессировании опухоли, были сформированы две группы: в 1-ю группу включили 14 (42 %) пациенток с низкой (<20 %) экспрессией CD163inv, во 2-ю группу – 19 (58 %) пациенток с высокой (≥20 %) экспрессией CD163inv. Статистический анализ показал (рис. 3, табл. 4), что у пациенток
Таблица 3/table 3
Проведение неоадъювантной химиотерапии при HeR2-low раке молочной железы и уровень иммунокомпетентных клеток
neoadjuvant chemotherapy for HeR2-low breast cancer and the level of immunocompetent cells
|
Параметры/Parameters |
neoХТ/neoСТ (-) (n=26) |
neoХТ/neoСТ (+) (n=7) |
p |
|
Tils, Me [LQ; UQ] % |
5 [5; 10] |
10 [7,5; 15] |
0,272a |
|
Внутриопухолевая субпопуляция Tils/Tils subpopulation in intra-tumor sites |
|||
|
CD68с, Me [LQ; UQ] % |
5 [2; 7] |
7 [2,5; 10] |
0,351a |
|
CD163с, Me [LQ; UQ] % |
15 [7; 30] |
10 [10; 20] |
0,824a |
|
CD4с, Me [LQ; UQ] % |
5 [5; 7] |
5 [5; 12,5] |
0,675a |
|
CD8с, Me [LQ; UQ] % |
2 [2; 5] |
5 [3,5; 11] |
0,066a |
|
Субпопуляции Tils в инвазивном крае/Tils subpopulations in the invasive margin |
|||
|
CD68inv, Me [LQ; UQ] % |
5 [2; 9,2] |
7 [2,5; 10] |
0,736a |
|
CD163 inv, Me [LQ; UQ] % |
20 [10; 40] |
20 [10; 30] |
0,579a |
|
CD4 inv, Me [LQ; UQ] % |
5 [5; 7] |
5 [5; 20] |
0,511a |
|
CD8 inv, Me [LQ; UQ] % |
2 [2; 5] |
5 [3,5; 11] |
0,147a |
Список литературы Иммуноморфологическая специфичность HER2-low рака молочной железы
- Pernas S., Tolaney S.M. Clinical trial data and emerging strategies: HER2-positive breast cancer. Breast Cancer Res Treat. 2022; 193(2): 281-91. https://doi.org/10.1007/s10549-022-06575-7.
- Denkert C., Seither F., Schneeweiss A., Link T., Blohmer J.U., Just M., Wimberger P., Forberger A., Tesch H., Jackisch C., Schmatloch S., Reinisch M., Solomayer E.F., Schmitt W.D., Hanusch C., Fasching P.A., Lübbe K., Solbach C., Huober J., Rhiem K., Marmé F., Reimer T., Schmidt M., Sinn B.V., Janni W., Stickeler E., Michel L., Stötzer O., Hahnen E., Furlanetto J., Seiler S., Nekljudova V., Untch M., Loibl S. Clinical and molecular characteristics of HER2-low-positive breast cancer: pooled analysis of individual patient data from four prospective, neoadjuvant clinical trials. Lancet Oncol. 2021; 22(8): 1151-61. https://doi.org/10.1016/S1470-2045(21)00301-6.
- Jin J., Li B., Cao J., Li T., Zhang J., Cao J., Zhao M., Wang L., Wang B., Tao Z., Hu X. Analysis of clinical features, genomic landscapes and survival outcomes in HER2-low breast cancer. J Transl Med. 2023; 21(1): 360. https://doi.org/10.1186/s12967-023-04076-9.
- Vtorushin S.V., Krakhmal' N.V., Zavalishina L.E., Kuznetsova O.A., Moskvina L.V., Frank G.A. Opredelenie HER2-statusa kartsinom razlichnykh lokalizatsii. Arkhiv patologii. 2023; 85(6): 31-46. https://doi.org/10.17116/patol20238506131.
- Schlam I., Tolaney S.M., Tarantino P. How I treat HER2-low advanced breast cancer. Breast. 2023; 67: 116-23. https://doi.org/10.1016/j.breast.2023.01.005.
- Moy B., Wolff A.C., Rumble R.B., Allison K.H., Carey L.A. Chemotherapy and Targeted Therapy for Endocrine-Pretreated or Hormone Receptor-Negative Metastatic Breast Cancer and Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: ASCO Guideline Rapid Recommendation Update Q and A. JCO Oncol Pract. 2023; 19(8): 547-50. https://doi.org/10.1200/OP.23.00047.
- Vaziri Fard E., Ali Y., Wang X.I., Saluja K., H Covinsky M., Wang L., Zhang S. Tumor-Infltrating Lymphocyte Volume Is a Better Predictor of Disease-Free Survival Than Stromal Tumor-Infltrating Lymphocytes in Invasive Breast Carcinoma. Am J Clin Pathol. 2019; 152(5): 656-65. https://doi.org/10.1093/ajcp/aqz088.
- García-Martínez E., Gil G.L., Benito A.C., González-Billalabeitia E., Conesa M.A., García García T., García-Garre E., Vicente V., Ayala de la Peña F. Tumor-infltrating immune cell profles and their change after neoadjuvant chemotherapy predict response and prognosis of breast cancer. Breast Cancer Res. 2014; 16(6): 488. https://doi.org/10.1186/s13058-014-0488-5.
- Skriver S.K., Jensen M.B., Knoop A.S., Ejlertsen B., Laenkholm A.V. Tumour-infltrating lymphocytes and response to neoadjuvant letrozole in patients with early oestrogen receptor-positive breast cancer: analysis from a nationwide phase II DBCG trial. Breast Cancer Res. 2020; 22(1): 46. https://doi.org/10.1186/s13058-020-01285-8.
- Salgado R., Denkert C., Campbell C., Savas P., Nuciforo P., Aura C., de Azambuja E., Eidtmann H., Ellis C.E., Baselga J., PiccartGebhart M.J., Michiels S., Bradbury I., Sotiriou C., Loi S. TumorInfltrating Lymphocytes and Associations With Pathological Complete Response and Event-Free Survival in HER2-Positive Early-Stage Breast Cancer Treated With Lapatinib and Trastuzumab: A Secondary Analysis of the NeoALTTO Trial. JAMA Oncol. 2015; 1(4): 448-54. https://doi.org/10.1001/jamaoncol.2015.0830. Erratum in: JAMA Oncol. 2015; 1(4): 544. https://doi.org/10.1001/jamaoncol.2015.1805. Erratum in: JAMA Oncol. 2015; 1(8): 1172. https://doi.org/10.1001/jamaoncol.2015.4229. Nucifero, Paolo [corrected to Nuciforo, Paolo]. Erratum in: JAMA Oncol. 2019; 5(1): 122. https://doi.org/10.1001/jamaoncol.2018.5678.
- Perez E.A., Ballman K.V., Tenner K.S., Thompson E.A., Badve S.S., Bailey H., Baehner F.L. Association of Stromal Tumor-Infltrating Lymphocytes With Recurrence-Free Survival in the N9831 Adjuvant Trial in Patients With Early-Stage HER2-Positive Breast Cancer. JAMA Oncol. 2016; 2(1): 56-64. https://doi.org/10.1001/jamaoncol.2015.3239.
- Fernandez-Martinez A., Pascual T., Singh B., Nuciforo P., Rashid N.U., Ballman K.V., Campbell J.D., Hoadley K.A., Spears P.A., Pare L., Brasó-Maristany F., Chic N., Krop I., Partridge A., Cortés J., Llombart-Cussac A., Prat A., Perou C.M., Carey L.A. Prognostic and Predictive Value of Immune-Related Gene Expression Signatures vs TumorInfltrating Lymphocytes in Early-Stage ERBB2/HER2-Positive Breast Cancer: A Correlative Analysis of the CALGB 40601 and PAMELA Trials. JAMA Oncol. 2023; 9(4): 490-9. https://doi.org/10.1001/jamaoncol.2022.6288.
- Pruneri G., Gray K.P., Vingiani A., Viale G., Curigliano G., Criscitiello C., Láng I., Ruhstaller T., Gianni L., Goldhirsch A., Kammler R., Price K.N., Cancello G., Munzone E., Gelber R.D., Regan M.M., Colleoni M. Tumor-infltrating lymphocytes (TILs) are a powerful prognostic marker in patients with triple-negative breast cancer enrolled in the IBCSG phase III randomized clinical trial 22-00. Breast Cancer Res Treat. 2016; 158(2): 323-31. https://doi.org/10.1007/s10549-016-3863-3.
- Kiselevskii M.V., Vlasenko R.Ya., Zabotina T.N., Kadagidze Z.G. Prognosticheskaya znachimost' opukhol'-infil'triruyushchikh limfotsitov. Immunologiya. 2019; 40(1): 73-82. https://doi.org/10.24411/0206-4952-2019-11009.
- Lundgren C., Bendahl P.O., Ekholm M., Fernö M., Forsare C., Krüger U., Nordenskjöld B., Stål O., Rydén L. Tumour-infltrating lymphocytes as a prognostic and tamoxifen predictive marker in premenopausal breast cancer: data from a randomised trial with long-term follow-up. Breast Cancer Res. 2020; 22(1): 140. https://doi.org/10.1186/s13058-020-01364-w.
- Gwak J.M., Jang M.H., Kim D.I., Seo A.N., Park S.Y. Prognostic value of tumor-associated macrophages according to histologic locations and hormone receptor status in breast cancer. PLoS One. 2015; 10(4). https://doi.org/10.1371/journal.pone.0125728.
- Mehraj U., Qayoom H., Mir M.A. Prognostic signifcance and targeting tumor-associated macrophages in cancer: new insights and future perspectives. Breast Cancer. 2021; 28(3): 539-55. https://doi.org/10.1007/s12282-021-01231-2.
- Hendry S., Salgado R., Gevaert T. et al. Assessing Tumor-infltrating Lymphocytes in Solid Tumors: A Practical Review for Pathologists and Proposal for a Standardized Method From the International Immunooncology Biomarkers Working Group: Part 1: Assessing the Host Immune Response, TILs in Invasive Breast Carcinoma and Ductal Carcinoma In Situ, Metastatic Tumor Deposits and Areas for Further Research. Adv Anat Pathol. 2017; 24(5): 235-51. https://doi.org/10.1097/PAP.0000000000000162.
- Wei C., Yang C., Wang S., Shi D., Zhang C., Lin X., Liu Q., Dou R., Xiong B. Crosstalk between cancer cells and tumor associated macrophages is required for mesenchymal circulating tumor cell-mediated colorectal cancer metastasis. Mol Cancer. 2019; 18(1): 64. https://doi.org/10.1186/s12943-019-0976-4.
- Anderson S., Bartow B.B., Siegal G.P., Huang X., Wei S. The dynamics of HER2-low expression during breast cancer progression. Breast Cancer Res Treat. 2023; 201(3): 437-46. https://doi.org/10.1007/s10549-023-07020-z.
- van den Ende N.S., Nguyen A.H., Jager A., Kok M., Debets R., van Deurzen C.H.M. Triple-Negative Breast Cancer and Predictive Markers of Response to Neoadjuvant Chemotherapy: A Systematic Review. Int J Mol Sci. 2023; 24(3): 2969. https://doi.org/10.3390/ijms24032969.
- Takada K., Kashiwagi S., Asano Y., Goto W., Kouhashi R., Yabumoto A., Morisaki T., Shibutani M., Takashima T., Fujita H., Hirakawa K., Ohira M. Prediction of lymph node metastasis by tumor-infltrating lymphocytes in T1 breast cancer. BMC Cancer. 2020; 20(1): 598. https://doi.org/10.1186/s12885-020-07101-y.
- Jeong H., Hwang I., Kang S.H., Shin H.C., Kwon S.Y. TumorAssociated Macrophages as Potential Prognostic Biomarkers of Invasive Breast Cancer. J Breast Cancer. 2019; 22(1): 38-51. https://doi.org/10.4048/ jbc.2019.22.e5.
- Kashyap D., Bal A., Irinike S., Khare S., Bhattacharya S., Das A., Singh G. Heterogeneity of the Tumor Microenvironment Across Molecular Subtypes of Breast Cancer. Appl Immunohistochem Mol Morphol. 2023; 31(8): 533-43. https://doi.org/10.1097/PAI.0000000000001139.
- Allison E., Edirimanne S., Matthews J., Fuller S.J. Breast Cancer Survival Outcomes and Tumor-Associated Macrophage Markers: A Systematic Review and Meta-Analysis. Oncol Ther. 2023; 11(1): 27-48. https://doi.org/10.1007/s40487-022-00214-3.
- Zhao X., Qu J., Sun Y., Wang J., Liu X., Wang F., Zhang H., Wang W., Ma X., Gao X., Zhang S. Prognostic signifcance of tumor-associated macrophages in breast cancer: a meta-analysis of the literature. Oncotarget. 2017; 8(18): 30576-86. https://doi.org/10.18632/oncotarget.15736.
- Muraro E., Martorelli D., Turchet E., Miolo G., Scalone S., Comaro E., Talamini R., Mastorci K., Lombardi D., Perin T., Carbone A., Veronesi A., Crivellari D., Dolcetti R. A diferent immunologic profle characterizes patients with HER-2-overexpressing and HER-2-negative locally advanced breast cancer: implications for immune-based therapies. Breast Cancer Res. 2011; 13(6). https://doi.org/10.1186/bcr3060.
- Mohamed R.F., Abdelhameed D.H., Mohamed M.A. Combination of Anatomical and Biological Factors to Predict Disease-Free Survival in Breast Cancer. JCO Glob Oncol. 2023; 9. https://doi.org/10.1200/GO.22.00269.
- Wang X., Zhang L., Zhang X., Luo J., Wang X., Chen X., Yang Z., Mei X., Yu X., Zhang Z., Guo X., Shao Z., Ma J. Impact of clinical-pathological factors on locoregional recurrence in mastectomy patients with T1-2N1 breast cancer: who can omit adjuvant radiotherapy? Breast Cancer Res Treat. 2021; 190(2): 277-86. https://doi.org/10.1007/s10549-021-06378-2.
- Mittendorf E.A., Ballman K.V., McCall L.M., Yi M., Sahin A.A., Bedrosian I., Hansen N., Gabram S., Hurd T., Giuliano A.E., Hunt K.K. Evaluation of the stage IB designation of the American Joint Committee on Cancer staging system in breast cancer. J Clin Oncol. 2015; 33(10): 1119-27. https://doi.org/10.1200/JCO.2014.57.2958.


