Impact of arsenic on the seedlings of Ranjit and Aijung, two most edible rice cultivars of Assam, India
Автор: Khan Zesmin, Thounaojam Thorny Chanu, Bhagawati Rupankar, Upadhyaya Hrishikesh
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 1 т.18, 2022 года.
Бесплатный доступ
Arsenic (As) toxicity profoundly affects the yield and quality of rice worldwide and it is one of the crucial threats in the world as rice being one of the major staple cereal crops. The present study was designed to investigate the growth (germination, growth of the seedlings), stress (H2O2 and lipid peroxidation) and biochemical parameters (amylase activity, enzymatic and nonenzymatic antioxidant activity) of Ranjit and Aijung, the two most edible cultivars of Assam under arsenic stress. Both the rice cultivars responded differentially to As treatments and showed differences in all noted parameters. With increasing arsenic concentration, reduction in germination, plumule-radicle length, reduced fresh and dry mass, and declination in amylase activity was prompted. The reductions of most of the observed parameters were higher in Ranjit than Aijung cultivar as compared to their respective control. Alteration of stress-related parameters and antioxidant enzymes were also inferred under As stress. Analysis of growth and biochemical study revealed that Aijung cultivar is more tolerant to As stress than Ranjit cultivar and that might be associated with a potent antioxidative defense system.
Arsenic stress, assam, most edible, rice cultivar, tolerant
Короткий адрес: https://sciup.org/143178336
IDR: 143178336
Текст научной статьи Impact of arsenic on the seedlings of Ranjit and Aijung, two most edible rice cultivars of Assam, India
Rice ( Oryza sativa L.) is a paramount food crop for almost 3 billion people of the world including Assam, India ( alita and Tanti, 2020). Several rice cultivars are grown in Assam, but Ranjit and Aijung are the most widely grown rice cultivars, and almost all the cultures, festivals of Assam depend on rice and its different products. However, its production is greatly impeded by Arsenic (As) toxicity because of the cultivation and irrigation of rice fields with As contaminated soil and water. Bag et al. , (2019) reported that early establishment and production of rice are significantly affected by the concomitant increase in As concentration in rice belt areas. Mridha et al. , (2021) reported that the intolerable levels of As in soil and application of As contaminated groundwater in rice cultivation are significantly affecting its production and also contaminates the food chain of living organisms including human beings. Rice is the more surplus accumulator of As than other crops due to the presence of As transporters like phosphate, aquaporins, and silicon transporters.
Many serious diseases and cancers are reported to be caused by exposure to As to human beings. Arsenic can occur in different inorganic (AsIII, AsV), organic (DMA, MMA) and gaseous (Arsine gas) forms, and inorganic forms are the most noxious to living organisms. Arsenic toxicity stimulated the aggravation of free radicals and reactive oxygen species (ROS) that cause oxidative damages (Alvarenga et al., 2020). Singh et al., (2017) mentioned that As stress hampers the plants from the germination stage to growth, and yield of plants. Chandrakar et al., (2017) reported that As stress-induced excessive ROS causes membrane damage, cellular oxidative injury that ultimately leads to hampering gaseous exchange, cellular functions, reduced uptake of nutrients and water, and thus plant growth is reduced. Plants defend themselves against oxidative stress provoked under stress conditions by elevating the production of enzymatic and nonenzymatic antioxidants such as superoxide dismutase (SOD), catalase (CAT), peroxidase (POD), ascorbate, etc (Shri et al., 2009). Understanding the effect of As on physico-biochemical processes of different rice cultivars would help provide in selecting rice cultivar which can withstand As stress to some extent in a particular area. The different approaches to mitigate As stress in rice is a great need of the time but for the application of As mitigation research approach in large scale is a very much time-consuming process. So it is very essential to select As-tolerant and As-sensitive cultivars. Although it is not safe to cultivate rice in As-prone areas because it accumulated a higher amount of As than other crops but it has been reported that the rice cultivar which can grow moderately well in As prone-areas, also accumulates less arsenic than the As sensitive cultivar. The objective of this study was to evaluate the comparative effect of As on growth, stress, and biochemical parameters of Ranjit and Aijung, the two most edible rice cultivars of Assam, India.
MATERIALS AND METHODS
Seed surface sterilization
The two rice cultivars (Ranjit and Aijung) were collected from the Regional Rainfed Lowland Rice Research Station (RRLRRS), Gerua, Hajo, Assam, India. The seeds were sterilized with 0.1% (w/v) HgCl 2 (mercuric chloride) solution for 3 minutes and washed 3 times thoroughly with distilled water. Two concentrations of As (sodium arsenate) were used as test solutions (50µM As, 100µM As). The seeds (20 seeds in each Petri plate) were set for germination on filter paper in Petri dishes (20 mm × 120 mm) containing 10ml of test solutions (0µM As, 50µM As, 100µM As). Distilled water was used in the control group (0µM As). The Petri plates were kept in an incubator at 28 ± 10 C. On the 4th day, germination percentage, growth, mass, and biochemical analysis were done. The experiment was repeated three times.
Growth parameters
Germination percentage
The germination percentage was analyzed using the following formula
Germination Percentage (%) = (Ng / Nt) × 100
Where, Ng - Number of total germinated seeds.
Nt - Total number of seeds tested.
Plumule, radicle length, and mass
Ten uniform rice seedlings were taken from each group, separated into plumule, radicle and their lengths were recorded separately and weighted for fresh mass. Dry mass of plumule and radicles were taken after oven drying at 80 ºc for 48 h.
Biochemical parameters
Estimation of amylase activity
To determine the α-amylase activity, 0.5gm sprouting samples of each treatment were homogenized in a chilled mortar and pestle with 5ml ice-cold sodium phosphate buffer (0.02M; PH = 6.9). All experimental procedures were performed at chilled environments (at 40 c) and the resulting homogenate was centrifuged at 10,000 g for 15 min. The collected supernatant was stored in a cold environment. 1% starch solution (substrate) was separately prepared by adding 1g starch in 100ml boiling distilled water and cooled it. 0.5ml enzyme extract of respective concentrations was taken in separate test tubes and after that in each test tube, 0.5ml starch solution was added. The test tubes were incubated for 30 minutes at 370 C. 1ml 3, 5-dinitrosalicylic acid (DNS) was added to all respective test tubes after incubation, and boiled for 5 minutes. Then test tubes were allowed to cool down at room temperature and, 10ml distilled water was added. The optical density of each sample was taken at 540nm (Bernfield et al. , 1955).
Estimation of lipid peroxidation levels
The level of lipid peroxidation was estimated as 2-thiobarbituric acid (TBA) reactive metabolites chiefly MDA (malondialdehyde) accumulation as described by Heath and Packer (1968). To determine MDA, 0.2g sample tissue was grounded and extracted in 10 ml TBA (0.25%) made in 10ml of 20% trichloroacetic acid (TCA). The resulting extract was heated at 950C for 30 min and then rapidly cooled in ice. Then after centrifugation at 10,000g for 10 min, the OD (Optical density) of the supernatant was taken at 532 nm. Correction of nonspecific turbidity was done by subtracting the absorbance value taken at 600 nm. Lipid peroxidation level is expressed as nmol of MDA formed using the extinction coefficient of 155 mM-1 cm-1.
Determination of hydrogen peroxide (H2O2) level
The method of Lin and ao, (2001) was used to estimate the hydrogen peroxide (H 2 O 2 ) level. Briefly, 0.1g plant tissues were homogenized in 2 ml phosphate buffer (0.05 M; pH 7.4) and the homogenate was centrifuged at 15000 g for 25 minutes. Then 1.5 ml of collected supernatant was added with 0.5 ml of 0.1% TiCl 2 (Titanium chloride). The resulting mixtures were again centrifuged at 15000 g for 15 minutes. The absorbance was recorded at 410 nm and the H 2 O 2 level was calculated using the extinction coefficient.
Determination of ascorbate content
The method of Oser (1979) was used for the extraction and estimation of ascorbate. The sample tissue was homogenized in 5ml metaphosphoric acid (5% w/v) and centrifuged at 10,000g for 10 min. Then the collected supernatant was used for ascorbate estimation. The reaction mixture consisted of 2ml Na-molybdate (2%), 2ml 0.15 N H 2 SO 4 , 1ml 1.5 mM Na 2 HPO 4 and 1ml tissue extract. It was mixed and incubated at 600C in a water bath for 40 min. After that, it was cooled to room temperature and centrifuged at 3,000g for 10 min. The absorbance was recorded at 660 nm.
Extraction and assay of enzyme
The superoxide dismutase (SOD) activity was assayed using the method of Giannopolitis and Reis (1977). For extraction of the enzyme, 0.1 g sample tissue was ground with pre-chilled mortar and pestle at 40C with 2ml ice-cooled phosphate buffer (0.05 M; pH 7.8). The homogenate was then centrifuged at 15,000g for 15 min and the collected supernatant was used as the enzyme (sample) extract for the superoxide dismutase (SOD) assay. The assay mixture for SOD contains 2.5ml methionine, 0.3ml riboflavin, 0.1ml nitroblue tetrazolium (NBT), and 0.1ml enzyme extract. By placing the test tubes in between two tube lights, the reaction mixtures were illuminated (Philips 20 W). By switching the light on and off, the reaction mixtures were illuminated and terminated. The increase in absorbance due to formazan formation was read at 560 nm. The increase in absorbance in the absence of enzyme was taken as 100 and 50% initial was taken equivalent to 1 unit of SOD activity. The OD was taken at 560nm.
The method of Chance and Maehly (1955) was used to estimate the catalase (CAT) activity by measuring the disappearance of H 2 O 2 . The assay enzyme was extracted by grinding 0.1g sample tissue in a mortar and pestle with 2ml chilled phosphate buffer (0.1M, PH 7) at 40 c. Then the extract was centrifuged at 15000 rpm for 15min and the resulting supernatant was used as enzyme extract. The assay mixture contains 0.05ml enzyme extract, 1.5ml buffer, 0.5ml H 2 O 2 (10mM), and 0.95ml distilled water. By placing the test tubes in between two tube lights the reaction mixtures were illuminated (Philips 20 W). The alteration in absorbance was taken at 470 nm after 3 min of incubation at room temperature, and activity was expressed in terms of mmol min-1 mg-1 protein.
Statistical analysis
All the experiments were repeated thrice and the data presented are mean ± standard errors (SE). Analysis of data was carried out using MS Excel and SPSS 20. LSD test was done to compare significant mean difference from control.
RESULTS
Germination percentage
The germination percentage of the two cultivars exposed to different concentrations of As (0 µM, 50µM, 100 µM) are shown in Table. 1. Yuan et al. , (2020) reported that seed germination is the critical point in seedling establishment and successive plant health under stress conditions. The germination percentages of both the cultivars were declined as the concentration of As increases that may reveal inhibition of germination of seeds by As stress as reported by Liu et al. , (2005). Under 50µM As stress, the germination percentage of Ranjit and Aijung cultivars were reduced by 10.73% and 9% as compared to their respective control and at 100µM As stress, the germination percentages were decreased by 15% and 11.22%. The more detrimental effect of As in seed germination was shown in Ranjit cultivar than Aijung signifying its more sensitivity towards As stress.
Plumule, radicle length, and biomass
The substantial reduction of plumule, radicle elongation, fresh and dry mass of Ranjit and Aijung cultivar with increasing As concentrations are shown in Table. 1. and Fig. 1. Maximum reduction of plumule and radicle length was observed at 100µM As stress, by 38.38 % and 42.24 % in Ranjit cultivar and by 30.18 % and 40.47% in Aijung compared to their respective control. Otherwise at 50µM As stress, the plumule and radicle length was reduced by 8.48% and 18.6% and in the Aijung cultivar, the length of plumule and radicle was decreased by 6.03% and 11.90% as compared to their control. The same trend of decrease in fresh and dry mass was seen in both the cultivars but radicle length and biomass (both fresh and dry) showed significant declination in both the cultivars that might be associated with improper uptake of water and nutrients and reduction in cell elongation under As stress (Yadu et al., 2019). The data of the growth parameters reveal more tolerance of Aijung cultivar towards As stress than Ranjit cultivar.
Biochemical parameter
Amylase activity
The progressive decline of α-amylase activity with increasing As concentration hamper germination of seeds and their growth. The obtained results of α-amylase activity in both the rice cultivars under different concentrations of As are illustrated in Fig. 2. The α-amylase activity of the Ranjit cultivar was reduced by 26.66% and 34.58% and in the Aijung cultivar it is reduced by 8.71% and 18.79% under 50µM and 100µM As stress as compared to their respective control. The α-amylase activity of the Aijung cultivar was less reduced than the Ranjit cultivar in comparison with their control revealing its tolerance towards As stress during germination.
Lipid peroxidation level / MDA content
Lipid peroxidation levels reveal the impact of heavy metal toxicity triggered by oxidative stress on plants and used as a sign of membrane lipid damage under stress conditions (Maiti et al., 2012). Lipid peroxidation level / MDA content was elevated with the addition of different concentrations of As and are represented in Fig. 3. The MDA content of plumule, radicle of Ranjit cultivar was increased by 225.16% and 217.70% under 50µM As stress and 430.4% and 395.83% at 100µM As stress compared to their control. Otherwise, in the Aijung cultivar, MDA content of plumule and radicle was enhanced by 187.64% and 213.33% at 50µM As and 335.95% and 302.5% at 100µM As. The data of the present study showed less MDA content in Aijung cultivar than Ranjit that might be correlated with less membrane lipid damage in Aijung cultivar than Ranjit under As stress.
Hydrogen peroxide level
The effect of As on H 2 O 2 content in both Ranjit and Aijung cultivars are shown in Fig. 4. The H 2 O 2 level increased in both the cultivars under all concentrations of As in comparison with their control. At 50µM As, the H 2 O 2 level was increased by 161.70% and 177.77% in the plumule and radicle of the Ranjit cultivar. However, in the Aijung cultivar at 50µM As, the H 2 O 2 level was enhanced by 140.30% and 144.73% in its plumule and radicle. Under 100µM As stress, H 2 O 2 content of plumule and radicle of Ranjit cultivar was increased by 255.31% and 346.66% and by 219.23% and 247.36% in plumule and radicle of Aijung cultivar that may signify more tolerance of Aijung cultivar towards As stress than Ranjit cultivar.
Ascorbate activity
Ascorbate catalyzes the detoxification of H 2 O 2 levels under stress conditions. Noctor and Foyer, (1998) reported that the function of catalase (CAT) is performed by ascorbate–glutathione cycle in cytosol and chloroplasts. The obtained results indicated the positive correlation between non-enzymatic antioxidant activities and As concentrations in both the rice cultivars shown in Fig. 5. The ascorbate content of plumule, radicle of Ranjit and Aijung cultivar was increased at 50µM As by 121.42%; 110% and 131.25%; 116.66%. However, at 100µM As stress, the ascorbate content of plumule and radicle of Ranjit cultivar was reduced by 25% ; 20% and by 25% ; 16.67% in plumule and Radicle of Aijung cultivar under 100µM As stress.
Enzymatic antioxidant activity
The oxidative stress induced by the heavy metal toxicity causes the impairment of different biomolecules and metabolic interruptions that resulted in the elevation of various protective measures of plants like upregulation of antioxidant defense system to scavenge the excessive reactive oxygen species triggered under stress conditions (Pandey et al., 2016; Rui et al., 2016).
The superoxide anion (O2-) which is very harmful to biomolecules and chloroplasts of plants is scavenged by SOD or superoxide dismutase into oxygen (O 2 ) and hydrogen peroxide (H 2 O 2 ). The effect of As on the SOD activity of Ranjit and Aijung cultivars is shown in Fig. 6. SOD activity upregulated in both the cultivars at 50µM As stress. However, at 100µM As stress, there was a reduction in SOD activity comparing to their respective control. Under 50µM As stress, SOD activity was enhanced by 107.54%, 106.5% in plumule and radicle of Ranjit cultivar, but under 100µM As stress, SOD content of plumule and radicle was decreased by 18.31% and 50%. Otherwise, in the Aijung cultivar, SOD content of both plumule and radicle was enhanced by 111.1% and 107.4% under 50µM As stress and reduced by 17.78% and 48.14% under 100µM As stress.
Catalase (CAT) enzymes catalyze the conversion of H 2 O 2 into H 2 O and O 2 . The two rice cultivars showed a differential response in CAT activity with increasing concentration of As and are illustrated in Fig. 7. The CAT activity of both the cultivars upregulated at 50µM As and downregulated at 100µM As stress. In Ranjit cultivar, CAT activity of plumule and radicle was increased by 122.93% and 138.84% under 50µM As stress. Under 100µM As stress, CAT activity was decreased by 12.3% and 38.69%. In Aijung cultivar at 50µM As, CAT activity of plumule and radicle was enhanced by 124.82% and 141.74% but at 100µM As stress, CAT activity of plumule and Radicle was reduced by 15.04% and 14.57% as compared to control.Overall SOD and CAT activity was higher in Aijung cultivar than Ranjit cultivar signifying its more strong antioxidative response than Ranjit cultivar under As stress.
Table 1. Effect of arsenic (0, 50 & 100 µM As) on germination percentage, plumule, radicle length, fresh and dry mass of germinating rice seedlings (Ranjit and Aijung cultivar). Data presented are mean ±SE (n=3). * indicates significant difference from control at P<0.05 by LSD test.
|
Name of Rice cultivar |
Treatment ( µM As) |
Germinati on % |
Plumule length (cm) |
Radicle length (cm) |
Fresh mass of plumule (mg) |
Fresh mass of radicle (mg) |
Dry mass of plumule (mg) |
Dry mass of radicle (mg) |
|
Ranjit |
0 |
98.33 ± 0.78 |
1.65 ± 0.02 |
5.16 ± 0.19 |
11.16± 0.09 |
6.16 ± 0.11 |
1.11±0.06 |
0.70±0.01 |
|
50 |
87.60 ± 1.29 |
1.51 ± 0.04* |
4.20 ± 0.15* |
9.51± 0.13* |
4.78±0.06* |
1.00±0.01 |
0.60±0.02* |
|
|
100 |
85.00 ± 1.86* |
1.16 ± 0.02* |
2.98 ± 0.10* |
7.61±0.13* |
3.23±0.05* |
0.88±0.01* |
0.10±0.01* |
|
|
Aijung |
0 |
98.00 ±0.51 |
1.16 ±0.03 |
4.20 ± 0.10 |
2.61± 0.13 |
0.68±0.04 |
0.44 ±0.02 |
0.40 ±0.01 |
|
50 |
89.00 ±1.36* |
1.09±0.01* |
3.70 ±0.05* |
2.35±0.06* |
0.63 ±0.01* |
0.41 ±0.01 |
0.34 ±0.02 |
|
|
100 |
87.00 ±0.51* |
0.81±0.01* |
2.56 ±0.17* |
1.51±0.18* |
0.41 ±0.02* |
0.23 ±0.02 |
0.10 ±0.01 |
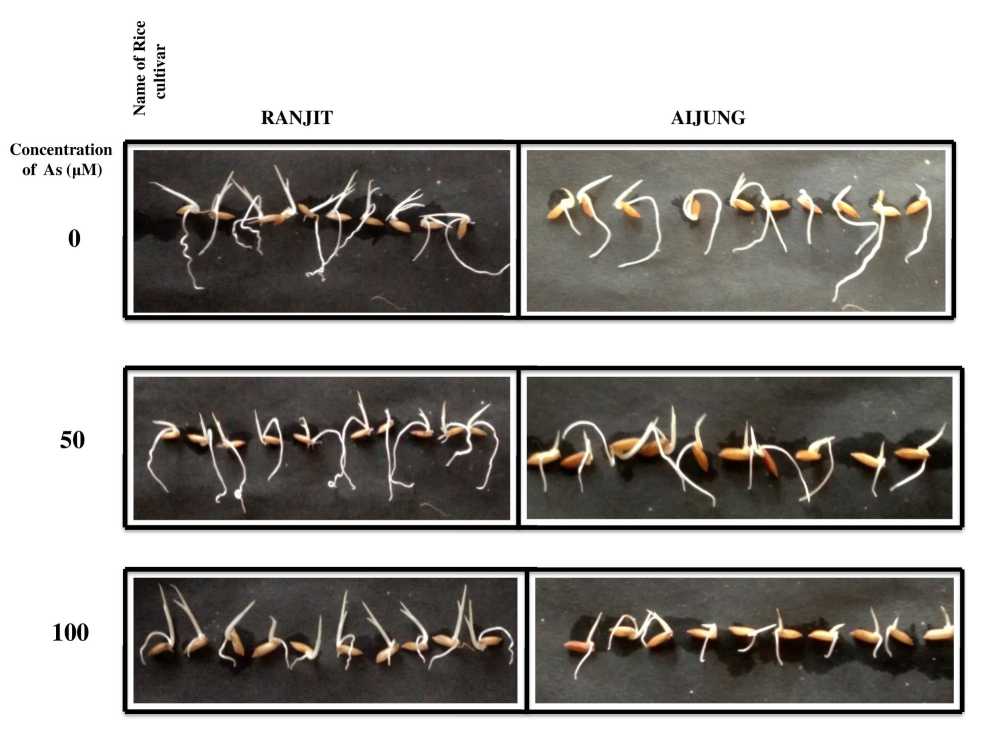
Figure 1. Effect of arsenic (0, 50 & 100 µM As) on germinating rice (Ranjit and Aijung cultivar) seeds
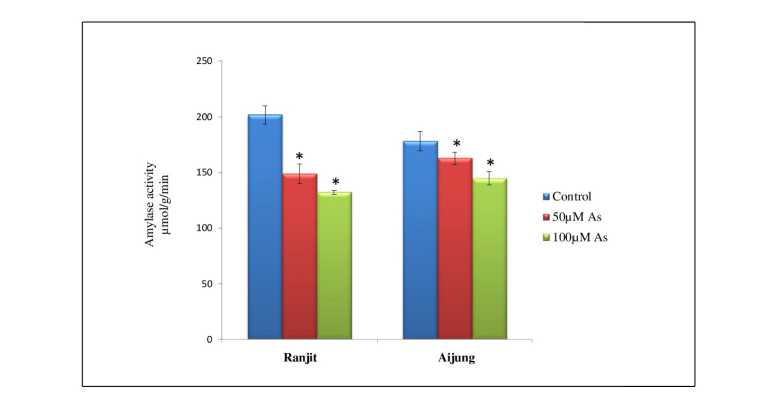
Figure 2. Effect of arsenic (0, 50 & 100 µM As) on amylase activity of germinating rice of Ranjit and Aijung cultivar. Data presented are mean ±SE (n=3). * indicates significant difference from control at P<0.05 by LSD test.
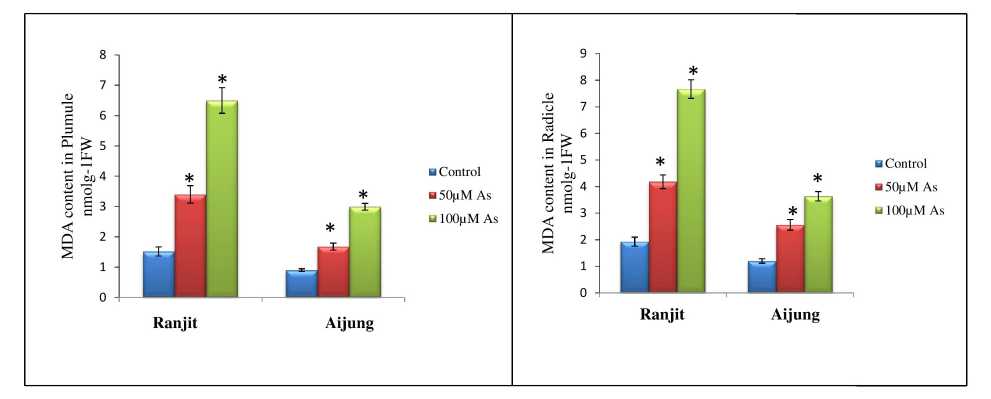
Figure 3. Effect of arsenic (0, 50 & 100 µM As) on MDA content of plumule and radicle of germinating rice seedlings (Ranjit and Aijung cultivar). Data presented are mean ±SE (n=3). * indicates significant difference from control at P<0.05 by LSD test.
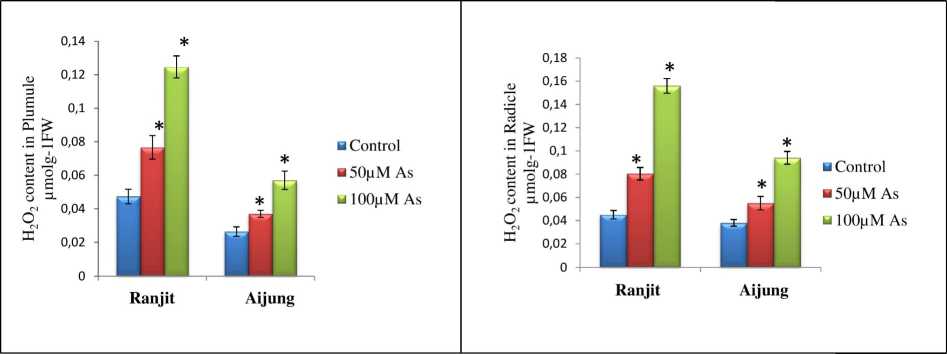
Figure 4. Effect of arsenic (0, 50 & 100 µM As) on hydrogen peroxide (H2O2) content of plumule and radicle of germinating rice seedlings (Ranjit and Aijung cultivar). Data presented are mean ±SE (n=3). * indicates significant difference from control at P<0.05 by LSD test.
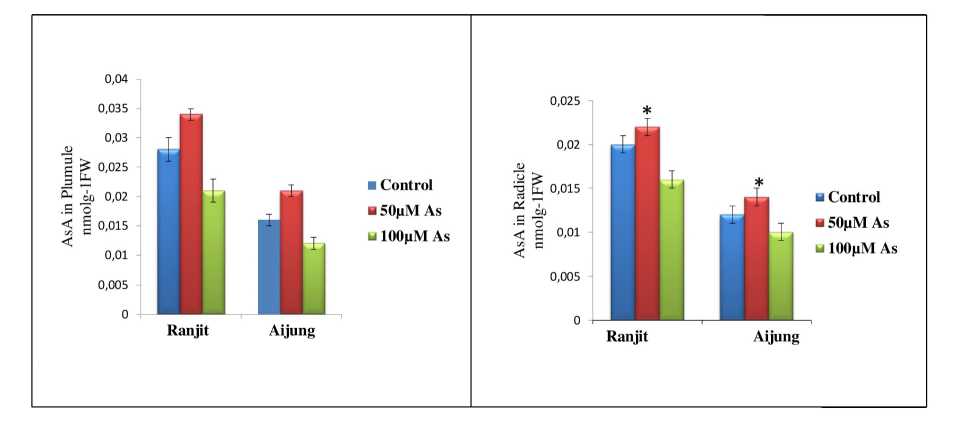
Figure 5. Effect of arsenic (0, 50 & 100 µM As) on ascorbate content of plumule and radicle of germinating rice seedlings (Ranjit and Aijung cultivar). Data presented are mean ±SE (n=3). * indicates significant difference from control at P<0.05 by LSD test.
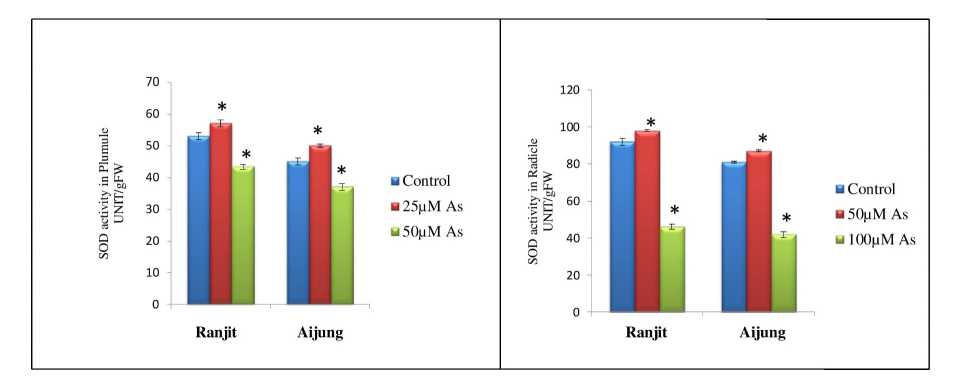
Figure 6. Effect of arsenic (0, 50 & 100 µM As) on superoxide dismutase (SOD) activity of plumule and radicle of germinating rice seedlings (Ranjit and Aijung cultivar). Data presented are mean ±SE (n=3). * indicates significant difference from control at P<0.05 by LSD test.
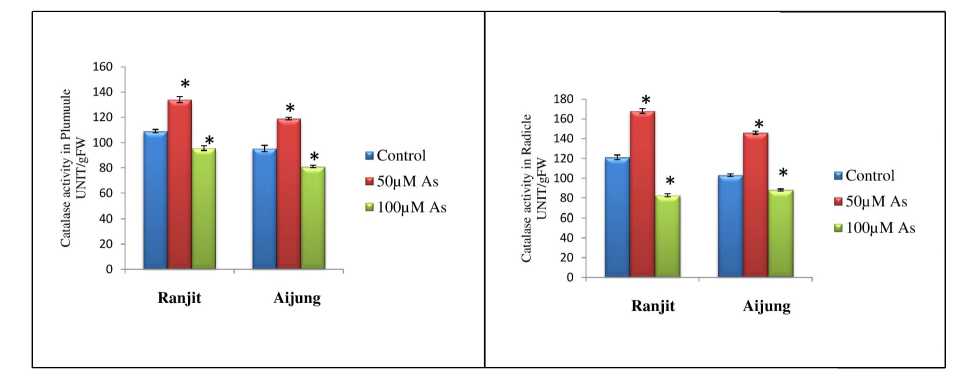
Figure 7. Effect of arsenic (0, 50 & 100 µM As) on catalase (CAT) activity of plumule and radicle of germinating rice seedlings (Ranjit and Aijung cultivar). Data presented are mean ±SE (n=3). * indicates significant difference from control at P<0.05 by LSD test.
DISCUSSION
A concentration-dependent marked decrease in germination percentage, elongation of the shoot, roots (most notably) as well as reduced plant biomass were noted in both the rice cultivars with the increase in arsenic treatments, as compared to their respective control. The result of the present study showed a higher germination percentage in Aijung cultivar than in Ranjit. Under As stress, germination of seeds is reported to be very sensitive and is considered as the first physiological process affected by As stress (Liu et al , 2005; Shankar et al. , 2006). The decrease in germination percentage and growth parameters may be attributed to lowered uptake of water, nutrients, and reduction of cell elongation under As stress as reported by Yadu et al. , (2019). aneko et al. , (2002) reported that under As stress, the conversion of endospermic stored starch into metabolizable sugars by α-amylase is not adequate for use for germination of seeds and growth of roots and shoots. Our result is in agreement with the study on rice by umar et al. , (2021).
The radicles were more affected than the plumule of the Ranjit cultivar but in the Ajiung cultivar, mostly plumules were affected by the exposure to As and the Aijung cultivar showed more growth and biomass than Ranjit cultivar compared to their respective control. It is reported that As induced oxidative stress decreased radicle length by generating excessive oxidative stress that elevated lipid peroxidation levels (Harminder et al. , 2007). Ekmekçi et al. , (2009) reported that water deficit conditions attributed due to the disturbances in water balance and loss of permeability of membranes due to molecular modifications of lipid bilayer under heavy metal stress are some of the main reasons for the decrease in plant growth. The successful growth and development of the plant are limited in every possible way by the severe phytotoxic effects caused by the oxidative stress under heavy metal stress (Maiti et al. , 2012). Our result is following the study on rice by Wu et al. , (2020).
The reduction of α-amylase activity was more in Ranjit cultivar than Aijung and that might reveal less mobilization of stored sugar during germination in Ranjit cultivar in exposure to As. He et al., (2010) mentioned that due to the reduction of amylase activity under stress conditions, mobilizations of endospermic products are also reduced and that inhibits seedling growth. Our result is in accordance with the study on Phaseolus vulgaris under cadmium (Cd) stress by Yuan et al., (2019).
Maiti et al. , (2012) reported that under various biotic and abiotic stresses, Hydrogen peroxide (H 2 O 2 ) may act as a secondary messenger to trigger many physiological mechanisms. The reaction of O2- with H 2 O 2 generates hydroxyl radicals that play a major role in lipid peroxidation of the membrane. The H 2 O 2 level was higher in the Ranjit cultivar than Aijung that suggests more oxidative damage in the Ranjit cultivar. Increased production of H 2 O 2 under stress conditions has also been reported in Triticum aestivum ( han et. al., 2007) and Brassica juncea by Mobin and han, (2007).
han and Panda, (2008) reported that lipid peroxidation is measured as the amount of malondialdehyde (TBARS or MDA) produced when the polyunsaturated fatty acids in the membrane undergo oxidation by the accumulation of free oxygen radicals. The MDA content of radicles was more than the plumule of both cultivars but the overall MDA content of both plumule and radicles were higher in Ranjit revealing the more damaging effect of As on its membrane than the Aijung cultivar. Finnegan and Chen, (2012) reported that As stress causes membrane damage that leads to electrolyte leakage and increased production of MDA (malondialdehyde). Our result is in agreement with the study on Brassica juncea by Ahmad et al. , (2021).
Nonenzymatic antioxidant pathway (ascorbate) catalyzes the detoxification of H2O2 by directly reacting with the AOS (Allene oxide synthase) in photosynthetic tissues, recycles α-tocopherol, and protects the prosthetic metal ions containing enzyme that is utilized as an ascorbate peroxidase substrate. The data of our present study showed more ascorbate activity in the Aijung cultivar than in the Ranjit cultivar. Yuan et al., (2020) reported that the nonenzymatic antioxidant activity of the rice cultivars under metal stress conditions implies their inherent capacity to cope with the stressed condition and that is considered as the major indicator of the potentiality of a species. Our result is in agreement with the study on rice by han et al., (2021).
The enhancement of SOD activity under metal stress conditions implies a clear indication of detoxification of the O2 - and other free radicals in different cell organelles in downstream pathways (Maiti et al. , 2012). The result of our present study implies that the two cultivars ingrained their differential antioxidant pathway under the same concentration of As and more efficiency is shown by the Aijung cultivar. Under moderate concentration of As (50µM As), SOD activity upregulated in both the cultivars but lowered at 100µM As stress, more inhibition was observed in Ranjit cultivar. The elevated activity of superoxide dismutase (SOD) under As stress was also reported by Shri et al. , (2019).
Under 100µM As stress, CAT activity was decreased in roots of both the cultivars, and that might have upregulated H 2 O 2 accumulation and form hydroxyl radicals that lead to damage of membrane lipids. But the upregulation of CAT activity at 50µM As stress was more prominent in Aijung cultivar and downregulation of its activity at 100µM As stress was more in Ranjit cultivar compared to their respective control signifying the more potentiality of Aijung cultivar to tolerate As stress than Ranjit cultivar. The declination of SOD and CAT activity at higher concentrations of As are also reported in Vigna radiata by atiyar et al. , (2020).
Both of the rice cultivars showed variation in enzymatic and nonenzymatic antioxidant activity signifying their limits of As stress tolerance and it can be considered to characterize sensitivity or tolerance to heavy metal stress. The data of our present study reveal that the Aijung cultivar showed better As stress tolerance as compared to Ranjit cultivar that might be due to less accumulation of ROS, higher amylase activity, restriction of membrane damage with the decrease in MDA and H 2 O 2 content by enhancement of antioxidative defense system.
CONCLUSION
The results of our study reveal that arsenic stress influenced germination, α- amylase activity, growth, non-enzymatic and enzymatic antioxidative activity in both the rice cultivars. The significant impact of As-induced stress was more prominent in the Ranjit rice cultivar than the Aijung cultivar. Ranjit cultivar showed more H2O2 and lipid peroxidation level and less enzymatic and non-enzymatic activity than Aijung under As stress that may suggest it to be less tolerant than Aijung cultivar. The comparative tolerance of Aijung cultivar to As stress than Ranjit cultivar might be associated with inherent high amylase and antioxidative activity under As stress. CONFLICTS OF INTEREST
The authors declare that they have no potential conflicts of interest.
Список литературы Impact of arsenic on the seedlings of Ranjit and Aijung, two most edible rice cultivars of Assam, India
- Ahmad A., Khan W.U., Shah A.A., Yasin N.A., Naz S., Ali A., Tahir A., Batool AI. (2021). Synergistic effects of nitric oxide and silicon on promoting plant growth, oxidative stress tolerance and reduction of arsenic uptake in Brassica juncea. Chemosphere, 262,128384.
- Alvarenga I.F., Dos Santos F.E., Silveira G.L., Andrade-Vieira L.F., Martins G.C., Guilherme L.R. (2020). Investigating arsenic toxicity in tropical soils: A cell cycle and DNA fragmentation approach. Sci. Total Environ., 698, 134272.
- Bag A.G., Nandi R., Chatterjee N., Dolui S., Hazra G.C., Ghosh M. (2019). Toxicity of arsenic on germination and seedling growth of indigenous aromatic rice varieties of India. Int. J. Chem. Sci., 7, 2889-96.
- Bernfeld P. (1955). Amylases, a and p.149-158.
- Chandrakar V., Yadu B., Meena R.K., Dubey A., Keshavkant S. (2017). Arsenic-induced genotoxic responses and their amelioration by diphenylene iodonium, 24-epibrassinolide and proline in Glycine max L. Plant Physiol. Biochem., 112, 74-86.
- Chance B., Maehly A.C. (1955). Assay of catalases and peroxidases.-Methods Enzymol. 2, 764-775.
- Ekmek?i Y., Tanyola? D., Ayhan, B. (2009). A crop tolerating oxidative stress induced by excess lead: maize. Acta physiologiae plantarum, 31(2), 319-330.
- Finnegan P.M., Chen W. (2012). Arsenic toxicity: the effects on plant metabolism. Front Physiol., 3, 182199.
- Giannopolitis C.N., Ries S.K. (1977). Superoxide dismutases: II. Purification and quantitative relationship with water-soluble protein in seedlings. Plantphysiol., 59(2), 315-318.
- Harminder P.S., Daizy R.B., Ravinder K.K., isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys., 125, 189-198.
- Komal A. (2007). Arsenic-induced root growth inhibition in mung bean (Phaseolus aureus Roxb.) is due to oxidative stress resulting from enhanced lipid peroxidation. Plant Growth Regul., 53: 65-73.
- He J., Ren Y., Pan X., Yan Y., Zhu C., Jiang D. (2010). Salicylic acid alleviates the toxicity effect of cadmium on germination, seedling growth, and amylase activity of rice. J. Plant Nutr. Soil Sci., 173(2), 300-5.
- Kalita J., Tanti B. (2020). Screening of some traditional rice cultivars of Assam, India, for their response to arsenic-induced abiotic stress. Acta Agrobotanica, 73(1).
- Kaneko M., Itoh H., Ueguchi-Tanaka M., Ashikari M., Matsuoka M. (2002). The a-amylase induction in endosperm during rice seed germination is caused by gibberellin synthesized in epithelium. Plant Physiol, 128(4), 1264-1270.
- Keshavkant S. (2020). Titanium nanoparticles attenuates arsenic toxicity by up-regulating expressions of defensive genes in Vigna radiata L. J. Environ. Sci., 92, 18-27.
- N., Irfan M., Sehar Z., Khan N.A. (2021). Crosstalk of plant growth regulators protects photosynthetic performance from arsenic damage by modulating defense systems in rice. Ecotoxicol. Environ. Sal., 222,112535.
- Khan M.H., Panda S.K. (2008). Alterations in root lipid peroxidation and antioxidative responses in two rice cultivars under NaCl-salinity stress. Acta Physiol. Plant., 30(1), 81-89.
- Khan N.A.S., Singh S., Nazar R. (2007). Activities of antioxidative enzymes, sulphur assimilation, photosynthetic activity and growth of wheat (Triticum aestivum) cultivars differing in yield potential under cadmium stress. J. Agron. Crop Sci., 193(6), 435-444.
- Kumar J., Kumar S., Mishra S., Singh, A. K. (2021). Role of zinc oxide nanoparticles in alleviating arsenic mediated stress in early growth stages of wheat. J. Environ. Biol, 42, 518-523.
- Kumar A., Basu S., Kumar, G. (2021). Evaluating the effect of seed-priming for improving arsenic tolerance in rice. J. Plant Biochem. Biotechnol., 1-5.
- Lin C.C., Kao C.H. (2001). Abscisic acid induced changes in cell wall peroxidase activity and hydrogen peroxide level in roots of rice seedlings. Plant Sci.,160(2), 323-9.
- Liu H., Probst A., Liao B. (2005). Metal contamination of soils and crops affected by the Chenzhou lead/zinc mine spill (Hunan China). Sci Total Environ., 339, 153-166.
- Maiti S., Ghosh N., Mandal C., Das K., Dey N., Adak, M.K. (2012). Responses of the maize plant to chromium stress with reference to antioxidation activity. Braz. J. Plant Physiol., 24(3), 203-212.
- Mobin M., Khan N.A. (2007). Photosynthetic activity, pigment composition and antioxidative response of two mustard (Brassica juncea) cultivars differing in photosynthetic capacity subjected to cadmium stress. J. Plant Physiol, 164(5), 601-610.
- Mridha D., Paul I., De A., Ray I., Das A., Joardar M., Chowdhury N.R., Bhadoria P.B.S. Roychowdhury, T. (2021). Rice seed (IR64) priming with potassium humate for improvement of seed germination, seedling growth and antioxidant defense system under arsenic stress. Ecotoxicol. Environ. Saf., 219, 112313.
- Noctor G., Foyer C.H. (1998). Ascorbate and glutathione: keeping active oxygen under control. Annu. Rev. Plant biol., 49(1), 249-79.
- Oser B.L. (1979). Care and maintenance of animals. Hawks Physiological Chemistry, McGraw Hill Inc, New York.1372-77.
- Pandey C., Khan E., Panthri M., Tripathi R.D., Gupta, M. (2016). Impact of silicon on Indian mustard (Brassica juncea L.) root traits by regulating growth Heath R.L., Packer L.J. (1968). Photoperoxidation in Katiyar P., Yadu B., Korram J., Satnami M.L., Kumar M., Khan M.I., Jahan B., AlAjmi M.F., Rehman M.T., Iqbal parameters, cellular antioxidants and stress modulators under arsenic stress. Plant Physiol. Biochem., 104, 216-225.
- Rui M., Ma C., Hao Y., Guo J., Rui Y., Tang X., Zhao Q., Fan X., Zhang Z., Hou T., Zhu, S. ( 2016).Iron oxide nanoparticles as a potential iron fertilizer for peanut (Arachis hypogaea). Front. Plant Sci., 7, 815.
- Shankar U. (2006). Seed size as a predictor of germination success and early seedling growth in 'hollong' (Dipterocarpus macrocarpus Vesque). New For., 31, 305-320.
- Shri M., Kumar S., Chakrabarty D., Trivedi P.K., Mallick S., Misra P., Shukla D., Mishra S., Srivastava S., Tripathi R.D., Tuli, R. (2009). Effect of arsenic on growth, oxidative stress, and antioxidant system in rice seedlings. Ecotoxicol. Envirol. Saf., 72(4), 11021110.
- Singh A.P., Dixit G., Kumar A., Mishra S., Kumar N., Dixit S., Singh P.K., Dwivedi S., Trivedi P.K., Pandey V., Dhankher O.P. (2017). A protective role for nitric oxide and salicylic acid for arsenite phytotoxicity in rice (Oryza sativa L.). Plant Physiol. Biochem., 115,163-73.
- Wu F., Fang Q., Yan S., Pan L., Tang X., Ye W. (2020). Effects of zinc oxide nanoparticles on arsenic stress in rice (Oryza sativa L.): germination, early growth, and arsenic uptake. Environ. Sci. Pollut. Res., 27(21), 26974-26981.
- Yadu B., Chandrakar V., Tamboli R., Keshavkant S. (2019). Dimethylthiourea antagonizes oxidative responses by upregulating expressions of pyrroline-5-carboxylate synthetase and antioxidant genes under arsenic stress. Int. J. Environ. Sci. Technol., 16, 8401-8410.
- Yuan Y., Imtiaz M., Rizwan M., Dong X., Tu, S. (2020). Effect of vanadium on germination, growth and activities of amylase and antioxidant enzymes in genotypes of rice. Int. J. Environ. Sci. Tech., 17(1), 383-394.


